A review of aromatic l-amino acid decarboxylase (AADC) deficiency in Taiwan
Funding information: Agilis Biotherapeutics; National Taiwan University Hospital; National Research Programme for Biopharmaceuticals
Abstract
Aromatic l-amino acid decarboxylase deficiency (AADCD) is a rare inherited disease prevalent in South East Asia. This disease is due to the founder mutation IVS 6 + 4A > T (c.714 + 4A > T), which accounts for most alleles. Patients with this mutation have severe phenotypes. About 90 % of these patients in South East Asia do not have head control and cannot sit, stand, or speak from birth to the time of observation. In 2012, a gene study to treat these patients with intraputamen injection of adeno-associated virus2-human AADC showed prominent motor improvement and an increased PDMS-2 score 12 months after treatment. In addition, systemic gene therapy in a mouse model of AADCD achieved widespread correction of the Ddc gene. In this article, we review the natural history, clinical course, and treatment effects seen in these clinical and mouse studies. Future studies focusing on noninvasive viral vector delivery or alternative emerging treatments may also benefit patients with AADCD.
1 INTRODUCTION
Aromatic l-amino acid decarboxylase deficiency (AADCD, OMIM #608643) is an autosomal recessive neurotransmitter disease caused by pathogenic variants in the DDC gene (OMIM *107930) (Kniffin, 2001). The DDC gene encodes the pyridoxine-dependent homodimer enzyme (EC. 4.1.1.28), which converts 5-hydroxytryptophan into serotonin (5-hydroxytryptamine) and 3,4-dihydroxyphenylalanine (l-dopa) into dopamine (DA; (Barth et al., 2012; Bowsher & Henry, 1986; Burkhard, Dominici, Borri-Voltattorni, Jansonius, & Malashkevich, 2001).
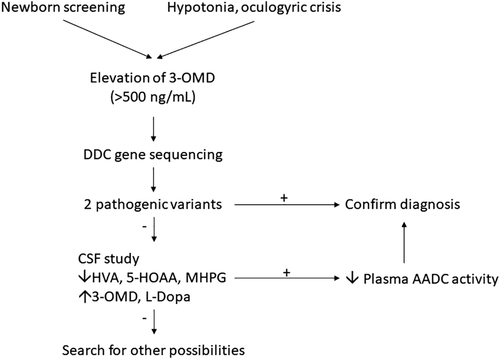
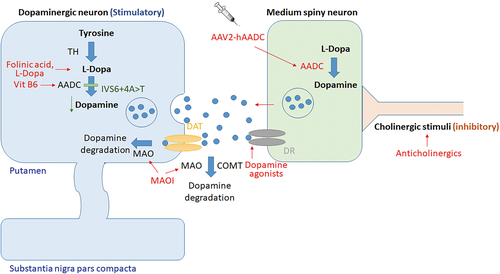
Since Hyland and Clayton first reported AADCD in 1990 (Hyland & Clayton, 1990), only 120 cases of this ultrarare disease have been reported worldwide (Brun et al., 2010; Dai, Ding, & Fang, 2018; Portaro et al., 2018; Sherazi et al., 2017; Wassenberg et al., 2017; Zhu & Yu, 2017). A wide range of clinical presentation, from severe (profound hypotonia, developmental delay, and oculogyric crisis [OGC]), moderate, to mild (mild developmental delay, ambulatory without assistance, mild intellectual disability), or atypical cases, make the diagnosis difficult, though the majority of cases present before 18 months of age with hypotonia or floppiness (Brun et al., 2010; Kojima et al., 2016; Portaro et al., 2018; Spitz et al., 2017; Wassenberg et al., 2017). The diagnosis mainly depends on low 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA), high 3-O-methyldopa (3-OMD) and l-dopa in the cerebrospinal fluid (CSF), and low plasma AADC enzyme activity, followed by identification of compound heterozygous or homozygous pathogenic variants in the DDC gene (Brun et al., 2010; Wassenberg et al., 2017) (Figure 1). Although several drugs have been suggested as first-line treatments, such as Vitamin B6, DA agonists, and monoamine oxidase (MAO) inhibitors (Wassenberg et al., 2017) (Figure 2), the efficacy has not been satisfactory for severe cases, for example, those in South East Asia (Hwu, Chien, Lee, & Li, 2018).
Of the reported cases with AADCD, 30% (37 of the 120 cases) are Taiwanese (Hwu et al., 2018), highlighting the high prevalence of this disease in this area. In this review, we will address the genetic aspects of AADCD, update the results of gene therapy, and discuss the unmet needs for addressing this disease.
2 GENOTYPIC AND PHENOTYPIC CHARACTERISTICS
The most common genotype of Taiwanese AADCD is the splice-site pathogenic variant (IVS6 + 4A > T; c.714 + 4A > T), which accounts for 76% of all DDC mutations, followed by c.1297dupA (6.8%), c.1234C > T (p.R412W; 4.1%) (Hwu et al., 2018). All these three variants are first found in Chinese descents, although the c.1297dupA has been found as heterozygous in one non-Finish European, and c.1234C > T has been found as heterozygous in one African, while IVS6 + 4A > T are solely from East Asian according to gnomAD database (Hwu et al., 2018; Lee, Tsai, Chi, Chang, & Lee, 2009; Lek et al., 2016). The IVS6 + 4A > T variant was first reported by Tay et al. in two unusually mild Chinese siblings in Singapore in 2007 and was subsequently found to be the most common DDC mutation in the Chinese population (Brun et al., 2010; Tay et al., 2007). The initial cases harbored IVS6 + 4A > T/c.853C > T (p.R285W) compound heterozygous variants that were responsive to MAO inhibitor and DA agonist because the c.853C > T variant causes milder impact on enzyme function (Montioli et al., 2014; Tay et al., 2007).
An mRNA study showed this IVS6 + 4A > T change creates a cryptic splicing donor site at the +38 nucleotide of intron 6, resulting in a 37 nucleotide insertion between Exons 6 and 7 followed by a downstream frameshift (Lee et al., 2009). The IVS6 + 4A > T variant causes the severe phenotypes of AADCD (Hwu et al., 2018; Wassenberg et al., 2017). Most patients with this variant fail to gain weight, have profound motor deficits, and are unable to reach any developmental milestones (Hwu et al., 2018; Wassenberg et al., 2017). Compared with literature reports that 95% of patients have hypotonia, 86% experience OGC, and 39% show ptosis, the Taiwanese cohort included 36 of 37 patients (97.3%) with hypotonia, OGC, and ptosis (Brun et al., 2010; Hwu et al., 2012; Hwu et al., 2018). Unlike patients with milder phenotype that may respond to DA agonists, MAO inhibitors, or pyridoxine (Kojima et al., 2016; Swoboda et al., 1999; Wassenberg et al., 2017), patients with severe phenotype (such as with IVS64 + A > T) show poor response to these medication, and none reach motor milestones under treatment with these medications (Hwu et al., 2018). Death occurs at a mean age of 4.6 years (range 1.0–7.0 years) with 50% of deaths due to multiple organ failure and 20% to sepsis, followed by heart failure and pneumonia (Hwu et al., 2012).
The allele frequency of IVS6 + 4A > T is estimated to be one allele in 254 healthy Taiwanese, indicating the incidence of homozygous IVS6 + 4A > T is 1 in 258,064 in Taiwan (Lee et al., 2009). However, the prevalence of AADCD identified via newborn screening showed an even higher incidence of 1 in 32,000 (Chien et al., 2016). Three of the four newborns carrying homozygous IVD64 + A > T developed typical symptoms of AADCD while one case harboring IVS6 + 4A > T/c.752 T > C (p.F251S) were still asymptomatic at latest follow-up (2 years of age) (Chien et al., 2016). Based on a case series study showing 56.8% (21/37) homozygous and 35.1% (13/37) heterozygous for IVS6 + 4A > T (Hwu et al., 2018), larger studies are necessary to resolve this apparent discrepancy and delineate phenotype spectrum in this disease.
3 GENE THERAPIES FOR PATIENTS WITH AADC DEFICIENCY
Recently, gene therapy has become an emerging strategy to treat human diseases. In 2009 to 2010 (Christine et al., 2009; Muramatsu et al., 2010), clinical studies using adeno-associated virus (AAV) serotype 2 vector-mediated delivery of human DDC gene into putamen in Parkinson's disease had demonstrated the safety and tolerability in adults. Because of the severe phenotype of Taiwanese patients with AADCD, the same treatment using intra-putamen injection of AAV2-AADC in AADCD had been performed since 2010 (Figure 2) (Hwu et al., 2012). For the first 4 patients (Hwu et al., 2012), the mean age that they received treatment was 4.75 years (range 4–6 years). The stereotactic surgery delivery method is safe, and no notable intracerebral hemorrhage was observed in postoperative computed tomography (CT) or magnetic resonance imaging (MRI). After surgery, these patients gained weight and exhibited gradual improvement in motor function, with positive PET scans for FDOPA uptake. Additionally, a reduction in the severity of OGC was also observed with an increase in CSF HVA and 5-HIAA, although the levels of l-DOPA and 3-OMD were still elevated. Based on these data, a phase 1/2 clinical trial was started in 2014 (Chien et al., 2017). A total of 10 patients at younger ages (median age 2.71 years old) were treated. One patient died due to endemic influenza B encephalitis 10 months after treatment. At 12 months after treatment, a median increase of 62 points (p = .005) on the Peabody Developmental Motor Scale (PDMS-2 score) and a median elevation of HVA of 25 nM (p = .012) were observed. The most common adverse reactions were fever (16%) and orofacial dyskinesia (10%; (Chien et al., 2017). All patients had transient dyskinesia and were responsive to risperidone. Until now, a total of 25 AADCD patients have received gene therapy, with 1.8 × 1011 vg total of AAV2-AADC (n = 21) or 2.4x1011 vg total of AAV2-AADC (n = 4).
4 DEVELOPING OTHER THERAPEUTIC STRATEGIES
Intra-putamen injection of AAV2-AADC only targets motor functions affected by AADCD. However, DDC is also expressed in other dopaminergic and serotoninergic systems, and gene therapy studies have not been applied to these areas. Additionally, because intra-putamen injection is an invasive procedure, noninvasive methods of vector delivery should be considered for secondary gene therapy. To perform future research on the treatment for AADCD, we generated an AADC deficiency mouse model (DDCKI mice) by knocking in the common mutation (IVS6 + 4 A > T) found in South East Asian patients with AADCD (Lee et al., 2013). In the brain of the DDCKI mice, AADC mRNA showed exon 6 skipping, and the activity was <0.3% of wild-type (WT) mice. The DA level of the mice brains at 2 weeks of age was 9.39% of WT. Half of the DDCKI mice were live-born but two-thirds showed poor growth and tended to die before weaning (Brun et al., 2010).
Using this model, intracerebral ventricular (ICV) injection of neonatal mice with widespread AAV vector serotype 9 (AAV9) expressing the human AADC gene (AAV9-hAADC) resulted in improved growth and survival rates of DDCKI mice (Lee et al., 2014). In addition, treated DDCKI mice show DA levels at 100% and serotonin levels at 40% of WT mice as well as normalization of hind limb clasping and cardiovascular dysfunction. However, slightly more activity was observed in DDCKI mice (Lee et al., 2014). Due to concerns that this hyperactivity may have been due to excess ectopic DA production and the invasive procedure in the neonatal period, we modified our viral vector delivery strategy to use intraperitoneal injection of the neuronal preferential viral vector (yfAAV9/3-Syn-I-mAADC) in 7-day-old DDCKI mice (Lee et al., 2015). The yfAAV9/3-Syn-I-mAADC-treated mice showed greater neuronal transduction, higher brain DA level and less hyperactivity compared with AAV9-CMV-hAADC-treated mice (Lee et al., 2015). Further large animal experiments are in progress to develop the next generation of gene therapies for patients with AADCD.
In addition to gene supplement treatment, other strategies to increase wild-type DDC expression have been tested, especially targeting the unique splice site mutation in the Chinese cohort. We designed U1 snRNA vectors to adapt the U1 snRNA binding sequences of the mutated DDC gene (Lee, Lee, Chen, Byrne, & Hwu, 2016). In vitro, the modified U1 snRNA (IVS-AAA) completely matching both the intronic and exonic U1 binding sequences of the mutated DDC gene can correct the splicing error. We further performed the injection of the AAV9-IVS-AAA viral vector in DDCKI mice, and they showed better survival, but the efficiency still needs to be improved (Lee et al., 2016). Another attempt using antisense oligonucleotides targeting the IVS6 + 4 A > T site on lymphoblastoid cells showed an increase in DDC protein and serotonin levels (Tsai, Lee, Chi, Yang, & Hsu, 2018).
5 CONCLUSION
AADCD due to the founder splice site mutation is a unique disease model regarding both pathophysiology and phenotype. AADCD is also a good candidate for pharmaceutical research on the development of drugs targeting central neuronal diseases. The success of intraputamen gene therapy is a promising ray of light for these patients, and we believe that research in this area will lead to a cure for them in the future.
ACKNOWLEDGMENTS
W.-L.H. and Y.-H.C. received grants from the National Research Programme for Biopharmaceuticals and the AADC Research Fund at National Taiwan University Hospital (Taipei, Taiwan) during the conduct of the study, as well as grants from gene therapy for AADCD licensing to Agilis Biotherapeutics and grants from Agilis Biotherapeutics outside of the submitted work. Additionally, W.-L.H has a pending patent for gene therapy for AADC deficiency.
CONFLICT OF INTEREST
No potential conflicts of interest are declared by the other authors.




