Megalencephaly and hemimegalencephaly: Breakthroughs in molecular etiology
Abstract
Megalencephaly (MEG) is a developmental disorder characterized by brain overgrowth that occurs due to either increased number or size of neurons and glial cells. The former may be due to either increased neuronal proliferation or decreased apoptosis. The degree of brain overgrowth may be extensive, ranging from generalized MEG affecting the entire cortex–as with mutations in PTEN (phosphatase and tensin homolog on chromosome ten)–to unilateral hemispheric malformations–as in classic hemimegalencephaly (HME). On the other hand, some lesions are more focal or segmental. These developmental brain abnormalities may occur in isolation in some individuals, whereas others occur in the context of a syndrome involving dysmorphic features, skin findings, or other organ system involvement. Brain overgrowth disorders are often associated with malformations of cortical development, resulting in increased risk of epilepsy, intellectual disability, and autistic features, and some are associated with hydrocephalus. The past few years have witnessed a dramatic leap in our understanding of the molecular basis of brain overgrowth, particularly the identification of mosaic (or post-zygotic) mutations in core components of key cellular pathways such as the phosphatidylinositol 3-kinase (PI3K)-vakt murine thymoma viral oncogene homolog (AKT)-mTOR pathway. These molecular insights have broadened our view of brain overgrowth disorders that now appear to span a wide spectrum of overlapping phenotypic, neuroimaging, and neuropathologic features and molecular pathogenesis. These molecular advances also bring to light the possibility of pathway-based therapies for these often medically devastating developmental disorders. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
The etiologies of focal malformations of cortical development have long been a puzzle. Unlike most forms of lissencephaly and microcephaly where brain morphology appears affected globally in most forms, suggesting a genetic disorder affecting the entire cortex, focal malformations such as focal cortical dysplasia (FCD), hemimegalencephaly (HME), and focal megalencephalies suggest another pattern in which only part of the developing brain appears to have experienced a genetic aberration. Brain overgrowth phenotypes range from very localized lesions to more diffuse multifocal forms. These are conditions were asymmetry is the rule, and where etiology had long been elusive. Non-genetic etiologies were previously sought to explain the presence of a severely overgrown and dysplastic portion of the brain with the neighboring cortex seemingly normal. However, one of the earliest and first recognized neurogenetic syndromes, tuberous sclerosis complex (TSC), featured just this type of patchy malformation, the cortical tuber. For over a decade, it has been known that germline loss of function mutations in TSC1 and TSC2, causing hyperactivation of the mammalian target of rapamycin (mTOR) signaling cascade, are associated with cytomegaly and disorganized lamination within the cerebral cortex, the hallmark features of TSC. Furthermore, loss of function mutations of PTEN, an upstream phosphatase that inhibits the PI3K-AKT-mTOR pathway, are well known causes of generalized megalencephaly (MEG) in syndromic (Cowden and Bannayan–Riley–Ruvalcaba syndromes) and non-syndromic phenotypes (MEG with autism). In this context, the identification of somatic mosaicism in HME and MEG phenotypes makes sense but has only recently come to attention. Mosaicism, as exemplified by these disorders, provides a new set of mechanisms previously under-appreciated as causes of neurological disease [Poduri et al., 2013]. Single cell sequencing of cortical tubers and the detection of somatic TSC1/2 mutations was a foreshadowing of our very rapidly improved understanding of the genetics of these disorders [Crino et al., 2010].
In this review, we will highlight genetic etiologies of brain overgrowth disorders broadly and then review the clinical spectrum and molecular pathogenesis of a recently emerging class of brain overgrowth phenotypes associated with mutations in the PI3K-AKT-mTOR pathway. Identification of new genes is ongoing. However, the biologic effects of these mutations on brain and body overgrowth and genotype–phenotype correlations are still under investigation, and we anticipate that these will be areas of continued discovery in the coming years.
SYNDROMES AND GENES ASSOCIATED WITH BRAIN OVERGROWTH: A GENERAL OVERVIEW
Megalencephaly (MEG) is classically defined as an oversized and overweight brain that exceeds the age-related mean by 2 or more standard deviations. Clinically, the distinction between megalencephaly (enlarged brain) and macrocephaly (enlarged head overall) relies on neuroimaging examination of the brain and recognition of enlarged cerebral structures. Whereas MEG is associated with specific syndromes, macrocephaly can be caused by a myriad of causes such as hydrocephalus or ventriculomegaly, enlarged extra-axial spaces, and thickened skull bones. Therefore, the distinction between MEG and macrocephaly is clinically helpful towards accurate diagnosis.
Megalencephaly (MEG) is classically defined as an oversized and overweight brain that exceeds the age-related mean by 2 or more standard deviations. Clinically, the distinction between megalencephaly (enlarged brain) and macrocephaly (enlarged head overall) relies on neuroimaging examination of the brain and recognition of enlarged cerebral structures.
MEG has long been classified based on pathogenesis into metabolic and non-metabolic (or anatomic) subtypes [DeMyer, 1972, 1986]. Cellular hypertrophy due to cellular edema or accumulation of metabolic substrates can cause MEG in a wide range of neurometabolic syndromes, such as Canavan disease, glutaric aciduria type I, lysosomal storage disorders, among others (Table I). A growing number of developmental (or non-metabolic) genetic syndromes are known to be associated with generalized or focal MEG, including HME. Table II represents a broad overview of genetic disorders where MEG is a defining/diagnostic or common feature. Brain overgrowth in these syndromes varies widely in severity, distribution and co-occurrence of other malformations of cortical development from a mild (and often relatively) enlarged brain with a normal cortex to bilateral MEG with diffuse cortical dysplasia, as discussed below. Finally, reciprocal copy number changes are known to be associated with brain growth dysregulation (i.e., MEG and microcephaly) (Table III).
| Syndrome | Gene | Clinical features | Neuroimaging findings | Metabolic abnormalities |
|---|---|---|---|---|
| Cerebral organic acid disorders and disorders of lysine metabolism | ||||
| N-Acetylaspartic aciduria (Canavan disease)a | ASPA | Progressive severe ID, SZ, OA, spasticity, opisthotonus | Diffuse symmetric WM abnormalities | ↓Aspartoacylase (ASPA), ↑N-acetyl-aspartic acid (NAA) |
| Glutaric aciduria (GA) type Ia | GCDH | Neonatal MAC, ID, dyskinesia, choreoathetosis, dystonia | Frontotemporal atrophy (95%), delayed myelination, high signal intensity in the dentate nucleus, subdural effusion/hemorrhage | ↓Glutaryl-CoA dehydrogenase, ↑Glutaryl-CoA, ↑Acylcarnitines:free carnitine, ↑Urinary dicarboxylic acids |
| L-2-Hydroxyglutaric aciduria | L2HGDH | Progressive MAC (50%), ID, SZ, extrapyramidal signs | Swollen subcortical WM, progressive loss of arcuate fibers, severe cerebellar atrophy, signal intensities in the dentate nuclei and globi pallidi, low signal intensities in the thalami | ↓L-2-hydroxyglutarate dehydrogenase, ↑L-2-hydroxyglutaric acid (CSF> plasma), ↑hydroxydicarboxylic acids (CSF), ↑Lysine (CSF, blood) |
| D-2-Hydroxygylatric aciduria | D2HGDH | Neonatal epileptic encephalopathy with severe ID, hypotonia, CM to mild DD/no symptoms | Delayed and abnormal gyration, myelination and opercularization, VMEG, cysts over head of the caudate nucleus | ↓D-2-hydroxyglutaric acid dehydrogenase, ↑D-2-hydroxyglutaric acid |
| Lysosomal storage diseases | ||||
| aDisorders of Sphingolipid Metabolism | ||||
| Generalized gangliosidosis GM1 (early infantile)a | GLB1 | ID, HSM, SZ, tone abnormalities, DYSM, HSM, macular cherry red spot | Diffuse hypomyelination, mild T2 hyperintensities of the caudate nucleus and putamen | ↓β-galactosidase, ↑GM1 ganglioside, asialo-GA1 (neurons), ↑oligosaccharide, minor glycolipids, glycopeptides (visceral organs) |
| GM2 gangliosidosis | ||||
| Tay-Sachs disease (infantile)a | HEXA | Hypotonia, motor weakness, SZ, hyperacusis, macular cherry red spot, blindness, spasticity, MAC by 18 months of age | Similar to GM1 | ↓Hexosaminidase A, ↑GM2-ganglioside (neurons) |
| Sandhoff diseasea | HEXB | Organomegaly and bony abnormalities less common | Similar to GM1 | ↓Hexosaminidase A and B, ↑GM2-ganglioside, asialo-GM2 (neurons), ↑Globosides, oligosaccharides (viscera) |
| Krabbe disease (globoid cell leukodystrophy) (early infantile)a | GALC | PN, opisthotonus, SZ, hyperpyrexia, blindness, loss of bulbar functions, hypotonia | Diffuse WM abnormalities, diffuse cerebral atrophy, calcifications (thalamus, BG, periventricular WM) | ↓Galactosylceramidase, ↑Galactosylceramide (globoid cells), ↑Galactosylphingosine (oligodendrocytes, Schwann cells) |
| Mucopolysaccharidoses (MPS) | ||||
| Hurler syndrome (type IH) | IDUA | HSM, CNS, DM, DYS, OPH, CAR | WM abnormalities, cerebral atrophy, cervical myelopathy | ↓Iduronidase, ↑Heparan sulfate, ↑Dermatan sulfate |
| Hunter syndrome (type II) | IDS | HSM, CNS, DM, DYS, OPH, CAR, SK | WM abnormalities, cerebral atrophy, cervical myelopathy | ↓Iduronate-2-sulfatase, ↑Heparin sulfate, ↑Dermatan sulfate |
| Sanfilippo syndrome (type III) | SGSH(IIIA), NAGLU(IIIB), HGSNAT(IIIC), GNS(IIID) | CNS, DM (+/−), DYS (+/−) | WM abnormalities, cerebral atrophy, cervical myelopathy | ↓Heparan N- sulfatase (IIIA), ↓N-acetyl-glucosaminidase (IIIB), ↓Acetyl CoA glucosamine N-acetyl transferase (IIIC), ↓N-acetyl-glucosamine-6-sulfatase (IIID), ↑Heparan sulfate |
| Morquio syndrome (type IV] | GALNS(IVA), GLB1(IVB) | DM, CAR, OPH (+/−) | WM abnormalities, cerebral atrophy, cervical myelopathy | ↓N-acetylgalactosamine-6-sulfatase (IVA), ↓β-galactosidase (IVB), ↑Keratan sulfate |
| Maroteaux-Lamy syndrome (type VI) | ARSB | HSM, DM, DYS, OPH, CAR | WM abnormalities, cerebral atrophy, cervical myelopathy | ↓N-acetyl-galactosamine-4-sulfatase, ↑Dermatan sulfate |
| Mucolipidosesb | ||||
| Mucolipidosis type II (I-cell disease) | GNPTAB | HSM, CNS, DM, DYS, OPH, CAR | Cerebral atrophy, WM abnormalities (occasionally) | ↓Transferasec |
| Mucolipidosis type III | GNPTAB (α/β), GNPTG (γ) | HSM (+/−), CNS (+/−), DM, DYS (+/−), CAR | Cerebral atrophy, WM abnormalities (occasionally) | ↓Transferasec |
| Mannosidosis | MAN2B1 (α), MANBA (β) | HSM, DM, DYS, CAR, CNS (+/−) | Partially empty sella turcica, cerebellar atrophy, WM abnormalities (α) | ↓α-mannosidase (α), ↑α-mannosides (α), ↓β-mannosidase (β), ↑β-mannosides (β) |
| Leukoencephalopathiesa | ||||
| aAlexander disease (infantile and juvenile forms) | GFAP | ID, SZ, paraparesis, feeding problems | WM abnormalities (frontally-predominant), calcification of the BG, cerebellar changes, HYD | — |
| Megalencephalic leukoencephalopathy with subcortical cysts | MLC1, HEPACAM | Progressive spasticity, ataxia | Extensive symmetric, WM changes with subcortical cyst | — |
- BG, basal ganglia; CAR, cardiovascular involvement; CC, corpus callosum; CM, cardiomyopathy; CNS, central nervous system regression; ID, developmental delay; DM, dysostosis multiplex; DYS, dysmorphic features; HL, hearing loss; HSM, hepatosplenomegaly; MAC, macrocephaly; OA, optic atrophy; OPH, ocular anomalies [corneal clouding, ophthalmoplegia]; PN, peripheral neuropathy; SK, dermatological findings; SZ, seizures; VMEG, ventriculomegaly; WM, white matter.
- Inheritance: Most of these disorders are AR in inheritance with the exception of Hunter syndrome (XL), Alexander disease (AD), and megalencephalic leukoencephalopathy with subcortical cysts due to HEPACAM mutations.
- a Other leukoencephalopathies associated with megalencephaly (as indicated in the table): Canavan disease, glutaric aciduria type I, infantile generalized, or GM1, gangliosidosis, infantile GM2 gangliosidosis (Tay-Sachs; Sandhoff diseases), infantile Krabbe disease.
- b May not be true MAC.
- c Lysosomal UDP-N-acetylglucosamine-I-phosphotransferase.
- Reference: Mirzaa et al. [2012].
| Gene | Protein function | Inheritance | Syndrome | Clinical features | Neurologic findings | MRI findings |
|---|---|---|---|---|---|---|
| PI3K-AKT-MTOR | ||||||
| PTEN | Phosphatase, tumor suppressor | De novo/dominant | Megalencephaly-autism syndrome | Mild DYSM (frontal bossing, midface hypoplasia, biparietal narrowing) | ASD, ID | MEG |
| Cowden syndrome | Mucocutaneous lesions, malignancy risk (breast, thyroid, endometrium) | ID (10%) | Cerebellar dysplastic gangliocytoma (Lhermitte-Duclos disease) | |||
| Bannayan–Riley–Ruvalcaba syndrome | Overgrowth, hamartomatous intestinal polyposis, lipomas, penile pigmented macules, malignancy risk similar to CS | Autistic features, ID (70%), SZ (25%), proximal myopathy (60%) | — | |||
| HME | Macrocephaly (asymmetric; maybe seen in HME) | ID (severe), SZ (intractable), hemiparesis | HME: VMEG, MCD, WM abnormalities (ipsilateral) | |||
| PIK3CA | Kinase | Post zygotic/mosaic (rare germ line) | MCAP syndrome | MEG, capillary malformations, digit anomalies (polydactyly, syndactyly), segmental somatic overgrowth, connective tissue/skin laxity | ID, SZ, hypotonia (variable) | HYD, VMEG, CBTE, PMG, thick CC |
| HME | As above | As above | As above | |||
| Somatic overgrowth: CLOVES, Fibroadipose hypoplasia, Isolated macrodactyly | Variable segmental somatic overgrowth, digital anomalies, spinal anomalies, cutaneous vascular malformations; also isolated macrodactyly in some cases | Some have ID | HME and Chiari malformation reported in some individuals | |||
| Klippel–Trenaunay syndrome (KTS) | Cutaneous VM (capillary, venous, lymphatic), varicose veins, unilateral hypertrophy of bones and soft tissues | ID/SZ (rare) | HYD, calcifications, HME reported in some individuals | |||
| PIK3R2 | Kinase | De novo/dominant | MPPH syndrome | Postaxial polydactyly, MEG | ID, epilepsy, tone abnormalities | MEG, perisylvian PMG, HYD, mega CC |
| AKT1 | Kinase | Post-zygotic/mosaic | Proteus syndrome (associated with HME) | Asymmetric and disproportionate hamartomatous overgrowth of multiple tissues, connective tissue and epidermal nevi, dysregulated adipose tissue, VM, hyperostosis | ID (20%), SZ (13%) | Calcifications, abnormalities of the CC, HYD, HME reported in some individuals |
| AKT3 | Kinase | De novo/dominant | MPPH | As above | As above | As above |
| HME | As above | As above | As above | |||
| STRADA/LYK5 | STE20-related kinase adaptor (“pseudokinase”) | Recessive | Polyhydramnios, MEG, symptomatic epilepsy (PMSE) syndrome | DYSM, strabismus, skeletal muscle hypoplasia, nephrocalcinosis | ID, hypotonia, SZ, ASD | VEMG (mild), subependymal dysplasia, WM abnormalities |
| TSC1, TSC2 | Tumor suppressor | De novo/dominant (somatic mosaicism described) | Tuberous sclerosis complex (TSC) (associated with HME, FCD) | Skin (hypomelanotic macules, facial angiofibromas, shagreen patches, fibrous facial plaques, ungal fibromas), angiomyolipomas, rhabdomyomas | SZ (80%), ID (50%), ASD/PDD (40–50%), ADHD | SEN, cortical tubers, SEGAs, WM abnormalities, HME/FCD |
| TBC1D7 | GTPase-RHEB | Recessive | ID, macrocephaly, patellar dislocation, celiac disease | Osteoarticular problems, celiac disease, myopia, astigmatism | ID (mild), behavioral abnormalities, LD | Cerebral calcifications |
| MTOR | Kinase | Post-zygotic/mosaic | HME | As above | As above | As above |
| CCND2 | Cyclin; cell cycle control | De novo/dominant | MPPH | As above | As above | As above |
| Ras/mitogen-activated protein kinase (MAPK) pathway “the RASopathies” (associated with absolute or relative macrocephaly) | ||||||
| NF1 | RasGAP | De novo/dominant | Neurofibromatosis 1 | CALs, axillary freckling, cutaneous neurofibromas, short stature | LD (50-75%), (severe ID 3%), ADHD, headaches (20%), SZ (10%) | Optic glioma (15%), UBOs, CC abnormalities, HYD |
| SPRED1 | Sprouty-related | De novo/dominant | Legius syndrome | CALs, freckling, lipomas, macrocephaly, no tumor manifestations | ID/LD, ADHD, headaches, SZ | — |
| HRAS | GTPase | De novo/dominant | Costello syndrome | FTT, short stature, coarse facial features, fine, curly or sparse hair, papillomata, HCM, CHD, malignancy risk (15%) | ID (∼100%), hypotonia (most), SZ (20-50%) | CBTE, VMEG/HYD |
| BRAF | Kinase | De novo/dominant | Cardiofaciocutaneous (CFC) syndrome | Cardiac abnormalities (VHD, HCM, dysrhythmias), DYSM, multiple cutaneous abnormalities | ID (80%), SZ (50%), hypotonia | HYD/VMEG, cortical atrophy, ACC, NMD |
| Noonan syndrome (NS) | Short stature, CHD (PVS, HCM), characteristic facies, webbed neck, coagulation defects, lymphatic dysplasias | ID (variable), language delay | VMEG, CBTE | |||
| MAP2K1 | Kinase | De novo/dominant | CFC syndrome | As above | As above | As above |
| Noonan syndrome | As above | As in the above | As in the above | |||
| MAP2K2 | Kinase | De novo/dominant | CFC syndrome | As above | As above | As above |
| KRAS | GTPase | De novo/dominant | CFC syndrome | As above | As above | As above |
| Noonan syndrome | As above | As above | As above | |||
| PTPN11 | Phosphatase | De novo/dominant | Noonan syndrome | As above | As above | As above |
| NRAS | GTPase | De novo/dominant | Noonan syndrome | As above | As above | As above |
| RAF1 | Kinase | De novo/dominant | Noonan syndrome | As above | As above | As above |
| SOS1 | RasGEF | De novo/dominant | Noonan syndrome | As above | As above | As above |
| RIT1 | GTPase | De novo/dominant | Noonan syndrome | As above | As above | As above |
| SHOC2 | Scaffolding | De novo/dominant | Noonan syndrome with loose anagen hair | Skin and hair abnormalities (sparse, thin, slow growing hair with pigment abnormalities), features of NS | As above | As above |
| Transcriptional regulation | ||||||
| NSD1 | Histone methyltransferase | De novo/dominant | Sotos syndrome | Prenatal and postnatal overgrowth, characteristic facial gestalt, advanced bone age | Hypotonia, ID/behavioral problems (very common), SZ (25%) | Prominent trigone, VMEG/HYD, XAX, CC abnormalities, CSP |
| EZH2 | Histone methyltransferase | De novo/dominant | Weaver syndrome | Characteristic facies (prominent hypertelorism, micrognathia, deep horizontal skin crease), camptodactyly | ID (81%) | Pachygyria, VMEG, cysts of the septum pellucidum (rare) |
| MED12 | Mediator complex | X-linked | Opitz–Kaveggia (FG) syndrome | Imperforate anus, characteristic facial features, broad thumbs | ID (97%), hypotonia (90%), SZ (70%) | Abnormalities of CC, VMEG, HET |
| Lujan (Lujan–Fryns) syndrome | Marfanoid habitus, maxillary hypoplasia, palate and dental problems, long hands, hyperextensible digits | ID (mild-mod), behavioral abnormalities | Dysgenesis of the CC | |||
| Glypicans | ||||||
| GPC3 | Glypican; cell surface heparan sulfate proteoglycans | X-linked | Simpson-Golabi-Behmel syndrome | Prenatal overgrowth, characteristic facies (macroglossia, macrostomia, central groove of lower lip, ocular hypertelorism), supernumerary nipples | Hypotonia, ID (variable), SZ | HYD, CBTE, ACC (all rare) |
| Mitotic regulation, centrosome and microtubule assembly | ||||||
| KIF7 | Kinesin | De novo/dominant | Acrocallosal syndrome | Polysyndactyly, hypertelorism | SZ, ID (very common) | ACC |
| OFD1 | Centriole-associated | X-linked | Simpson-Golabi-Behmel syndrome (type 2) | Ciliary dyskinesia, respiratory problems, DYSM, short fingers | Severe ID, hypotonia | VMEG* |
| DIS3L2 | Exoribonuclease | Recessive | Perlman syndrome | Fetal gigantism, renal hamartomas, nephroblastomatosis, risk for Wilms tumor | ID (most), (poor survival) | Abnormalities of the CC, HET, WM abnormalities, cerebral atrophy |
| Sonic Hedgehog (Shh) signaling | ||||||
| PTCH1 | Patched; receptor for sonic hedgehog | De novo/dominant | Nevoid basal cell carcinoma (Gorlin) syndrome | Jaw keratocysts, basal cell carcinomas (BCCs), coarse facial features, facial milia, skeletal anomalies (bifid ribs, wedge-shaped vertebrae) | — | Ectopic calcifications (in falx >90%), medulloblastoma (PNET) (5%) |
| GLI3 | Zinc finger | Recessive | Acrocallosal syndrome | As above | As above | As above |
| De novo/dominant | Greig cephalosyndactyly | Polydactyly (pre-, post-axial, mixed), ocular hypertelorism, craniosynostosis | ID, SZ (<10%) | HYD (uncommon) | ||
| Other less common genes and disorders | ||||||
| RIN2 | Ras effector protein | Recessive | MACS syndrome (macrocephaly, alopecia, cutis laxa, and scoliosis) | Coarse facial features, gingival hyperplasia, severe joint hypermobility, soft redundant skin, sparse hair, short stature, severe scoliosis | — | — |
| RAB39 | Rab GTPase; cellular endocytosis | X-linked | XLID, autism, epilepsy and macrocephaly | Macrocephaly | ID/MR, ASD, SZ | — |
Notes
- 1. This table includes only gene-known MEG/HME disorders and does not include others where underlying genetic etiology is unknown as of writing this manuscript (such as Macrocephaly, megalocornea, motor and mental retardation (MMMM) syndrome, Macrosomia, obesity, macrocephaly, and ocular abnormalities (MOMO), for example). This list also does not include skeletal dysplasias known to be associated with MEG (such as achondroplasia and thanatophoric dysplasia).
- 2. HME has also been reported with other somatic manifestations such as hypomelanosis of Ito and linear nevus sebaceous syndrome.
- Reference: Mirzaa et al. [2012].
- ACC, agenesis of the corpus callosum; ADHD, attention-deficit-hyperactivity disorder; ASD, autism spectrum disorder; CAL, café au lait macules; CBTE, cerebellar tonsillar ectopia; CC, corpus callosum; CHD, congenital heart disease; CLOVES, congenital lipomatous asymmetric overgrowth of the trunk, lymphatic, capillary, venous, and combined-type vascular malformations, epidermal nevi, skeletal and spinal anomalies; CSP, cavum septum pellucidum; DYSM, dysmorphic features; FCD, focal cortical dysplasia; FTT, failure to thrive; HCM, hypertrophic cardiomyopathy; HET, heterotopias; HME, hemimegalencephaly; HYD, hydrocephalus; ID, intellectual disability; LD, learning disability; MEG, megalencephaly; MPPH, megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome; NMD, neuronal migration disorder; PDD, pervasive developmental disorder; PMG, polymicrogyria; PNET, primitive neuroectodermal tumor; PVS, pulmonary valve stenosis; SZ, seizures; UBO, unidentified bright object; VHD, valvular heart disease; VM, vascular malformation; VMEG, ventriculomegaly; WM, white matter; XAX, enlarged extra-axial space; XLID, X-linked intellectual disability.
| Locus | CNV | Gene | Size | Syndrome/pathway | Refs. |
|---|---|---|---|---|---|
| 1q21.1 | Duplication | — | MAC | — | Brunetti-Pierri et al. [2008] |
| Deletion | — | MIC | — | Brunetti-Pierri et al. [2008] | |
| 1q43.44 | Duplication | AKT3 | MEG | PI3K-AKT; MEG and HME | Poduri et al. [2012], Wang et al. [2013], Chung et al. [2014] |
| Deletion | — | MIC | PI3K-AKT; postnatal MIC | Ballif et al. [2012] | |
| 2q24.3a | Duplication | MYCN | MEG | Regulates growth an apoptosis | Malan et al. [2010] |
| Deletion | — | MIC | Feingold syndrome | Van Bokhoven et al. [2005] | |
| 5q35.5 | Duplication | — | MIC | — | Rosenfeld et al. [2013] |
| Deletion | NSD1 | MEG | Sotos syndrome, transcriptional regulator | Tatton-Brown et al. [2005] | |
| 10q22–23 | Duplication | — | MIC | — | Aalfs et al. [1995] |
| Deletion | — | MAC | — | Van Bon et al. [2011] | |
| 16p11.2 | Duplication | — | MIC | — | Shinawi et al. [2009] |
| Deletion | — | MAC | — | Shinawi et al. [2009] |
- HME, hemimegalencephaly; MAC, macrocephaly; MEG, megalencephaly; MIC, microcephaly.
- a This disorder is associated with triphalangeal thumb, and dysmorphic facial features similar to Weaver syndrome.
Other important overgrowth conditions include Sotos syndrome, Weaver syndrome, Simpson Golabi Behmel syndrome, and nevoid basal cell carcinoma syndrome. While they are beyond the scope of this review, many of the same principles apply to these disorders as to the conditions that affect the brain predominantly.
MOLECULAR PATHWAYS OF MEGALENCEPHALY AND HEMIMEGALENCEPHALY
Cellular growth of neuronal elements is an intricately orchestrated process, as discussed by Drs. Alcantara and O'Driscoll in this series. Dysregulation of a number of critical pathways is known to be associated with human brain overgrowth phenotypes, as highlighted in Table II. Dysregulation of two particular critical cellular pathways, the Ras/mitogen-activated protein kinase (MAPK) pathway and the PI3K-AKT-mTOR pathway, appear to account for the largest number of known MEG/HME syndromes. Both pathways are associated with multiple diverse cellular functions including cellular proliferation, differentiation, cell cycle regulation, survival, and metabolism. Not surprisingly, these two pathways are functionally related. Given their critical developmental roles, germline mutations of genes in both pathways are believed to be embryonic lethal. When dysregulated, regardless of the specific gene or protein alteration, the ensuing syndromes exhibit numerous overlapping phenotypic features spanning many organ systems. Furthermore, both pathways have been extensively studied in the cancer field and constitute very attractive targets for pathway (small molecule) inhibitor therapy to treat various malignancies.
Whereas most mutations in the RASopathies are germline, the emerging spectrum of PI3K-AKT-mTOR pathway phenotypes (particularly in upstream components such as PIK3CA) includes predominantly post-zygotic mutations present in a mosaic pattern. The related MEG syndromes involve almost every organ system in the body including the brain (intellectual disability, autism, epilepsy, hydrocephalus, Chiari malformation), heart and vascular system (conduction defects, heart-great vessel anomalies), skin (capillary malformations, epidermal nevi), connective tissue (skin laxity, joint hypermobility), skeleton (polydactyly, syndactyly), and others. Mutations within the PI3K-AKT-mTOR pathway are associated with the most severe brain overgrowth phenotypes including marked brain overgrowth (occipito-frontal circumference, OFC, more than 4 standard deviations above the mean) and HME, a serious medical condition typically associated with severe early onset intractable epilepsy and poor developmental outcome. While some syndromes in both pathways are considered cancer syndromes, as identified mutations are “activating” causing enhanced pathway activation, some of the novel germline and mosaic mutations are not as robustly activating as those associated with oncogenesis and require further study. We will specifically focus on an area of rapidly increasing knowledge related to PI3K-AKT-mTOR-related brain overgrowth phenotypes, their clinical and neuroimaging features, and molecular pathogenesis.
PI3K-AKT RELEATED MEG AND HME: THE CLINICAL AND NEUROIMAGING SPECTRUM
In addition to PTEN-related disorders, upstream mutations in core components of the PI3K-AKT-mTOR pathway have now been identified in classic HME and a variety of MEG syndromes including the MEG-capillary malformation syndrome (MCAP) and the MEG-polymicrogyria-polydactyly-hydrocephalus syndrome (MPPH). Collectively, these disorders not only overlap molecularly but also share clinical, neuroimaging, and neuropathologic features.
PTEN-Related Disorders
Loss of function mutations of PTEN have been identified in a wide range of MEG phenotypes including two well-known cancer predisposition syndromes: Cowden and Bannayan–Riley–Ruvalcaba syndrome (BRRS) syndromes [Liaw et al., 1997; Marsh et al., 1997]. These disorders constitute a clinical spectrum associated with prenatal onset MEG, hamartomas, lipomas, intestinal polyps, and various types of cutaneous vascular malformations [Gorlin et al., 1992; Tan et al., 2011]. Neurologically, affected individuals have hypotonia, delayed gross motor skills and, in some, proximal myopathy [Marsh et al., 1999]. Prenatal onset progressive MEG is typical and OFCs are usually 4–5 (and up to 8) standard deviations above the mean, whereas body overgrowth is typically mild (+1–3 SD). PTEN mutation carriers are at increased risk for various tumors, most notably of the breast, thyroid, and endometrium. Finally, PTEN mutations constitute the largest single gene defects in autistic children with MEG, with estimates of mutations between 1% and 17% in this cohort [Butler et al., 2005; Buxbaum et al., 2007]. While OFCs in this cohort vary, one of the earliest reports showed OFCs of +7–8 SD above the mean [Butler et al., 2005]. An average OFC of +4.35 SD was recently reported in 6 PTEN mutation-positive individuals with autism [Hobert et al., 2014]. Pten loss of function mutant mice develop macrocephaly and behavioral abnormalities such as reduced social activity, increased anxiety and sporadic seizures, closely resembling the human phenotype of PTEN-related disorders [Kwon et al., 2001; Ogawa et al., 2007].
Pten loss of function mutant mice develop macrocephaly and behavioral abnormalities such as reduced social activity, increased anxiety and sporadic seizures, closely resembling the human phenotype of PTEN-related disorders.
While most children with PTEN mutations have uniform bilateral MEG with grossly normal cortical cytoarchitecture (Fig. 1A–C), there are several published cases of asymmetric or focal brain phenotypes in individuals with germline PTEN mutations. These include HME and linear epidermal nevi in a child whose family has features of BRRS, and FCD with focal intractable epilepsy in a child with features of Cowden syndrome [Merks et al., 2003; Elia et al., 2012] (Fig. 1D–F).
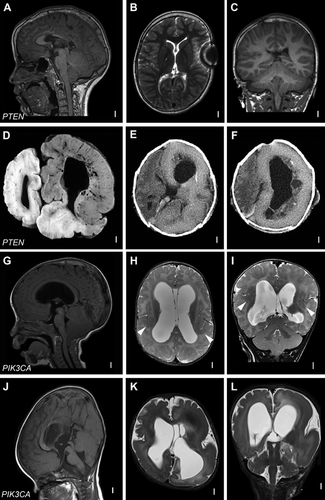
PIK3CA-Related Disorders
Post-zygotic gain-of-function mutations in PI3KCA have been recently identified in a growing number of segmental brain and body overgrowth disorders (Table IV). These phenotypes include somatic overgrowth disorders such as CLOVES syndrome (congenital lipomatous asymmetric overgrowth of the trunk, lymphatic, capillary, venous, and combined-type vascular malformations, epidermal nevi, skeletal and spinal anomalies), fibroadipose hyperplasia, isolated macrodactyly and Klippel–Trenaunay syndrome, and predominantly brain overgrowth phenotypes such as HME and MCAP syndrome [Kurek et al., 2012; Lindhurst et al., 2012; Poduri et al., 2012; Rivière et al., 2012; Lee et al., 2012; Rios et al., 2013]. The severity and extent of brain and body overgrowth in these phenotypes is variable. This is particularly true with brain involvement that ranges from bilateral generalized MEG with PMG (as with classic MCAP), to HME to dysplastic MEG (Fig. 1G–L). The tissue distribution and types of post-zygotic mutations are expected to be among the primary determinants of the developmental phenotypes that are often severe, as we discuss below.
| Gene | Type of mutation | Inheritance | CNS phenotype | Non-CNS phenotype |
|---|---|---|---|---|
| PTEN | Loss of function | De novo/dominant | MEG-autism, HME, FCD | Cowden, BRRS |
| AKT3 | Gain of function | Post-zygotic/mosaic, De novo/dominant | HME, MPPH | — |
| PIK3CA | Gain of function | Post-zygotic/mosaic | Megalencephaly (MCAP), HME | Somatic overgrowth (CLOVES/FH, macrodactyly, MCAP) |
| PIK3R2 | Gain of function | De novo/dominant | MPPH | Polydactyly |
| CCND2 | Gain of function | De novo/dominant | MPPH | Polydactyly |
| AKT1 | Gain of function | Post-zygotic/mosaic | HMEa | Proteus syndrome |
- BRRS, Bannayan–Riley–Ruvalcaba syndrome; FH, fibro-adipose hyperplasia; HME, hemimegalencephaly; MCAP, megalencephaly-capillary malformation syndrome.
- a HME reported in Proteus syndrome, but no AKT1 mutations have been identified in affected brain tissues to our knowledge.
The classic neuroimaging features of MCAP are marked diffuse MEG with bilateral perisylvian polymicrogyria (PMG), although this latter feature may not be present in a large number of children. Some individuals also have marked cerebellar enlargement, with a large and crowded posterior fossa and ensuing cerebellar tonsillar ectopia that may necessitate surgical decompression [Conway et al., 2007a,b; Mirzaa et al., 2012a]. Somatic manifestations of MCAP include cutaneous vascular malformations (typically capillary malformations), polydactyly, syndactyly, and focal somatic overgrowth—overlapping with but typically milder than other somatic PIK3CA-related disorders [Clayton-Smith et al., 1997; Moore et al., 1997; Conway et al., 2007b; Mirzaa et al., 2012a].
The classic neuroimaging features of MCAP are marked diffuse MEG with bilateral perisylvian polymicrogyria (PMG), although this latter feature may not be present in a large number of children. Some individuals also have marked cerebellar enlargement, with a large and crowded posterior fossa and ensuing cerebellar tonsillar ectopia that may necessitate surgical decompression
The Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus (MPPH) Syndrome
This rare developmental syndrome is characterized predominantly by prenatal onset MEG and bilateral perisylvian PMG. Hydrocephalus and postaxial polydactyly are more variable features seen in nearly half of reported individuals [Kariminejad et al., 2012; Mirzaa et al., 2013]. A subset of children also has a distinctly thick corpus callosum (mega-corpus callosum). De novo germline mutations in three core PI3K-AKT-mTOR pathway genes are now known to be associated with MPPH including, in order of frequency, PIK3R2, CCND2, and AKT3 [Rivière et al., 2012; Nakamura et al., 2014; Mirzaa et al., 2014] (Fig. 2A–C, G–L). These mutations are mostly germline mutations with a narrow mutational spectrum. For example, a single recurrent PIK3R2 (p.Gly373Arg) mutation has been reported in most MPPH children. Postaxial polydactyly is a more frequent feature in PIK3R2 versus CCND2-positive children.
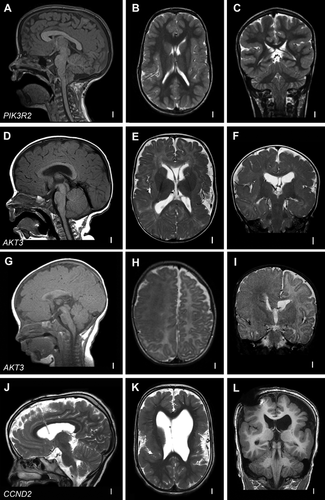
Hemimegalencephaly
HME is a severe brain malformation characterized by overgrowth of all or part of a cerebral hemisphere, often with ipsilateral severe cortical dysplasia or dysgenesis, white matter hypertrophy and a dilated and dysmorphic lateral ventricle. It is often an isolated congenital abnormality, but there are sporadic associations with neurocutaneous and overgrowth syndromes in the literature including with Proteus syndrome, Klippel–Trenaunay syndrome, linear nevus sebaceous (LNS) syndrome, TSC, neurofibromatosis type 1, and hypomelanosis of Ito [Cristaldi et al., 1995; Sharma et al., 2009; Pavlidis et al., 2012]. HME constitutes the most severe brain overgrowth phenotype not only morphologically but also because most children with HME experience early onset intractable epilepsy, typically within the first few months of life. Children with HME can present with focal seizures or epilepsy syndromes such as infantile spasms. Developmental delay is often early and severe. Within the affected hemisphere, neuroimaging reveals regions of apparent PMG, pachygyria, subcortical, and periventricular gray matter heterotopia. However, various morphological abnormalities outside the involved cerebral hemisphere have been reported such as ipsilateral cerebral vascular dilatation, ipsilateral and bilateral cerebellar enlargement with dysplastic folia, and ipsilateral olfactory nerve enlargement [Sato et al., 2007]. Moreover, contralateral volume loss (or hemimicrencephaly) with white matter abnormalities have been reported [Shiroishi et al., 2010]. Although it is not determined whether these abnormalities are developmental or acquired, these and other more widespread or asymmetric malformations are believed to partially account for poor seizure control and poor post-hemispherectomy outcome in some individuals.
Activating mosaic mutations in three PI3K-AKT-mTOR pathway genes have now been reported in isolated HME including PIK3CA (four patients), AKT3 (two patients), and MTOR (one patient) [Lee et al., 2012; Poduri et al., 2012]. Further, duplications of 1q encompassing AKT3 have been identified in two HME patients with presumed activation of the gene [Poduri et al., 2012]. Duplications of AKT3 have also been reported in children with macrocephaly, focal PMG, and intellectual disability [Wang et al., 2013; Chung et al., 2014].
Interestingly, other less common patterns of focal MEG with cortical dysplasia have been described in the literature such as total or diffuse HME, localized MEG (hemi-hemimegalencephaly), and multilobar cortical dysplasia that share similar neuropathological findings to HME including large neurons, cortical dyslamination, with or without dysmorphic and ectopic neurons, heterotopia, balloon cells, and abnormal white matter [Barkovich and Chuang, 1991; Nakahashi et al., 2009; Blümcke and Mühlebner, 2011]. It is therefore expected that these more focal manifestations may share the same molecular pathogenesis.
PI3K-AKT-mTOR RELEATED MEG AND HME: MOLECULAR SPECTRUM AND INSIGHTS INTO MOLECULAR PATHOGENESIS
Mutations of the above mentioned PI3K-AKT-mTOR pathway genes, whether loss of function mutations of PTEN or gain of function mutations of PIK3CA, AKT3, and PIK3R2, all share a common functional endpoint, namely activation of the pathway (Table IV). Mutations of PTEN, PIK3R2, and AKT3 have been predominantly germline. Whereas mutations of PIK3CA and the Proteus syndrome gene, AKT1, have been mosaic, providing a molecular explanation for the wide phenotypic variability in their attendant overgrowth phenotypes [Lindhurst et al., 2011]. The phenotypic spectrum of PIK3CA-related disorders is particularly wide and, while the exact mechanisms by which mutations result in these manifestations are currently under study, some preliminary genotype–phenotype correlations can be suggested. For example, the same PIK3CA mutation (p.Glu545Lys) has been identified in the four children with HME so far [Lee et al., 2012]. The mutational spectrum of MCAP syndrome, on the other hand, is wide and has not included any of the so-called mutation “hotspots” seen in cancer (p.Glu542Lys, p.Glu545Lys, p.His1047Arg, p.His1047Leu) [Samuels and Ericson, 2006; Samuels and Waldman, 2010; Rivière et al., 2012; Mirzaa et al., 2013]. While these mutations are all activating, they may not as robustly activating as those seen in cancer, given that the cancer risk in these phenotypes does not appear to be much increased; although natural history, and particularly cancer risk, data are lacking.
Mutations of the above mentioned PI3K-AKT-mTOR pathway genes, whether loss of function mutations of PTEN or gain of function mutations of PIK3CA, AKT3, and PIK3R2, all share a common functional endpoint, namely activation of the pathway.
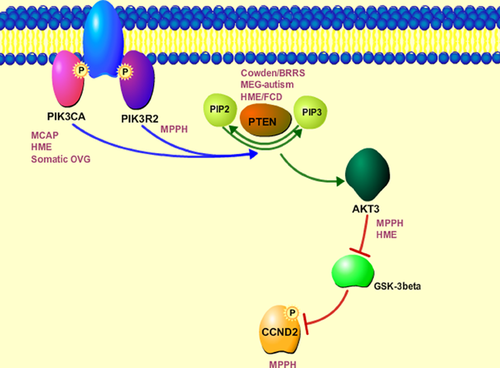
For more than a decade now, hyperactivation of the mTOR signaling downstream of PI3K-AKT (due to loss of function mutations of TSC1 and TSC2, for example) have been considered to provide a pathological link between TSC, HME, and FCD by extensive studies [Crino, 2007; Lim and Crino, 2013]. Further, mTOR inhibition reversed neuronal hypertrophy in Pten-deficient mice and ameliorated a subset of Pten-associated abnormal behaviors, thereby substantiating evidence that the mTOR pathway downstream of PTEN is critical for its complex phenotype [Kwon et al., 2003; Zhou et al., 2009]. However, the recent identification of mutations in CCND2 in MPPH syndrome sheds a novel insight into the molecular pathology of these phenotypes [Mirzaa et al., 2014]. CCND2 is a member of the D-type cyclin family critically required for G1/S transition during the cell cycle [Matsushime et al., 1991; Inaba et al., 1992; Ross et al., 1996; Glickstein et al., 2006, 2009]. Identified mutations within CCND2 affect highly conserved terminal residues that include targets for glycogen synthase kinase 3β (GSK-3β)-phosphorylation and, ultimately, its' ubuiquitin mediated degradation [Kida et al., 2007]. Recent data demonstrate accumulation of degradation resistant CCND2 in individuals with MPPH and also, interestingly, in lymphoblastoid cell lines of individuals with upstream mutations in PIK3CA, PIK3R2, and AKT3 [Mirzaa et al., 2014]. These data implicate for the first time the involvement of another critical effector pathway downstream of PI3K-AKT: the cell cycle pathway downstream of GSK3b (Fig. 3). Clearly, further investigation is necessary to determine the detailed molecular mechanisms of brain overgrowth in this pathway and how these various critical downstream pathways interact in these phenotypes.
Recent data demonstrate accumulation of degradation resistant CCND2 in individuals with MPPH and also, interestingly, in lymphoblastoid cell lines of individuals with upstream mutations in PIK3CA, PIK3R2, and AKT3.
THERAPIES AND FUTURE DIRECTIONS
The past few years have witnessed exciting advances in our understanding of the molecular pathogenesis of brain overgrowth. It follows that individuals with disorders of the PI3K-AKT-mTOR and the interacting Ras/MAPK pathways may have the option in the future of pathway-based rational treatment. Some of the key functional deficits of these disorders, such as intellectual disability, autism, epilepsy, hydrocephalus, are naturally attractive targets for treatment that today are still treated empirically. For example, the epilepsy associated with some MEG syndromes is treated with anti-epileptic drugs that do not specifically address the molecular defects that we now know to be the basis of their disease. The use of numerous small molecule PI3K-AKT-mTOR pathway inhibitors to alleviate some of these developmental defects is currently under study. While specific therapy is already available for the downstream TSC-mTOR pathway by everolimus [Kingwell, 2013; Krueger et al., 2013], it is clear that the brain and somatic overgrowth phenotypes associated with upstream PI3K-AKT-mTOR pathway mutations result from increased activation of multiple pathways downstream of AKT, leading us to predict that successful treatment strategies will need to downregulate more than one of these pathways.
Some of the key functional deficits of these disorders, such as intellectual disability, autism, epilepsy, hydrocephalus, are naturally attractive targets for treatment that today are still treated empirically. For example, the epilepsy associated with some MEG syndromes is treated with anti-epileptic drugs that do not specifically address the molecular defects that we now know to be the basis of their disease.
ACKNOWLEDGMENTS
The authors thank our patients and their families for their valuable and ongoing contributions and support of our research. A.P. was supported by the NINDS (NS069784).




