Nocturnal sleeping habits of the Yunnan snub-nosed monkey in Xiangguqing, China
Abstract
Weather, predation, and social organization are hypothesized to influence sleeping habits of nonhuman primates at night. To investigate how the Yunnan snub-nosed monkey (Rhinopithecus bieti) prepares for and behaves during cold nights in their harsh alpine forest habitat (above 3,000 m), we studied the sleeping habits of the 171 one-male units (OMU) in one group for 12 months at Xiangguqing in the Baimaxueshan Nature Reserve, China. It took 20.2 min from the time the study group entered a sleeping site until they fell asleep. This duration was consistent over seasons. On average, sleeping time was 11.5 hr per night over the year. Seasonal mean lengths of sleeping time varied significantly, however, and ranged from 10 to 13 hr per night, correlating with night length. Two sleeping styles were distinguishable: solitary sleeping and huddled sleeping. That adult males in OMUs principally slept alone. This is likely to reflect night-time guarding behavior. Female–juvenile and female–infant dyadic huddles were the most prevalent sleeping unit (42% of all observed data), and the monkeys employed female-biased huddling during nocturnal sleep. Huddled sleeping group size showed significant seasonal variation, with the largest huddle (eight individuals) occurring in winter. Climate and social organization profoundly influence the nocturnal sleeping habits of R. bieti, while huddling behavior may help shield animals from cold nights and provide additional protection against predators. Am. J. Primatol. 72:1092–1099, 2010. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Primate sleeping habits are highly variable and accommodate the survival, safety, and sleep quality of individuals [Altmann, 1980]. Many factors may relate to sleeping habits in primates. Climate and weather [Aquino & Encarnación, 1986], i.e. temperature, day length, rain, and snow, affect the sleep habits of temperate primates for they must cope with low temperatures and food scarcity in winter. For example, François' Langurs (Trachypithecus françoisi) entered sleeping sites earlier in winter and spring than in summer and autumn in response to seasonal changes in photoperiod [Zhou et al., 2009]. In addition to climatic factors, predator avoidance is likely a major influence [Anderson, 1998; Di Bitetti et al., 2000; Zhang, 1995]. Animals' behavior should minimize their vulnerability to predation [Anderson, 1984; Reichard, 1998]. Studies of individual patterns of night-time sleeping behavior are also related to social organization, and the nature of social relationships among individuals [Di Bitetti et al., 2000]. These social relationships may be less frequently or less clearly expressed during daytime activities [Altmann, 1980]. Investigating the sleeping behavior of diurnal primates also contributes to understanding social stability at night [Takahashi, 1997]. Other factors that may influence sleeping site location include proximity to major feeding sites [Chapman et al., 1989; Huang et al., 2003; von Hippel, 1998; Zhou et al., 2009], parasite avoidance [Hausfater & Maede, 1982; Li et al., 2006], comfort [Anderson, 1984, 1998; Liu & Zhao, 2004], and ranging patterns [Chapman, 1989; Cui & Xiao, 2006; Heymann, 1995; Ramirez, 1989].
Our study investigated sleeping behavior in the Yunnan snub-nosed monkey (Rhinopithecus bieti), a rare colobine inhabiting high-altitude temperate forests (3,000–4,500 m above sea level) in the Hengduan Mountains of Northwest Yunnan and Southeast Tibet [Long et al., 1994, Xiang et al., 2007]. This species, the highest-altitude primate in the world, faces significant environmental pressure through low temperature, especially at night. Previous studies, which focused on choice of sleeping sites on a macro- and microhabitat basis, have shown that R. bieti is highly selective about night sleeping sites, favoring conifer forests, and mixed deciduous/conifer forests [Cui & Xiao, 2006; Li et al., 2006; Liu & Zhao, 2004; Xiang, 2005]. R. bieti live in a nested social system in which one-male units (OMUs) and all-male units (AMUs) associate to form larger groups [Grueter & van Schaik, 2010; Kirkpatrick et al., 1998]. However, previous studies have not provided data on sleeping behavior per se, i.e. activities performed at the sleeping sites and arrangement of sleeping clusters. Although R. bieti has been observed to frequently form huddling clusters in the daytime [Kirkpatrick, 1996; Ren et al., 2008], it is not known whether this behavior occurs at night and whether this relates to thermoregulation or social cohesion and stability [Hanya et al., 2007; Wada et al., 2007].
Our study aimed to determine the effects of weather and seasonality, predator pressure, and social cohesion/stability on the sleeping habits of R. bieti. We evaluated three sets of hypotheses related to these factors: (1) Monkeys will sleep for longer periods in winter, depart from sleeping sites later in the morning after snowy or rainy nights, and huddle in larger groups as air temperature decreases. (2) Vulnerable individuals such as females with an infant will enter the sleeping tree first to find a safe place of refuge or shelter. (3) Individuals in an OMU are expected to huddle together, with female–juvenile and female–infant dyads being the most frequent. Adult males are expected to sleep alone to keep watch and potentially drive off non-OMU members.
METHODS
Study Site and Climate
We conducted the study between June 2008 and May 2009 at Xiangguqing [27°37′N, 99°22′E], which is in the Samage Forest in the Baimaxueshan National Nature Reserve, PRC (Fig. 1). The research area covers almost 90 km2 of subtropical to temperate forest, including previously clear-cut areas and cattle-grazing land. Forest cover is a mosaic of mixed coniferous and deciduous broadleaf forest (2,900–3,600 m), subalpine fir forest (Abies georgei, 3,500–4,000 m), montane sclerophyllous oak forest (Quercus pannosa, 3,200–3,500 m), subtropical evergreen broadleaf forest (Cyclobalanopisis spp., 2,500–3,000 m), and pine forest (Pinus yunnanensis, 2,500–3,100 m). Seasons are classified as spring (March–May), summer (June–August), autumn (September–November), and winter (December–February) [Grueter et al., 2009; Li et al., 2008]. Some diurnal and nocturnal raptors are potential predators of R. bieti at Baimaxueshan Nature Reserve [Bai et al., 1987]. Infants and immature individuals are particularly vulnerable. Although predators of R. bieti are now rarely found in the study area [Kirkpatrick, 1996; Li et al., 2006], Cui [2003] reported a buzzard attempting to hunt juvenile R. bieti.
Map of natural groups of Rhinopithecus bieti and the study site.
The study area is characterized by distinct seasonality in precipitation and temperature. We collected detailed climatic data at the study camp (located at a height of 3,038 m), within the core area of the primate group's home range. Annual rainfall was 1,371 mm over the course of the study, with 70% of the rain falling between June and October 2008 (Fig. 2). There was a dry period from November 2008 to January 2009, with only 11 mm of precipitation in total. Most snowfalls occurred at the end of February 2009, during which time snow accumulated to depths of 50 cm at elevations above 3,000 m.
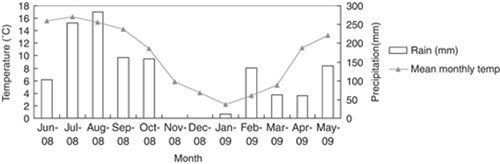
Mean monthly temperature and monthly precipitation at Xiangguqing (3,038 m) from June 2008 to May 2009.
The mean annual temperature was 9.8°C. Temperatures dropped to −9.3°C in January 2009 and reached 27.7°C in July 2008. The month with the highest average temperature (16.2°C) was July, and the month with the lowest average temperature (2.2°C) was January (Fig. 2). There were marked day–night fluctuations in temperature, especially during winter (mean range=15.7°C).
Study Group and Data Collection
We studied sleeping habits for a single R. bieti group at Xiangguqing. The group has been well habituated since 2006 and could be approached to 20–30 m almost every day. We classified individuals into four age/sex categories based on their body size and pelage color: (1) Adult males were the largest individuals in the group, with long white hair on their flanks obscuring ischial callosities, a strong contrast of black-and-white hair, hair on the top of the head falling forward, and a long and bushy tail. (2) Adult females had a body length ≤1/2 adult males, possessed long black nipples, and were often with infants. (3) Juveniles had backs and limbs that were light gray, and their tail hair was short. (4) Infants were the smallest individuals, had predominantly gray–white pelage, and were often suckling.
We sampled sleeping behavior for five full nights each month from June 2008 to May 2009. We followed the group on each observation day from the time it left a sleeping site in the morning until it entered a new sleeping site at dusk. We camped overnight at a distance of about 20 m to maximize data collected but minimize disturbance. We used 8×40 binoculars and a flashlight to record sleeping behavior of the group at night. The group had become accustomed to a flashlight and did not flee before the onset of data collection.
We recorded pre-sleeping time, including the period from when the first monkey entered the sleeping site to when no member of the entire group moved or vocalized. We also recorded the sequence in which members of each OMU entered the sleeping trees. We defined sleeping time as the interval between when the entire group had settled at night until >50% of group members had left the sleeping site the following morning. We recorded the time and sequence of group members stirring the following morning. We defined waking time as when the first individual left its sleeping tree until more than 50% of group members had left the sleeping site. Time of darkness and daybreak were recorded daily to calculate night length.
As the group often sleeps over a relatively large area of roughly 15,000 m2 at night [Li et al., 2006], only 2–4 OMUs could be observed each night for the collection of sleeping behavior data (e.g. sleep style and huddle size). Additionally, as AMUs settle about 50 m away from OMUs and rarely exhibit group or huddled sleeping, data on their huddles were not included in this study. Solitary sleeping meant the individual slept separately from others, whereas huddling consisted of two or more individuals embracing each other either belly-to-belly or belly-to-back. The number of huddling clusters and solitary individuals sleeping alone were counted after the whole group fell asleep (i.e. when movement ceased and the whole group became quiet). We also recorded whether solitary sleeping and huddling clusters changed in terms of size and composition throughout the season. This group never changed sleep configuration over the course of the night [Li, unpublished data].
Data Analysis
We performed statistical tests using SPSS 12.0. All statistical analyses were two-tailed and the alpha level was set at 0.05. We used descriptive statistics to examine the distribution of huddle size and solitary sleeping among the four age/sex categories. We used a one-way ANOVA test to examine differences among pre-sleeping time, waking time, and sleeping time across seasons. We used non-parametric correlation analysis to measure the relationship between sleeping time and the length of night, as well as the correlation between huddling size and unit size. We tested differences in the sequence of entering the sleeping site via the χ2 test, and the sequence of leaving sleeping site via the Kruskal–Wallis test. We used the χ2 test to determine whether there was a preference for solitary sleeping or huddling by age–sex category. We used the Mann–Whitney U test to compare the huddle size in different seasons.
All research protocols of this study were reviewed and approved by the Institute of Zoology, Chinese Academy of Sciences. Our research complied with protocols approved by the animal care committees of the Wildlife Protection Society of China. This study was also permitted by the regulatory requirements of The Baimaxueshan National Nature Reserve, China, where the study was conducted, and adhered to the American Society of Primatologists principles for the ethical treatment of nonhuman primates.
RESULTS
Pre-sleeping Behavior
The study group entered its nocturnal sleeping site when the sun set each day. We recorded 60 pre-sleeping behavioral bouts in total. On average, the group entered a sleeping site and stopped moving by 19:29 (range: 18:29–20:36, N=60) each day, 27 min before dark (range: −5 to 76 min, N=60). It took an average of 20.2 min (range: 10–36 min, N=60) for the whole group to cease movement and vocalization. Pre-sleeping time did not vary significantly over the four seasons (one-way ANOVA test: F4,60=1.127, P=0.346; Table I).
| Pre-sleeping time (min) | Sleeping time (hr) | Waking time (min) | |||||
|---|---|---|---|---|---|---|---|
| Seasons | Mean±SD | Range | Mean±SD | Range | Mean±SD | Range | N |
| Spring | 20.2±5.6 | 10–32 | 11.0±0.9 | 9.8–12.2 | 12.6±3.8 | 5–18 | 15 |
| Summer | 20.9±8.3 | 11–36 | 10.1±0.3 | 9.5–10.6 | 11.9±2.9 | 5–15 | 15 |
| Autumn | 17.9±3.7 | 12–27 | 12.0±0.6 | 10.7–12.8 | 13.3±4.8 | 5–20 | 15 |
| Winter | 21.7±5.6 | 13–34 | 13.0±0.7 | 12.4–15.0 | 11.6±3.5 | 6–20 | 15 |
| Mean | 20.2±6.0 | 10–36 | 11.5±1.3 | 9.5–15.0 | 12.3±3.8 | 5–20 | 60 |
Individuals usually entered sleeping trees one by one. Juveniles and infants vocalized before the whole group became quiet. Over the 60 nights of observation, adult females and mother–infant dyads entered sleeping trees before adult males and juveniles (adult female N=56; adult female with an infant N=107; juvenile N=17; χ2=67.90, df=2, P<0.01). Adult males of OMUs were always the last to enter the sleeping site tree (N=180).
Sleeping Time
The group spent an average of 11.5 hr per night (range: 9.5–15.0 hr, N=60) sleeping throughout the study period (Table I). Sleeping time varied significantly over the four seasons (one-way ANOVA test: F4,60=54.58, P<0.01; Table I). The average sleeping time was 10.1 hr (range: 9.5–10.6) in summer, the shortest seasonal average for the year. The longest seasonal average was 13.0 hr (range: 12.4–15.0) in winter (Table I). Variation in sleeping time were positively correlated with the length of night throughout the year (rs=0.833, P<0.01, N=60), as the group did not stir before daybreak.
Sleeping Time Influenced by Weather
The presence of snow significantly influenced the length of sleeping time and delayed departure from the sleeping site. In February 2009, for example, the group slept 12.7 hr (N=3) during nights with no snow yet slept 15.1 hr (N=2) during nights with snow (Mann–Whitney U tests: Z3,2=−2.121, P<0.05). In contrast to snow, rainfall did not influence sleeping duration (Mann–Whitney U tests: Z7,8=1.462, P=0.144).
Waking from Night Sleep
On average, the group left the sleeping site at 07:08 (range: 06:06–09:39, N=60) each day, 27 min after daybreak (range: −10 to 132 min, N=60). It took 12.3 min (range: 5–20 min) for the whole group to wake and leave the sleeping site (Table I). Waking (from night sleep) time was consistent across seasons (one-way ANOVA test: F4,60=0.583, P=0.629).
We had 216 records of the first individuals in OMUs to leave sleeping trees. The sequence of site departure was different than the entering sequence, with individuals of all age–sex categories observed leaving the sleeping site first (adult males N=42; adult females N=78; female–infant dyads N=40; juveniles N=56; χ2=3.000, df=3, P=0.392).
Solitary Sleeping and Size of Huddles
During the study period, we made 1,385 individual records based on the 171 OMUs. Individual monkeys in each OMU were then recorded an average of eight times. Most OMUs (N=163) shared one sleeping tree at the site, with only eight recorded instances of members of the same OMU sleeping in adjacent trees.
At night, some individuals slept alone and some slept in a huddle. In total, we observed 582 solitary and huddling clusters during the study period (Table II). There were 102 records of solitary sleepers, and these records showed seasonal variation, (χ2=7.25, df=3, P=0.064). The mean group size of a huddling cluster was two (N=480), with the largest huddle of eight individuals observed in winter (N=3, Table II). The huddling together of an entire OMU was also observed only in winter (N=6). Although a large huddle could be formed by a large OMU at night (rs=0.352, P<0.01, N=171), OMU size did not determine the night huddling size since dyadic huddles dominated in most cases (Table II). Huddling during sleeping occurred more often in winter than the other three seasons (Mann–Whitney U tests: Z113,135=−2.405, P<0.05 for spring; Z113,174=−3.726, P<0.01 for summer; Z113,160=−4.225, P<0.01 for autumn).
| Lone sleeping | No. of individuals in the huddle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean per huddle | Total groups observed | |
| Spring | 24 | 61 | 30 | 14 | 2 | 2 | 2 | 0 | 2.43 | 135 |
| Summer | 32 | 87 | 45 | 10 | 0 | 0 | 0 | 0 | 2.19 | 174 |
| Autumn | 31 | 86 | 36 | 6 | 1 | 0 | 0 | 0 | 2.13 | 160 |
| Winter | 15 | 43 | 23 | 13 | 4 | 5 | 7 | 3 | 3.05 | 113 |
| Total | 102 | 277 | 134 | 43 | 7 | 7 | 9 | 3 | 2.38 | 582 |
Age–Sex Categories and Sleeping Arrangements
Among the 102 records of solitary sleeping, 91 were adult males, 6 were adult females, and 5 were juveniles. Infants were never observed sleeping alone. Adult males slept alone significantly more than any other age/sex category (χ2=139.46, df=2, P<0.01).
Thirteen huddle compositions were recorded according to the age/sex classes of the group members (Table III). There was a significant difference in the frequency of the 13 types of huddle by the study group (χ2=377.24, df=12, P<0.01), with most huddles formed by adult females and immature individuals (Mann–Whitney U test: Z2,11=2.171, P<0.05). Although juveniles in the same OMU huddled together at night (N=67, 14% of 480 records), adults seldom huddled at night (5% of 480 records).
| Code | Age/sex categorya | Observed value (%) |
|---|---|---|
| 1 | Female+Juvenile | 27 |
| 2 | Female+Infant | 16 |
| 3 | Juvenile+Juvenile | 14 |
| 4 | Female+Juvenile+Infant | 9 |
| 5 | Female+Female+Infant | 7 |
| 6 | Male+Female+Juvenile | 5 |
| 7 | Female+Female+Juvenile+Infant | 4 |
| 8 | Female+Female+Juvenile | 4 |
| 9 | Male+Juvenile | 4 |
| 10 | Male+Female+Juvenile+Infant | 3 |
| 11 | Male+Female | 3 |
| 12 | Male+Female+Infant | 3 |
| 13 | Female+Female | 1 |
DISCUSSION
Sleep Behavior and Weather
As a diurnal primate residing in temperate forests, the Yunnan snub-nosed monkey (Rhinopithecus bieti) is profoundly influenced by seasonality in most aspects of its routine behavior, including species-specific sleeping habits. Within their habitat, day length and night length display significant seasonal variation [Ren et al., 2009], which in turn influences nocturnal sleeping time. We found that R. bieti slept for a longer night-time period during winter and least in summer. In addition, weather may influence R. bieti sleeping time, with heavy rain and snow slowing down the speed of the group's movements to sleeping locations [Anderson, 1984] and poor weather delaying morning departure from sleeping sites [Ren et al., 2009]. We found that only the presence of snow-delayed sleeping site departure, with other weather (e.g. rain, sunshine, and overcast) having no apparent influence. As heavy snowfalls are always accompanied by cold temperatures, the prolonged sleep time may be a strategy of R. bieti to conserve energy in a low-temperature environment [Schoener, 1971]. This suggests that the harsh winter conditions prevalent in R. bieti habitat require the development of specific behavioral strategies to overcome the cold. Furthermore, our R. bieti study showed that non-seasonal variation in time spent entering and leaving the night sleeping sites was regulated by darkness and daybreak. Although similar results have been observed in other primate species, such as black crested gibbons (Nomascus concolor) [Fan & Jiang, 2008], retiring time can also be determined by seasonal photoperiod, as observed in François' langurs (T. françoisi) [Zhou et al., 2009].
Sleeping in a huddle may function as a strategy to combat low temperature at night [Anderson, 1984] by reducing the exposed surface area of the body [Altmann, 1980; Anderson & McGrew, 1984; Ogawa & Takahashi, 2003; Reichard, 1998; Southwick et al., 1965; Takahashi, 1997; Wada & Tokida, 1985]. Rest huddles during daytime in cold weather have been observed in Presbytis entellus [Bishop, 1979] and R. bieti [Kirkpatrick et al., 1998], and we found that R. bieti also formed and increased the number and size of sleeping huddles during cold nights. In addition, while we did not measure body heat loss of R. bieti, studies on Japanese macaques (Macaca fuscata) have shown that sleeping huddles reduce body heat loss during heavy snowfall [Nakagawa, 1989]. Although low temperature did not correlate with rest huddles in R. bieti in the daytime [Kirkpatrick et al., 1998], it positively correlated with night sleeping huddles. These results suggest that huddled sleeping can help shield R. bieti from low temperature at night.
Sleep Behavior and Predation Avoidance
Although predation pressure on R. bieti is low at the Samage Forest now [Li et al., 2006], past predation pressure may have helped shape present sleeping behavior for this species. Differences in approach to a sleeping site can be a reflection of predator threat and social pressure [Anderson, 1998]. Some primates, for example, always move rapidly and quietly when approaching a sleeping site to minimize detection by predators [Fan & Jiang, 2008; Reichard, 1998; Xiang et al., 2010; Zhou et al., 2009]. In addition, predation pressure may influence each age/sex class differently [Fan & Jiang, 2008], with females and infants often the first to enter sleeping trees in many primate species [N. concolor, Ellefson, 1974; Hylobates lar, Fan & Jiang, 2008; Reichard, 1998; T. françoisi, Zhou et al., 2009]. R. bieti followed this pattern when retiring for the night. Adult females with infants usually entered the sleeping trees first, which is likely related to protection of their young and selection of suitably safe resting places. Conversely, departure from the sleeping trees in the morning for the same group of R. bieti varied without a clear order, similar to H. lar [Reichard, 1998], probably because of their recovered agility after night-time resting and paucity of diurnal predators [Li et al., 2006]. Although orderly departure from night sleeping sites was not observed during our study, it has been adopted by other primates such as Papio ursinus [Buskirk et al., 1974; Davidge, 1978a,b] and is related to their rigid social hierarchy.
Sleep Behavior, Social Cohesion, and Stability
Combinations of different age/sex classes in huddles can reflect social relationships within a group [Li & Wang, 1994; Takahashi, 1997], such as social rank [Li & Wang, 1994; Ogawa, 1995a] and kinship [Chaffin et al., 1995; Takahashi, 1997; Wada & Tokida, 1985]. As OMUs are highly cohesive in R. bieti, huddles were evidently dominated by kinship or OMU selection (i.e. individuals in the same OMU huddled together). Mother–infant dyads were the most common huddles in R. bieti, which likely reflects maternal behavior, including nursing and protection against predation [Koyama, 1973] or falling from sleeping trees [Roonwal & Mohnot, 1977].
Age and sex also influenced huddle composition [Ansorge et al., 1992; Ogawa, 1995b]. While male Tibetan macaques of different ages have often been observed huddling together in groups of two or three [Li & Wang, 1994], only juvenile male R. bieti of similar age were observed huddling together during sleep. Huddling behavior is an important aspect of group living as it can facilitate social cohesion [Koyama, 1973] by increasing tolerance among individuals [Takahashi, 1997]. In addition, R. bieti formed larger huddles during winter nights and all members of the same OMU used the same sleeping tree [Li et al., 2006]. It is likely that body contact with several individuals when huddled can increase warmth, social safety, and sleep quality [Anderson, 1984; Li et al., 2006; Liu & Zhao, 2004].
To date, solitary sleeping has been reported only in bonnet macaques (M. radiata) in India [Koyama, 1973]. The reasons why individuals increase their vulnerability to predators by adopting such a sleeping habit remains unanswered. Our results showed that some R. bieti individuals exhibited solitary sleeping behavior, especially the adult male in an OMU (89% of solitary sleepers). The function of such solitary sleep remains unclear, particularly given the role of adult males in harem defense during the day. Why do they distance themselves from adult females during the night? This question might be clarified by understanding the social structure of this rare primate. Two basic units (OMUs and AMUs) form a typical group of R. bieti in the wild [Grueter & van Schaik, 2010; Ren et al., 2004] and only males who have established their own OMU have access to fertile females. Presumably night-time provides males from AMUs with the opportunity to furtively approach reproductive females. As sleeping in a huddle may reduce an individual's alertness, it is possible that adult males from OMUs would be more vigilant as solitary sleepers. We hypothesize, therefore, that solitary sleeping by OMU males may increase their ability to monitor conspecifics, drive invaders from their sleeping trees, and effectively manage their harems at night.
Various environmental and social factors have shaped the pattern of sleeping behavior in R. bieti. Huddling was confined to members of the same OMU and shaped the spatial distribution pattern of the group though partner selectivity. Furthermore, comparison between midday-resting patterns and nocturnal sleeping patterns may help clarify social organization and behavioral strategies important to the conservation of this endangered species, and elucidate relationships that may be displayed rarely during diurnal activity.
Acknowledgements
This study was granted by the NBRPC (973 Program: 2007CB411600), the key project of NSFC (No. 30630016), the project of NSFC (No. 30970442), China Program of TNC, and the IPCAS (KSCX2-YW-R-091). We are grateful to our field assistants Xinming He, Rong Yang, Jianhua Yu, Jianjun Yu and Xiaohua Yu, and also to the Baimaxueshan Nature Reserve for granting a work permit. Special thanks to Ali Krzton (Department of Anthropology, Texas A&M University) for English revision. We also thank Dr. Xiang ZF and anonymous reviewers for their comments.




