Catatonia responsive to corticosteroids in a patient with an SCN2A variant
Kimberly Senko and Kelsey L. Saddoris contributed equally to this study.
Abstract
Variants in SCN2A are a known risk factor for developing autism spectrum disorder (ASD). Catatonia is a complex neuropsychiatric syndrome, which occurs at a higher rate in individuals with ASD. Catatonia has also been associated with COVID-19 infection, though the majority of these cases are associated with increased serum inflammatory markers. We present a case of a 15-year-old female with ASD and corticosteroid responsive stuporous catatonia to explore the relationship between SCN2A variants, ASD, COVID-19 exposure, and treatment refractory catatonia. Despite a lack of significantly elevated serum or CSF inflammatory markers, this patient showed significant improvement following initiation of corticosteroid therapy. This case presents a novel approach to the work-up and treatment of catatonia in individuals with SCN2A variants independent of elevated inflammatory markers.
Abbreviations
-
- ASD
-
- autism spectrum disorder
-
- BFCRS
-
- Bush-Francis Catatonia Rating Scale
-
- COVID-19
-
- Coronavirus disease of 2019
-
- GABA
-
- gamma-aminobutyric acid
-
- LLN
-
- lower limit of normal
-
- SCN2A
-
- sodium voltage gated channel alpha subunit 2
-
- ULN
-
- upper limit of normal
1 INTRODUCTION
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder affecting 1 in 54 school-aged children in the United States (Maenner et al., 2021). Core symptoms include deficits in social interaction and social communication as well as stereotypical and repetitive behaviors. ASD has a strong genetic component. However, while hundreds of genes have been identified with a correlation to ASD phenotypes, most cases are attributed to a combination of multiple genetic and environmental factors (Satterstrom et al., 2020). Just over 100 high confidence ASD de novo risk genes have been identified, of which about 90 are due to haploinsufficiency variants (Satterstrom et al., 2020). One such gene is SCN2A, which encodes the alpha subunit of voltage-gated type II sodium channels (Nav1.2) and is highly expressed in the brain (Willsey et al., 2013).
Catatonia is a complex neuropsychiatric syndrome marked by abnormal movements, affect, and behaviors. A common metric for screening and determining catatonia severity is the Bush-Francis Catatonia Rating Scale (BFCRS) (Sienaert et al., 2014). The BFCRS is an observational scoring system, which consists of 23 behavioral observations scored from zero to three, with zero indicating the behavior is not present and three indicating the behavior is constantly present (Bush et al., 1996). If two or more of the first 14 behaviors are present, regardless of score, it is considered a positive screen, and a higher score indicates a more severe presentation (Bush et al., 1996). In patients with neurodevelopmental disorders, such as ASD, there is a higher prevalence of catatonia (Vaquerizo-Serrano et al., 2021). The diagnosis of catatonia in individuals with ASD may be complicated, as symptoms such as social indifference, mutism, abnormal speech, stereotypic mannerisms and echolalia, have significant overlap between the two conditions (Vaquerizo-Serrano et al., 2021). In patients with ASD, clinicians must have a comprehensive understanding of the patient's baseline level of functioning and how the current presentation differs from baseline, in order to accurately diagnose catatonia. This is necessary as many patients with ASD will screen positive on the BFCRS even when catatonia is not present, due to symptom overlap between ASD and catatonia.
The relationship between COVID-19 and catatonia is not yet fully understood, though there have been multiple reports of catatonia in patients following COVID-19 exposure (Scheiner et al., 2021). However, the utility of corticosteroids in the treatment of catatonia outside of the treatment of autoimmune encephalitis has not been explored. This case study aims to explore the relationship between likely pathogenic SCN2A variants, ASD, COVID-19 exposure, and catatonia responsive to corticosteroids.
2 CASE SUMMARY
A 15-year-old female with past medical history significant for high-functioning ASD, childhood febrile seizures, and developmental delay was admitted to the hospital for a two-and-a-half-week history of progressive withdrawal, staring spells, mutism, echolalia, stiffness, new stereotypies, auditory hallucinations, and regression in functioning. She was no longer attending independently to activities of daily living (ADLs) as she had been previously, with a 4.6-kg weight loss prior to admission. Her symptoms and the significant change from baseline functioning were concerning for the development of stuporous catatonia.
Approximately 3 weeks prior to her admission, she was exposed to a family member with an active COVID-19 infection. A few days after the exposure, she noted the inability to taste and smell, and her caregiver reported progressive functional decline and withdrawal. She had a pediatrician appointment 9 days prior to admission for hallucinations and poor oral intake, during which she had a negative rapid COVID-19 antigen test and was positive for group A streptococcus pharyngitis. She was subsequently treated with a 10-day course of amoxicillin, with no improvement.
An extensive workup, including hematologic (complete blood count, iron, ferritin), infectious (HIV, syphilis, Lyme antibodies, rapid group A streptococcus), metabolic (renal, liver function, creatine kinase, lactate, pyruvate, uric acid, vitamin levels including B12, B1, and B9, heavy metals panel), and autoimmune studies (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], anti-nuclear antibody [ANA], thyroid peroxidase antibody, ANCA-associated vasculitis profile, anti-ENA panel, anti-ds DNA antibody, ribosomal antibody, neuronal antibody panel) were unremarkable except for the following: very mildly elevated ESR (21 mm/h with upper limit of normal [ULN] 20 mm/h) and mildly elevated CRP (1.3 mg/dL with ULN <0.4 mg/dL) that both normalized prior to the initiation of corticosteroids, slightly elevated ceruloplasmin (44 mg/dL with ULN 43 mg/dL) and copper level (229.8 ug/dL with ULN 129 ug/dL), positive SARS-CoV2-Ab nucleocapsid and spike protein, and mildly abnormal complement proteins (C3 152 mg/dL with ULN 150 mg/dL, C4 14.5 mg/dL with lower limit of normal (LLN) 15.7 mg/dL, CH50 90 U with ULN 75 U). Complement proteins normalized during hospitalization prior to the patient receiving any corticosteroids, except C4, which decreased further to 11.2 mg/dL. Cerebral spinal fluid (CSF) studies were non-revealing (normal glucose, protein, cell counts, IgG index, no oligoclonal bands, negative meningitis/encephalitis PCR panel, negative autoimmune encephalopathy panel). Electroencephalogram was negative for epileptiform discharges (though note this was obtained post-lorazepam due to agitation interfering with ability to obtain prior). MRI and MRA brain were normal other than a subtle ill-defined signal within the subcortical and deeper white matter of the right frontal operculum, thought to be most compatible with an ill-defined gray matter heterotopia. Ultrasound of the pelvis was negative and ruled out ovarian teratoma. Whole body PET scan was significant for high attenuation in the deep abdomen with associated FDG uptake. An MRI abdomen was subsequently obtained and did not show any correlating anatomic abnormality, which was reassuring and suggested that the findings on PET scan likely represented enteric contents.
The patient's clinical presentation was consistent with catatonia of unknown etiology with an initial Bush-Francis Catatonia Rating Scale (BFCRS) score of 34 (out of a maximum possible score of 69). She had a positive intravenous lorazepam challenge (BFCRS decreased to 11; see Figure 1) and lorazepam dose was titrated to clinical effect throughout hospitalization (maximum dose 42 mg/day). She was transitioned to oral lorazepam prior to discharge. She had no known trauma history; however, due to concern that untreated anxiety may be contributing to her presentation, escitalopram was initiated and titrated to 15 mg daily. Due to continued significant symptoms and functional impairment, memantine (N-Methyl-D-Aspartate receptor subtype of glutamate receptor antagonist) was started as augmentation of catatonia treatment and titrated to 10 mg twice daily. Oral olanzapine was initiated and titrated to 7.5 mg daily for treatment augmentation and to target possible underlying psychosis given the patient's history of new-onset hallucinations prior to developing catatonia, which led to some symptom improvement. Despite these interventions, the patient continued to have significant functional impairment. Given the recent COVID-19 exposure and initial mildly elevated inflammatory markers, there was concern for a possible underlying neuroinflammatory process contributing to her presentation despite her overall reassuring work up and normalization of systemic inflammatory markers. Our differential included a post-COVID-19 inflammatory process, small vessel vasculopathy, pediatric acute-onset neuropsychiatric syndrome (PANS), or other immunopathologic processes. Seronegative encephalitis was thought to be unlikely given her reassuring cerebral spinal fluid studies. Small vessel vasculopathy could only be confirmed by brain biopsy and the risks of performing this procedure outweighed the potential benefits. PANS remains a controversial diagnosis and is a diagnosis of exclusion. The current working diagnostic criteria include abrupt onset of obsessive compulsive disorder (OCD) or severely restricted food intake as well as concurrent presence of neuropsychiatric symptoms from two out of seven categories (anxiety, emotional lability/depression, irritability/aggression/severely oppositional behaviors, behavioral regression, deterioration of school performance, sensory or motor abnormalities, and somatic signs/symptoms including sleep disturbances, enuresis, or urinary frequency) (Swedo et al., 2012). It is worth noting that a portion of the diagnostic criteria overlap heavily with the diagnostic criteria for catatonia. This patient did not fit the typical demographic for PANS, as she did not demonstrate an acute onset of OCD-like behaviors, PANS is typically diagnosed in prepubertal patients with a mean age at diagnosis of 7 years (SD 2 years), and is twice as prevalent in males than females (Gagliano et al., 2023).
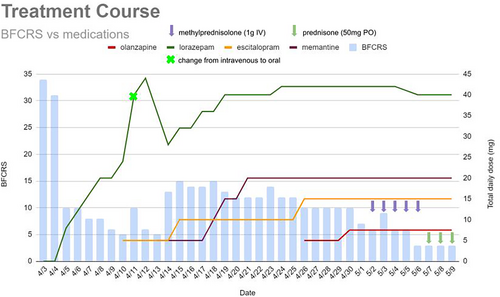
Despite a lack of diagnostic clarity, given the concern for a neuroinflammatory component of her presentation, she was initiated on a course of pulse dose steroids, starting at 1 g of methylprednisolone daily for 5 days. After two doses of intravenous methylprednisolone (~48 h), her BFCRS decreased to six with a notable decrease in ambitendency and automatic obedience as well as an increase in communication and oral intake. Specifically, she could feed herself with a steady hand and speech was more spontaneous, at a more appropriate volume, and in full sentences. She continued to have residual psychomotor slowing beyond her baseline and was placed on a prolonged oral steroid taper at discharge. She was discharged with plans to start outpatient electroconvulsive therapy (ECT) for treatment-resistant catatonia.
Prior to discharge, clinical whole genome sequencing was sent to determine if the patient had a genetic basis for her presentation. Following discharge from the hospital, results of rapid whole genome sequencing returned and were significant for heterozygosity for a likely pathogenic truncating variant in SCN2A; c.2509del or pGlu857Asnfs*31 (NM_021007.2). This variant is predicted to result in a frameshift and premature protein termination (loss-of-function variant) and has not been previously reported in the literature or in any large population databases. She was also found to be heterozygous for a ~7.6 kb duplication within the PLCB4 gene, which is a variant of uncertain significance and not thought to be related to her clinical presentation.
3 DISCUSSION
Genotypic variants in SCN2A generally result in one of three different phenotypes—infantile encephalopathy, benign (familial) infantile seizures, or ASD and/or intellectual disability with or without late onset seizures (Ben-Shalom et al., 2017; Sanders et al., 2018; Wolff et al., 2017). Patients with infantile encephalopathy (neonatal seizures followed by neurodevelopmental delay) typically have significant gain-of-function variants in SCN2A, while patients with benign infantile seizures typically have a milder gain-of-function variant in SCN2A (Ben-Shalom et al., 2017; Sanders et al., 2018). Conversely, patients with the ASD/intellectual disability phenotype are generally found to have a premature truncating or loss-of-function variant in SCN2A (Ben-Shalom et al., 2017). This phenotypic variability is likely due to the unique expression pattern of Nav1.2 in the developing brain. Though Nav1.2 is widely expressed throughout the central nervous system, it is predominately expressed on the unmyelinated axons of excitatory granule cells in the cerebellum and the myelinated axons of excitatory (glutaminergic) neurons in the cortex (Martínez-Hernández et al., 2013; Sanders et al., 2018). Nav1.2 expression varies throughout development in the cortex (Martínez-Hernández et al., 2013). Early in development (late in second trimester through 1–2 years of age), Nav1.2 is the sole sodium channel isoform present on the axon initial segment and is therefore the primary sodium channel responsible for action potential initiation in excitatory pyramidal cells (Gazina et al., 2015). As development progresses, Nav1.2 is replaced by Nav1.6 (SCN8A) in the distal axon initial segment and because Nav1.6 has a lower voltage threshold for depolarization, this becomes the primary site of action potential initiation in mature excitatory pyramidal neurons (Kole & Stuart, 2012; Spratt et al., 2019; Tian et al., 2014). At that point, Nav1.2 expression is largely limited to the proximal portion of the axon initial segment and is thought to be responsible for backpropagation of action potentials into the somatodendritic compartment (Hu et al., 2009). This backpropagation of action potentials depolarizes dendrites and can contribute to the formation of dendritic spikes (Spratt et al., 2019). In mouse models, Spratt et al. demonstrated that SCN2A haploinsufficiency impairs excitatory synapse function and plasticity (Spratt et al., 2019).
Catatonia can be caused by a wide array of both medical and psychiatric etiologies and has been associated with COVID-19 infections, whether as a manifestation or sequelae of the infection (Northoff, 2002; Scheiner et al., 2021). Though the etiopathogenesis of catatonia is not clear, the current leading hypothesis is that catatonia is due to an imbalance between excitatory (glutamate) and inhibitory gamma-aminobutyric acid (GABA) neurotransmitters (Dhossche et al., 2010). Given that in the mature cortex, Nav1.2 is primarily expressed on excitatory (glutaminergic) neurons, and SCN2A haploinsufficiency is thought to impair synapse function and plasticity, patients with loss-of-function mutations in SCN2A may have a baseline imbalance between excitatory and inhibitory neurotransmitters that may cause a predisposition to developing catatonia (Hu et al., 2009; Spratt et al., 2019). We suspect in this case, the patient's SCN2A variant predisposed her to developing catatonia, and her COVID-19 infection may have ultimately precipitated her presentation.
Though the relationship between SCN2A loss-of-function variants and autism has been well established, this is only the third report in the literature of a patient with an SCN2A variant developing catatonia (Sanders et al., 2012). The first case report describes a 4-year-old male with an SCN2A variant (p.Ala202Val) who presented with catatonia of unknown etiology that was partially responsive to corticosteroids, and almost completely resolved over 2 months with titration of lorazepam (Leroy et al., 2018). However, symptoms reoccurred when lorazepam was weaned slightly. The patient again had partial benefit from a second round of corticotherapy, but no benefit from five courses of plasma exchange (Leroy et al., 2018). He then had complete resolution of symptoms following the initiation of vigabatrin and subsequently tolerated a moderate lorazepam wean (Leroy et al., 2018). The second report describes a 20-year-old female with moderate intellectual disability and no known seizure history who initially presented at 14 years of age with catatonia of unknown etiology, though an autoimmune etiology was suspected (Colijn & Pirlot, 2024). Symptoms were not clearly responsive to intravenous immunoglobulin but were partially responsive to lorazepam, and ultimately improved following a course of ECT (Colijn & Pirlot, 2024). The patient was found to have a truncating variant of SCN2A (c.4669C > T or p.Gln1557*), and eventually required maintenance ECT to prevent recurrence of catatonia (Colijn & Pirlot, 2024). While the variants seen in the patient presented in this case report and the previously reported 20-year-old patient are both truncating (loss-of-function) variants, the variant reported in the 4-year-old is a gain-of-function variant that is predicted to reduce the rate of sodium channel inactivation, resulting in hyperexcitability (Colijn & Pirlot, 2024; Heron et al., 2002; Leroy et al., 2018).
The patient described in this case had catatonia that was incompletely responsive to high-dose benzodiazepines as well as glutamate antagonists and an antipsychotic, but later demonstrated significant clinical improvement following initiation of high-dose intravenous corticotherapy, despite a lack of identifiable biomarkers to indicate a significant inflammatory or autoimmune process underlying her presentation. Similarly, the previously reported four-year-old patient also partially responded to corticosteroids, despite a lack of inflammatory biomarkers (Leroy et al., 2018). It is unclear why catatonia symptoms in both patients responded to corticotherapy despite having different variants in SCN2A. If corticosteroids were effective because they were treating an unknown immunoglobulin-based autoinflammatory process, we would have anticipated plasma exchange might have benefitted the 4-year-old. Given his lack of response to plasma exchange, corticosteroids may treat catatonia in those with SCN2A variants through an alternative mechanism.
Studies have suggested that endogenous and exogenous glucocorticoids suppress glutamate release and facilitate GABA release, which may suggest a mechanism by which synthetic glucocorticoids may be effective in treating catatonia, even in the absence of significant identifiable inflammation (Stell et al., 2003; Wang et al., 2016). This may explain why both the patient presented in this case report and the previously reported four-year-old patient both exhibited a partial response to synthetic glucocorticoids despite the absence of identifiable elevated inflammatory biomarkers. However, synthetic glucocorticoids can be associated with significant adverse effects, especially if utilized long-term. Further research is needed to fully elucidate the relationship between SCN2A/Nav1.2, catatonia, and the effect of synthetic glucocorticoids. However, these cases suggest that the use of glucocorticoids may have a role in the treatment of patients with catatonia who are incompletely responsive to initial treatment measures.
AUTHOR CONTRIBUTIONS
Dr. Kimberly Senko and Dr. Kelsey Saddoris conceptualized the study, collected and interpreted relevant data, reviewed the literature, drafted the initial manuscript, and critically reviewed and revised the manuscript. Dr. Ella Baus collected and interpreted relevant data, reviewed the literature, assisted in drafting the initial manuscript, and critically reviewed and revised the manuscript. Dr. Katherine Soe collected and interpreted relevant data, reviewed the literature, supervised creation of the manuscript, and critically reviewed and revised the manuscript. Dr. Samuel Vaughn collected and interpreted relevant data, reviewed the literature, supervised creation of the manuscript, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
We wish to thank the patient and family for allowing us to share this case.
FUNDING INFORMATION
No funding was secured for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




