Use of space, activity patterns, and foraging behavior of red howler monkeys (Alouatta seniculus) in an Andean forest fragment in Colombia
Abstract
Howler monkeys are among the most studied primates in the Neotropics, however, behavioral studies including estimation of food availability in Andean forests are scarce. During 12 months we studied habitat use, behavior, and feeding ecology of two groups of red howler monkeys (Alouatta seniculus) in an isolated fragment in the Colombian Andes. We used a combination of focal animal and instantaneous sampling. We estimated fruit production (FP) using phenology transects, and calculated young leaf abundance by observing marked trees. The home range area used by each group was 10.5 and 16.7 ha and daily distances traveled were 431 ± 228 and 458 ± 259 m, respectively. We found that both groups spent most of their time resting (62–64%). Resting time did not increase with leaf consumption as expected using a strategy of energy minimization. We did not find a relationship between daily distances traveled and leaf consumption. However, howlers consumed fruits according to their availability, and the production of young leaves did not predict feeding time on this resource. Overall, our results are similar to those found on other forest types. We found that despite limited FP in Andean forests, this did not lead to a higher intake of leaves, longer resting periods, or shorter traveling distances for red howlers. Am. J. Primatol. 73:1062–1071, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Activity patterns in primates are influenced by physical and socioecological factors such as soil fertility, food distribution and availability, and competition for resources [Janson, 1985; Stevenson et al., 2000; Van Noordwijk & Van Schaik, 1987]. Seasonal and individuals changes in activity budget and diet highlight the ability of foragers to regulate their nutritional and metabolic requirements by reducing energy expenditure and/or selecting energy-rich or protein-rich food resources [Bronikowski & Altmann, 1996; Felton et al., 2009; McNab, 1978]. In the case of howler monkeys (Alouatta spp.) data provided by Gaulin and Gaulin [1982] indicate that diet composition differs between individuals living in lowland and montane forest. This is because plant species diversity and forest productivity decrease with elevation and at lower temperatures [Cavelier et al., 2001; Gentry, 1992]. The genus Alouatta is reported to exploit a range of forest types including highly disturbed forest fragments [Bicca-Marques, 2003; Cristóbal-Azkarate & Arroyo-Rodríguez, 2007]. However, little is known concerning the feeding ecology of howler's exploiting Andean forest fragments where fruit production (FP) is reported to be low [Gentry, 1992; Giraldo et al., 2007].
Alouatta is the most widely distributed ateline genus, with a geographic range extending from the dry, deciduous forests of the northern Argentine Chaco to Central America as far north as eastern Mexico [Di Fiore & Campbell, 2011]. In contrast to most other primates in the Neotropics (expect possibly Brachyteles), the diet of howler monkeys can include a large proportion of leaves [Bicca-Marques, 2003; Julliot & Sabatier, 1993]. Howlers have been described as facultative folivores because they seem to be frugivorous by choice but folivorous by necessity [Milton, 1980], switching from a leaf-based diet to a fruit-based diet in times of fruit scarcity [Stevenson et al., 2000]. This ability may be a primary reason why howler monkeys are able to survive in fragmented and isolated landscapes [Clarke et al., 2002; Crockett, 1996; Estrada et al., 1999; Mandujano & Escobedo-Morales, 2008; Schwarzkopf & Rylands, 1989], where they can maintain high population densities [Bravo & Sallenave, 2003].
Howler monkeys are reported to survive in home ranges as small as ≤1 ha [Gilbert, 2003; Terborgh et al., 2001], and as large as 182 ha [Palacios & Rodriguez, 2001]. Average daily distance traveled ranges between 340 and 1,150 m [Defler, 2004]. Several researchers have found a positive correlation between the amount of leaves howler monkeys eat, and the time they spent resting [Estrada et al., 1999; Gaulin & Gaulin, 1982; Silver et al., 1998]. This correlation is explained by suggesting that howlers are required to reduce energy costs, especially during periods of leaf eating in order to ferment, digest, and extract energy from the structural carbohydrates present in leaves [Milton, 1980]. Fermentation is facilitated by microbacteria in the howler gut. However, other studies have demonstrated that when exploiting fruit, howlers reduce the number of food sources exploited and time spent feeding, leading to a reduced feeding effort and increased time available for rest as occurs during leaf eating [Dunn et al., 2008].
Red howler monkeys (Alouatta seniculus) are broadly distributed in Colombia, inhabiting lowland forests in the Amazon basin, riverine forests along the Eastern Llanos, and all the Colombian Andes [Hernández-Camacho & Cooper, 1976]. Although the ecology of red howlers has been well studied in a variety of lowland forests (Tables I and II) only one long-term study has examined diet and feeding ecology in an Andean population of red howlers [Gaulin & Gaulin, 1982]. Despite the fact that red howler monkeys are not listed as endangered in Colombia [Defler, 2004], populations living in the Andes are at risk due to deforestation. Less than 30% of the original area of Andean forest remains, and most of these patches are highly fragmented and isolated [Kattan & Alvarez-Lopez, 1996] resulting in howler populations that are isolated in forest areas as small as 10 ha. In order to protect and manage these populations, detailed studies are needed on patterns of ranging and habitat use in relation to their population densities [Gómez-Posada & Roncancio, 2009], and red howler activity patterns and feeding behavior in relation to food availability.
| Taxon | Home range size (ha) | Average daily distance traveled (m) | Forest type | Population density (ind/km2) | Source | Number of groups |
|---|---|---|---|---|---|---|
| Alouatta seniculus | ∼22 | 706 | Mountain Andean forest | 15 | Gaulin and Gaulin [1982] | 1 |
| A. seniculus | 182 | 1,150 | Lowland tropical forest | 4 | Palacios and Rodriguez [2001] | 1 |
| A. seniculus | 14 | 412 | Mountain Andean forest | 72.6 | Gómez-Posada et al. [2007] | 1 |
| A. seniculus | 10.5 and 16.7 | 431 ± 228 and 458 ± 259 | Mountain Andean forest | 169 | This study and Goméz-Posada and Roncancio [2009] | 2 |
| A. seniculus | 5.1 | 375 | Lowland tropical forest | – | Sekulic [1982] | 1 |
| A. seniculus | 28.4 | ∼550 | Lowland tropical forest | – | Izawa [1997] | 1 |
| A. seniculus | 8 | ∼500 | Lowland tropical forest | 114.4 | Neville [1972] | 1 |
| Taxon | Foraging | Moving | Resting | Source |
|---|---|---|---|---|
| Alouatta belzebuth | 20 | 18.2 | 58.7 | Pinto [2002] |
| Alouatta caraya | 15.9 | 17.6 | 61.6 | Bicca-Marques [1993] |
| Alouatta guariba | 17.3 | 11 | 71.8 | Mendes [1989] |
| A. guariba | 19 | 18.8 | 57.6 | De Marques [1996] |
| Alouatta palliate | 16.2 | 10.2 | 65.5 | Milton [1980] |
| A. palliate | 17.3 | 2.2 | 79.7 | Estrada et al. [1999] |
| Alouatta pigra | 24.4 | 9.8 | 61.9 | Silver et al. [1998] |
| Alouatta Seniculus | 12.7 | 6.2 | 78.5 | Gaulin and Gaulin [1982] |
| A. seniculus | 22 | 11 | 67 | Neves and Rylands [1991] |
| A. seniculus | 22 | 10 | 63 | This study |
The objectives of this study are to describe the use of space, activity patterns, and foraging behavior of two groups of red howler monkeys (Alouatta seniculus) inhabiting an Andean forest fragment. At this site, howler population density is 169 ind/km2 [Gómez-Posada & Roncancio, 2009]. This value is five times greater than that commonly reported for this species. It remains unclear whether this high population density is unsustainable and a result of recent habitat disturbance (the same number of individuals living in a smaller area) or reflects the fact that Andean forests represent high-quality habitats for red howlers. Following Orihuela Lopez et al. [2005], we expect (1) a small home range area for our study groups, and a negative relationship between home range area and howler population density. Given that plant diversity decreases with elevation and the overall production of forests is lower at lower temperatures [Cavelier et al., 2001; Gentry, 1992], we expect that Andean red howler monkeys would (2) include a large proportion of leaves in their diet (more than 60% of total feeding time), and (3) use an energy minimizing strategy (e.g. a positive correlation between the time feeding on leaves and resting time).
METHODS
This research adhered to the legal requirements of Colombia and to the American Society of Primatologists, Principles for the Ethical Treatment of Non Human Primates.
Study Site
The study site is a pre-montane isolated forest fragment located in the Yotoco Reserve, Departamento del Valle del Cauca, Colombia (3°50′N and 16°20′W) (Fig. 1). It has approximately 559 ha and lies between 1,200 and 1,600 m above sea level. Mean temperature in this area is 20°C and there are two rainy seasons: between March and May, and between September and October. Yearly rainfall averages 1,500 mm [Escobar, 2001]. The Reserve is a protected area surrounded by pastures and cattle.
Geographical location of the study site in Colombia and the location of each study group's home range.
Data Collection
We collected data on activity, diet, and habitat use using a combination of focal animal and instantaneous sampling [Altmann, 1974] during a period of 12 months (July 2004–June 2005). We studied two groups of red howler monkeys: A0 and A9. At the beginning of the study, A0 consisted of two adult males, two adult females, one sub adult female, one juvenile female, and two infants. A9 had two adult males, two adult females, and one sub adult female. Subadult females were identified by body size and estimated to be 4 years of age. Both groups had a new born infant during February 2005. All group members were individually recognized, using facial features, sex, and body size.
We used focal sampling to study the activity patterns and diet of both groups. This was accomplished by continuous observational records for five consecutive days per month per group, devoting each day to a different individual (only the 5 adults in each group were chosen as focal animals). Each group was observed 12 hr (6:00–18:00) per day, which has been recommended for studies on feeding behavior [Felton et al., 2009]. During the observations we recorded the activity of the focal individual every 10 min and the duration of each feeding bout. The activities were classified into five categories: (1) feeding, (2) moving (any time the focal individual was traveling without feeding), (3) resting, (4) social activities (playing, grooming and intergroup encounters), and (5) others (any other behavior).
To study the diet of the groups, we recorded the species and the type of item (mature fruit, immature fruit, mature leaf, young leaf, flower, or bark) the focal individual was feeding on, and the total time the focal individual spent feeding on each food type. We recorded the location of the groups every 15 min using maps, marked trails, and a measuring tape. To determine the area of each group's home range, a quadrant system based on 50 m × 50 m plots was superimposed on our site map. All plots visited by the monkeys at least once were counted [NRC, 1981]. Although this method tends to overestimate home range because it is assumes that the entire area of each plot was visited by the monkeys, we feel that it represents a reasonable approximation of howler habitat use. We used the recorded locations of the group every 15 min to estimate the daily traveled distance. To identify the most frequently used plots and to examine relationships between the number of feeding and sleeping trees and patterns of habitat utilization, we marked all feeding and sleeping trees used by the groups during the study. Every time we saw other howler groups using an area also used by the study groups', we recorded the event on the map to calculate the amount of habitat shared across groups.
We used phenology transects and marked trees to estimate seasonal changes in food availability.
(1) Phenology transects: we identified two transects, t0 and t9, then selected our two groups of howler monkeys that used this area. The width of a given part of the transect was estimated by measuring the perpendicular distance between tree trunks and the transect, allowing us to adjust the width to the size of the tree (i.e. large plants are sampled in a larger area than small plants) [Stevenson, 2002; Stevenson & Link, 2010]. The total length of transects was 3.5 km (1.7 km = t0, t9 = 1.8 km) and the width varied between 0 and 14.5 m. Thus, the area of the transect was on average 3.8 ha. We identified all fruiting trees whose crowns were projected over the transects (N = 227). We calculated the approximate number of fruits in the selected trees by counting the number of fruits present on some branches, estimating an average and multiplying this by the number of similar-size branches on each tree (with the aid of binoculars). We measured the dry weight of five to ten fruits of each species found in transects to obtain an average dry weight (by achieving constant weight for the fruits placed in a wooden oven) and then, we estimated the fruit biomass produced by each tree. FP for each tree species was calculated by summing the production of each tree of that species and dividing by the sampling area. We checked transects every 15 days, from the beginning of July 2004 until the end of June of the following year.
(2) Marked trees: We used this method to estimate the production of new leaves in the forest. We chose seven of the most common tree species in the reserve following Escobar [2001], and marked 5–7 trees of each species (Total number of censused trees was 47). Trees were checked every 2 weeks from July 2004 until the end of June 2005. To estimate the availability of young leaves in the forest, we visually estimated the percentage of the crown with young leaves of these marked trees. The percentage of new leaves in each tree was used to estimate the monthly production of new leaves for each species.
Statistical Analysis
All tests were performed using SPSS for Windows 15.0 and Statistica 6.1. We considered statistical tests significant at the P<0.05 level. Diet and activity data are presented as a percentage of the overall time spent in each behavioral category over the study period.
We determined daily traveled distances by summing the straight-line distances measured between sequentially used trees during daily group follows, and we averaged daily traveled distances values for each month. Using this method, our daily travel distances represent minimal values because the howlers did not always travel in a straight-line between feeding and resting sites. We calculated home range overlap between groups as the percent area each group shared with neighbors relative to the total home range occupied by each of the study groups. We used a linear regression analysis to test that home range area diminished as population density increased. For this purpose, we included 13 sites where both variables were studied and the estimates were available in the published literature (references in Defler [2004]; DiFiore and Campbell [2011] and Stevenson [2001]). The model used log transformations for both variables. To investigate differences in activity patterns between months, we employed one way-ANOVAs. We used Spearman's correlations to determine (1) if activities (resting and moving time) were related to the time devoted to consume different food items (fruits and leaves); (2) if relationships existed between resource production (fruits and new leaves), their consumption, and monthly distances traveled; and (3) if FP and leaf consumption were correlated. We used a linear regression to investigate whether fruit consumption (FC) was predicted based on fruit availability.
RESULTS
Use of Space
A0 and A9 had home ranges of 10.5 and 16.75 ha (Fig. 2A and B) and traveled daily distances of 431 ± 228 and 458 ± 259 m, respectively. We found that both groups did not use quadrants within their home range evenly (Fig. 2A and B). When we analyzed published information for nine other red howler monkey populations (A. seniculus), we found a negative relationship between population density and home range area (F = 24.3, P<0.001, N = 13), indicating that our study groups showed relatively small home ranges and high population densities (Fig. 2C).
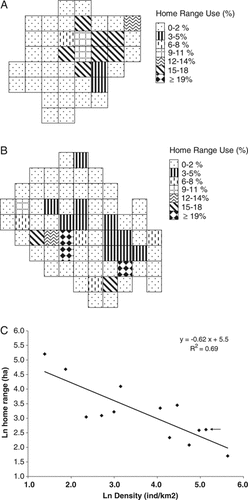
Pattern of home range use for two groups of Howler monkeys in the Yotoco reserve: (A) A0 and (B) A9. Each square equals a 50 m × 50 m plot. Percentages of home range use are given. (C) Relationship between population density and home range area in Howler monkey populations from published literature (references in Stevenson [2001], Defler [2004], and Di Fiore and Campbell [2011]). The arrow indicates our study population.
Activity Patterns
A0 and A9 spent most of their time resting (62.4 and 63.9%), followed by feeding (22.1 and 23.1%), moving (10.9 and 9.8%), and other activities (4.6 and 3.2%). Because the time devoted to feeding on mature leaves was low (less than 8% of all feeding time for both groups), we grouped mature and young leaves together as “leaves” for further analysis. Since the time devoted to feed on immature fruits was low (less than 5% of all feeding time for both groups) fruits (mature and immature) were grouped as “fruits” for further analysis.
Time spent by A0 feeding varied between months (F = 4.13, df = 1, P = 0.02) (Fig. 3A). The group spent 59.7% of their feeding time consuming new leaves, 30.1% fruits, 7.3% mature leaves, and 2.9% on other items (flowers, bark and soil). The time the group spent resting and moving varied between months (resting: F = 4.34, df = 1, P = 0.03; moving: F = 3.85, df = 1, P = 0.04). There was no correlation between time spent resting and the time spent feeding on leaves or fruits (leaves: rs = 0.36, P = 0.07, N = 12; fruits: rs = 0.10, P = 0.29, N = 12). Time spent moving and time spent feeding on leaves or fruits also was not correlated (Leaves: rs = 0.06, P = 0.38, N = 12; Fruits: rs = 0.05, P = 0.44, N = 12).
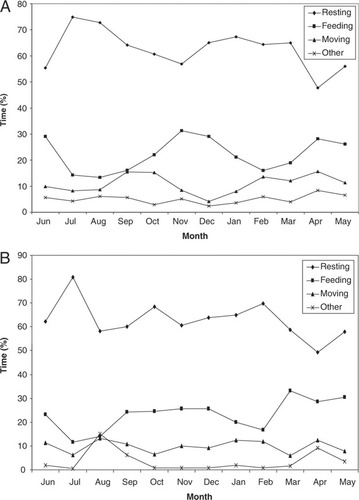
Activity patterns for two groups of howler monkeys in the Yotoco Reserve, in a premontane forest in Colombia: (A) A0 and (B) A9.
In the case of A9, time spent feeding also varied between months (F = 3.21, df = 5, P = 0.03) (Fig. 3B). This group spent 76.7% of their feeding time consuming new leaves, 13.3% fruits, 6.2% mature leaves, and 3.8% on other items (flowers, bark, and soil). The time A9 spent resting and moving varied between months (resting: F = 4.28, df = 5, P = 0.02; moving: F = 3.25, df = 1, P = 0.03). Time spent resting and time spent feeding on leaves or fruits showed no significant correlation (Leaves: rs = 0.03, P = 0.19, N = 12; Fruits: rs = 0.09, P = 0.36, N = 12). Similarly, time spent moving time and time spent feeding on leaves or fruits also was not correlated (Leaves: rs = 0.03, P = 0.42, N = 12; Fruits: rs = 0.06, P = 0.44, N = 12)
Leaf and FP and Consumption
The percentage of new leaves available (leaf production) varied significantly across the year (F = 2.85, N = 12, P = 0.04), it was lowest between July and October 2004, and highest between January and June 2005 (Fig. 4A). FP also varied during the year (F = 5.75, N = 12, P = 0.02). Peak FP was recorded from August until September, due to the production of Pouteria camito (Sapotaceae) (Fig. 4B).
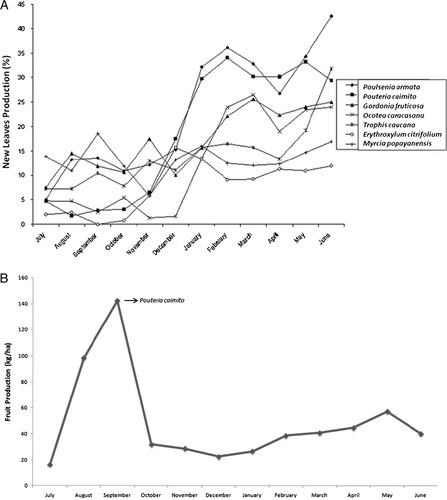
Patterns of resource availability during the study year in the Yotoco Reserve (Valle, Colombia). (A) Production of new leaves of seven common species consumed by howler monkeys. (B) Overall fruit production (kilograms of fruit /hectare).
For A0 and A9, significant correlations were found between FP and FC (A0 rs = 0.14, P = 0.03, N = 12; A9: rs = 0.31, P = 0.04, N = 12), but not between new leaf production (NLP) and new leaf consumption (NLC) (A0: rs = 0.02, P = 0.64, N = 12; A9: rs = 0.30, P = 0.07, N = 12). In A0, FP, NLC, and FC were significantly correlated with monthly distances traveled (FP: rs = 0.43, P = 0.02, N = 12; NLC: rs = 0.34, P = 0.04, N = 12; FC: rs = 0.33, P = 0.05, N = 12), while NLP showed no significant correlation with monthly distances traveled (NLP: rs = 0.07, P = 0.39, N = 12). For A9, neither FP, NLC nor FC were significantly correlated with monthly distances traveled (FP: rs = 0.16, P = 0.21, N = 12; NLC: rs = 0.33, P = 0.06, N = 12; FC: rs = 0.24, P = 0.07, N = 12). In this group, NLP and monthly distances traveled were significantly correlated (rs = 0.45, P = 0.02, N = 12), suggesting that there was an incentive to search for new leaves in different patches when they were available.(Fig. 5A and B show fruit, NLC, and monthly distances traveled for each group).
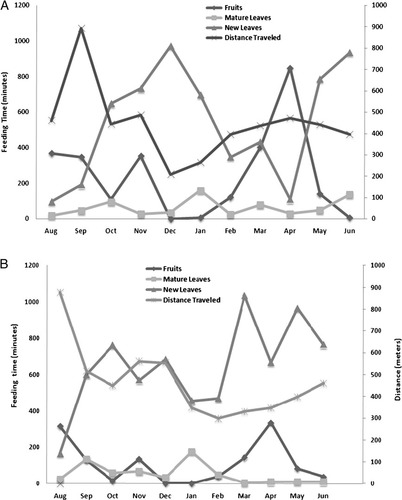
Fruit and leaf consumption in two groups of howler monkeys A0 (A) and A9 (B), based on time spent feeding and monthly distance traveled.
A linear regression to predict FC from fruit availability showed a positive slope (Fig. 6). The highest production corresponded to Pouteria caimito, followed by Poulsenia armata and Nectandra pichurin. The points with values lower than zero represent species that were consumed less than expected according to their availability (abundance). For both groups, FP and LC were negatively related (A0: rs = −0.72, P = 0.004, N = 12; A9: rs = −0.63, P = 0.03, N = 12).
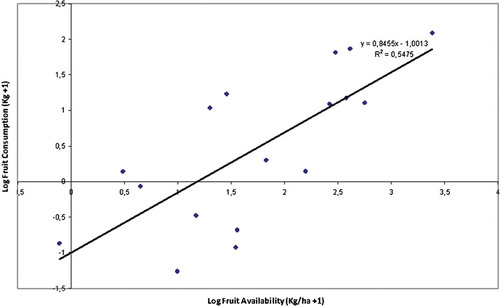
Regression analysis between fruit availability in the forest and FC by red howler monkeys during the study period in the Yotoco Reserve. FC, fruit consumption.
DISCUSSION
Given the high density of howler monkeys at our study site, we expected both groups to have small or highly overlapping home ranges. Our results confirmed our expectations, home ranges were generally small compared with red howler populations at other sites, and home range overlap among neighboring groups was 19.4% for A0 and 11% for A9. (Table I, Fig. 2C). Under conditions of high population density, red howlers exhibit reduced home range size. Population density and home range size or the total area used by an established social group over a period of time is expected to be affected by several factors such as habitat quality (usually defined for folivores as the density of resources with high nitrogen and low fiber components [Ganzhorn, 1992]), group size, anthropogenic disturbance and deforestation, hunting pressure, presence of competitors, and the percentage of home range overlap with other groups [Chapman et al., 2006; Janson & Goldsmith, 1995; Palacios & Rodriguez, 2001; Peres, 1997; Peres & Palacios, 2007; Snaith & Chapman, 2005]. In the case of red howlers at our study site, hunting is rare. The area has been protected from deforestation and selective logging for 60 years, and other species of leaf-eating primates are absent. Therefore, we argue that in relatively undisturbed forests, resource availability and inter-group competition represent the strongest factor affecting populations.
Time spent resting by both red howler study groups averaged 62–64%, and daily travel distances were similar to those reported in other studies (Table I). Overall, individuals in our study groups were characterized by an energy minimizing strategy typically reported for howlers [Milton, 1980]. Relatively long-resting periods appear to be a behavioral adaptation used to reduce energy expenditure during the digestion of leaves [Milton, 1998], which are a low energy resource that requires microbial fermentation to breakdown structural carbohydrates [Milton, 1980]. This is confirmed by studies that show that leaf consumption and resting time are highly correlated in howlers [Estrada et al., 1999; Gaulin & Gaulin, 1982; Silver et al., 1998]. Such a strategy is not only used by howler monkeys but it also has been reported in other folivorous primates [Dasilva, 1992; Milton, 1984]. However, we did not find a significant correlation between the time spent consuming leaves and the time spent resting for either group. Thus, even during periods of increased FC, red howlers maintained a relatively high level of inactivity and an energy minimizing pattern [Pavelka & Knoff, 2004]. For example, during months when fruits comprised more than 50% of red howler feeding time, day range continued to average 505 ± 230 m, and time spent resting was more than 60% of the activity budget for both groups. These results appear to contradict a leaf-eating, energy-saving strategy. During our study, the forest did not experience a long and marked period of fruit scarcity. For example, we estimated monthly FP during scarcity periods at approximately 30 kg/ha, while in another site, where the same methodology was implemented (Tinigua National Park, Colombia), the lowest monthly values were close to 10 kg/ha [Stevenson, 2005]. Thus, it is possible that in the Yotoco Reserve, the howlers did not alter their ranging and activity patterns when consuming fruit (i.e. maintained an energy-saving strategy) due to both the relative availability of fruit throughout the year, as well as a pattern of daily resource and range defence. We observed an average of 2.1 intergroup encounters per day.
Our results suggest that red howlers preferred fruits in time of abundance, while the intake of leaves although high (60–77% of feeding time) showed less seasonal variation. It is likely that red howler monkeys require a minimum daily intake of leaves in order to maintain protein demands, as have been documented for other atelids [Felton et al., 2009]. We found that time spent consuming different items was related to temporal variation in food availability. Although young leaves were available throughout all months of the study period, the amount of leaves eaten by the groups varied each month (although at least 8% of feeding time in each month was devoted to young leaves) with no relation to their availability. It is possible that red howlers consume at least 8% of young leaves each month in order to obtain sufficient protein, and then supplement the rest of their diet based on the availability and distribution of fruits, leaves, and flowers. Although young leaves may be difficult to locate, they are assumed to be richer in digestible protein content than mature leaves and have less fiber and toxic compounds [Ganzhorn, 1992]. In a study on Barro Colorado Island, Panama, Milton [1984] found that young leaves contained 33% more protein than mature leaves, and with only few exceptions the fiber content of young leaves was 36% lower than that of mature leaves. This may help to explain why both groups spent considerably more time eating young leaves than mature ones.
Our results on use of space, activity patterns, and foraging behavior are similar to those found for red howlers at other sites, suggesting that the red howler population in the Yotoco Reserve might be able to live sustainably in this isolated fragmented forest. Nonetheless, it is important to continue studying these groups, to determine if their current population is stable over time. Although howlers remain one of the most abundant primate species in the Neotropics, their populations are diminishing [Crockett, 1998], and continued habitat loss in the Andes is a major threat. We suggest that long-term studies in the Yotoco Reserve should be undertaken to see if the fragment's isolation has an impact on howler's population densities and home range stability, and to examine how the groups' home ranges change through time and in response to increases in population density and home range overlap.
ACKNOWLEDGMENTS
This research was funded by Fundación EcoAndina/WCS Colombia Program, Corporación Autónoma Regional del Valle del Cauca (CVC), grants from the John D. and Catherine T. MacArthur Foundation and from US Fish and Wildlife Service. We thank the CIRNY for their permission to work at the Reserve, Yesid Lozano, Francisco Hidalgo, and Valentín Hidalgo for their field assistance; and Joaquín Romero, Adolfo Velez, and Dr. Gustavo Kattan for their continuous advice and support to this project.




