Toward deconstructing the phenotype of late-onset Pompe disease†‡
Doctors Angela Schüller, Stephan Wenninger, and Nicola Strigl-Pill are members of Dr. Schoseŕs working group at the Friedrich-Baur-Institute.
How to cite this article: Schüller A, Wenninger S, Strigl-Pill N, Schoser B. 2012. Toward deconstructing the phenotype of late-onset Pompe disease. Am J Med Genet Part C Semin Med Genet 160C:80–88.
Abstract
Pompe disease (glycogen storage disease type 2 or acid maltase deficiency) is a rare autosomal recessive lysosomal storage disorder. Since the advent of ERT a lot has been learned about the phenotypic spectrum especially in the late onset patients. We describe in detail 44 patients diagnosed with late-onset Pompe disease (LOPD) at our neuromuscular department from 1985 to 2011 and compare them to patients with LOPD in the literature of the past 40 years. Study of the Munich LOPD group revealed varying musculoskeletal and cardio-cerebrovascular manifestation patterns. Several of these symptom patterns commonly appeared in conjunction with one another, highlighting the multisystem involvement of this condition. Common symptom patterns include: (i) Classic limb girdle and diaphragmatic weakness, (ii) rigid spine syndrome (RSS), scoliosis, and low body mass, and (iii) several cardio-cerebrovascular manifestation patterns. The most common presentation, limb girdle and diaphragmatic weakness, appeared in 78% (34/44) of our patients and over 80% of those in the literature. Sixteen percent (7/44) of our patients presented with rigid spine, scoliosis, and low body mass. Although scoliosis had a reported frequency of 33% in the general LOPD patient population, the literature only occasionally reported low body mass and RSS. Importantly, a multisystem extramuscular finding accompanied by cardio-cerebrovascular manifestations was found in 29% (13/44) of our LOPD patients; the literature showed an increasing prevalence of this latter finding. By examining the phenotype of patients with confirmed LOPD, we found a more subtle clinical multisystem involvement in LOPD. Whether patients presenting with the different symptom patterns respond differently to enzyme replacement therapy remains a key question for future research. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Pompe disease is broadly classified by clinical manifestations into two groups of affected patients: The classic infantile phenotype and the late-onset adult type. In contrast with infantile Pompe disease where patients present at birth or shortly thereafter and experience rapid disease progression, late-onset Pompe disease (LOPD) describes patients presenting symptoms anytime after the first few years of life. The late-onset cohort is often divided into childhood, juvenile, and adult-onset forms, although many patients recall symptoms becoming evident in childhood. LOPD is characterized by a spectrum of physical manifestations, most notably progressive deterioration of proximal truncal muscles particularly affecting lower limbs and respiratory function
The late-onset cohort is often divided into childhood, juvenile, and adult-onset forms, although many patients recall symptoms becoming evident in childhood. LOPD is characterized by a spectrum of physical manifestations, most notably progressive deterioration of proximal truncal muscles particularly affecting lower limbs and respiratory function
[Felice et al., 1995; Mellies et al., 2005; Hagemans et al., 2006; Müller-Felber et al., 2007; Mellies and Lofaso, 2009]. The clinical continuum cannot be distinguished by age of onset or by mutational analysis.
In the past 40 years, more than 1,000 LOPD patients have been described in the literature [Laforet et al., 2000; Ausems et al., 2001; Hagemans et al., 2004; Hagemans et al., 2005; Winkel et al., 2005; Müller-Felber et al., 2007; Bembi et al., 2010; Byrne et al., 2011; Gungor et al., 2011; Roberts et al., 2011]. Based on a recent study of 268 LOPD patients, median survival after diagnosis of LOPD was 27 years, with a median age at death of 55 years (range 23–77 years). Five-year survival rate for patients without wheelchair or respiratory support was 95% compared to 74% for those who were wheelchair-bound and used respiratory support [Gungor et al., 2011].
Since 2006 when enzyme replacement therapy with recombinant human acid alpha-glucosidase was approved, this treatment has been administered to an increasing number of LOPD patients [Schoser et al., 2008; Ravaglia et al., 2009; Bembi et al., 2010; Strothotte et al., 2010; van der Ploeg et al., 2010]. Thus, during the past 5 years, clinical diagnosis of LOPD has received major attention in the neuromuscular field and led to the identification of additional clinical features and numerous phenotype patterns. Our current understanding is that Pompe disease is multisystemic at any age. With that in mind, we reanalyzed our Munich LOPD cohort in order to determine if we could deconstruct symptom patterns from the general LOPD phenotype for a better understanding of the disease burden.
PATIENTS AND METHODS
We studied 44 patients diagnosed with LOPD at the Friedrich-Baur-Institute between 1985 and 2011. Diagnosis of Pompe disease was based on evidence of a severely reduced acid alpha-glucosidase activity in dried blood spots, lymphocytes, muscle tissue, and/or mutational analyses of the GAA gene. Demographic and clinical data are summarized in Tables I and II.
| Genetic background | |
| Caucasian | 44/44 |
| Male/female | 17/27 |
| Mean age at first symptom | 33.3 years (range 9–60 years) |
| Mean age at time of diagnosis | 42.8 years (range 17–69 years) |
| Family history of Pompe disease | |
| No family history | 34 |
| 1 or more family members | 10, including one sib pair |
| Symptom/Sign | No. of patients |
|---|---|
| Limb girdle muscle weakness and atrophy | 43 |
| proximal lower extremities | 43 |
| upper extremities | 23 |
| truncal | 35 |
| diaphragmatic insufficiency | 24 |
| Trendelenburg's sign | 26 |
| Gowers's sign | 17 |
| exercise intolerance | 35 |
| myalgia and cramps | 9 |
| Symptoms/signs of bulbar and cranial nerve involvement | 5 |
| facial weakness | 4 |
| ptosis | 2 |
| tongue atrophy | 2 |
| tongue hypertrophy | 7 |
| dysphagia | 3 |
| dysphonia | 1 |
| dysarthria | 6 |
| hearing alterations | 2 |
| asymmetrical muscle hypertrophy | 32 |
| CK elevation | |
| Rigid spine syndrome, scoliosis, and low body weight | 7 |
| scoliosis | 34 |
| lumbar hyperlordosis | 29 |
| rigid spine syndrome | 7 |
| scapular winging | 12 |
| contractures | 3 |
| pes equinus | 1 |
| underweight/low BMI (BMI between 11 and 19) | 8 |
| Cardio-cerebrovascular pattern | 13 |
| heart rhythm alterations | 13 |
| sinus arrhythmia | 7 |
| right bundle block | 2 |
| sick sinus syndrome | 1 |
| WPW syndrome | 3 |
| left ventricular hypertrophy | 6 |
| aortic dilatation/aneurysm | 1 |
| cerebral aneurysm | 1 |
| stroke | 2 |
- Cohort (total number) is 44.
An electronic search of MEDLINE and PUBMED was performed to identify all studies published in English from 1966 through December 2011. Medical subject headings for this search were “glycogen storage disease type II” along with the keywords: Pompe disease, adult, and acid maltase deficiency. Studies that did not report on patients were excluded. Two independent reviewers reviewed all retrieved articles and scored for relevance for inclusion. Relevant data were extracted from the retrieved publications in order to answer the specific question of phenotype in LOPD. All larger cohorts of adult patients with proven acid alpha-glucosidase deficiency and unique case reports found in the literature were reviewed and summarized; however, cohorts reporting less than three cases; historic small case series and case studies without morphological, enzymatic, or genetical confirmation of Pompe disease were excluded here. [Engel, 1970; Angelini and Engel, 1972; Martin et al., 1976; Francesconi et al., 1982; Miyamoto et al., 1985; Swash et al., 1985; Isaacs et al., 1986; Braunsdorf, 1987; van der Walt et al., 1987; Matsuoka et al., 1988; Kretzschmar et al., 1990; Fadic et al., 1997; Laforet et al., 2000; Martini et al., 2001; Toda et al., 2001; Tabarki et al., 2002; Hagemans et al., 2004; Hermans et al., 2004; Kamphoven et al., 2004; Anneser et al., 2005; Hagemans et al., 2005; Winkel et al., 2005; Hagemans et al., 2006; Kostera-Pruszczyk et al., 2006; Slonim et al., 2006; Kroos et al., 2007; Müller-Felber et al., 2007; Nemes et al., 2007; Slonim et al., 2007; Joshi et al., 2008; Laforet et al., 2008; Soliman et al., 2008; Wokke et al., 2008; van der Beek et al., 2008; Refai et al., 2008b; van der Beek et al., 2009; Kobayashi et al., 2010; Laforet et al., 2010; Ravaglia et al., 2010; Sacconi et al., 2010; Yanovitch et al., 2010; van Capelle et al., 2010; van der Ploeg et al., 2010; Chien et al., 2011; Dubrovsky et al., 2011; El-Gharbawy et al., 2011; Forsha et al., 2011; Gungor et al., 2011; Roberts et al., 2011; Yang et al., 2011; van den Berg et al., 2011; van der Beek et al., 2011].
Discerning Summary of LOPD Cohorts Found in the Literature
A major issue with given LOPD cohort data sets in the literature is that many patients are repeatedly presented. In order to avoid repetition, we sum up patient numbers according to their countries and centers for rare phenotype details. Data on common phenotype findings seen in LOPD were collected from all large cohorts; however, cohorts reporting less than three cases, historic small case series and case studies without morphological, enzymatic, or genetic confirmation of Pompe disease were excluded here. The results of this summary are presented in Table III.
| Finding | % of LOPD patients | Number of patients in cohort | Sources |
|---|---|---|---|
| Lower limb girdle, truncal, diaphragmatic muscular weakness with CK elevation | ∼80% | >1,000 |
Boerkoel et al. [1995]; Laforet et al. [2000]; Ausems et al. [2001]; Hagemans et al. [2004]; Hagemans et al. [2005]; Winkel et al. [2005]; Kroos et al. [2007]; Müller-Felber et al. [2007]; Joshi et al. [2008]; Van der Beek et al. [2009]; Bembi et al. [2010]; van der Ploeg and Reuser [2008]; Chien et al. [2011]; Roberts et al. [2011]; Hobson-Webb et al. [2011] |
| Respiratory muscular Insufficiency | 79% | 75 |
van der Beek et al. [2011] |
| Diaphragmatic weakness | 38% | 75 |
van der Beek et al. [2011] |
| Scoliosis | 33% | 235 |
Roberts et al. [2011] |
| Classic rigid spine syndrome with low BMI | 4 |
Fadic et al. [1997]; Kostera-Pruszczyk et al. [2006]; Laforet et al. [2010] |
|
| Low body weight (BMI <18) | 15 |
Kobayashi et al. [2010]; Ravaglia et al. [2010]; Strothotte et al. [2010]; Papadimas et al. [2011] |
|
| Cardiac Abnormalities | 7/87 |
Forsha et al. [2011] |
|
| Elevated left ventricular mass index | 4/74 | ||
| Left ventricular ejection fraction <55% | 6/74 | ||
| Left ventricular hypertrophy | 10/74 | ||
| Short PR interval | 8/74 | ||
| Wolff-Parkinson-White Syndrome cardiac arrhythmias | 9 |
Francesconi et al. [1982]; Tabarki et al., [2002]; Müller-Felber et al. [2007]; Soliman et al. [2008]; van der Beek et al. [2008]; Forsha et al. [2011]; van der Beek et al. [2011]; Müller-Felber et al., 2007 |
|
| Vascular abnormalities aortic and cerebral vessels | 33 |
Matsuoka et al. [1988]; Kretzschmar et al. [1990]; Toda et al. [2001]; Anneser et al. [2005]; Laforet et al. [2008]; Refai et al. [2008a]; Refai et al. [2008b]; Sacconi et al. [2010]; El-Gharbawy et al. [2011] |
RESULTS
The Munich Cohort
Our cohort encompasses 44 adult Caucasian LOPD patients, of whom 27 are female (61%) and 17 are male (39%), diagnosed between 1985 and 2011 at our neuromuscular center. Seventeen of our 44 patients have died during the past 15 years. Ten out of the 44 patients had a positive family history with at least one additional affected family member that could be detected. The Munich cohort included two brothers (Table I).
Age at Onset and Time for Diagnosis
The mean age of the patients at time of diagnosis was 42.8 years (range 17–69 years). First symptoms were evident at age 33.3 years (range 9–60 years). Onset of the disease was uncertain in six patients who reported symptoms such as poor performance in school sports. The mean time lag between onset of first symptoms and diagnosis was 9.6 years (range 0.1–31.6 years) (Table I). Seventeen of our 44 patients have died during the past 15 years. Mean age of death was 59 (range 52–78 years). For 12 of the deceased patients, the cause of death was undocumented. Three patients died (death at age 56, 56, and 73 years, respectively) from respiratory causes, specifically respiratory insufficiency and infections that may have been related to LOPD. One patient died of lung cancer at the age of 63 years, and another died due to chronic renal insufficiency at the age of 74 years. None of the patients with a documented cause of death had documented cardiac involvement. Autopsies were not performed on any of our patients.
The Limb Girdle and Diaphragmatic Weakness Pattern
The most common symptom pattern is lower limb girdle and truncal muscular weakness combined with exercise intolerance and CK elevation, which is seen in 78% (34/44) of the patients. This type of weakness is associated with a positive Trendelenburg's sign and Gowers´s sign in 59% (26/44) and 39% (17/44), respectively. Respiratory muscular insufficiency is found in combination with limb girdle weakness in 55% (24/44) of the patients (Table II). Additional involvement encompasses dysphagia, cranial nerve paresis, and facial muscle weakness. Most prominent manifestations here are swallowing difficulties in 16% (7/44) of our patients. Sensorineural hearing impairment was noted in 14% (6/44) (Table II, Fig. 1).
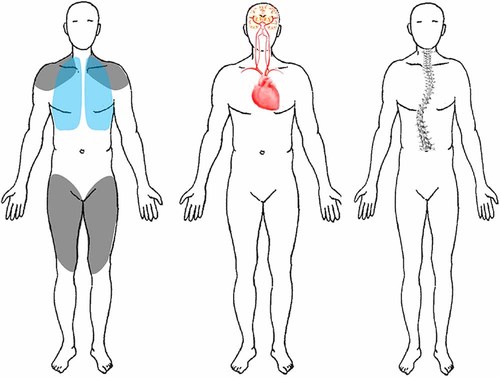
Clinical Manifestation Patterns by organ System Subtypes of Late-Onset-Pompe disease. Left: The Limb girdle and diaphragmatic muscle weakness manifestations. Middle: The Cardio-Cerebrovascular Pattern. Right: The Rigid Spine Syndrome, Scoliosis, and Low Body Weight Pattern. Please note that in all patterns of LOPD a muscular involvement could be found.
The Rigid Spine Syndrome (RSS), Scoliosis and Low Body Weight Pattern
In seven (16%, 7/44) of our patients, a combination of severe scoliosis with lumbar hyperlordosis, incomplete RSS and a low mean body mass index (BMI) was a predominant finding. In all patients, the RSS was combined with respiratory insufficiency requiring more than 16 hr of artificial ventilation daily. Disease-onset ranged from age 12 to 20 years. All patients presented with severe axial neck and trunk weakness, while proximal and distal muscle wasting and weakness of the extremities was mild to moderate. Additional neuromuscular features included overt scapular winging with distal contractures, including of the Achilles tendons, was found in 27% (12/44) and 9% (4/44) (Table II, Fig. 1). None of the patients with low body weight had swallowing difficulties or evidence of gastrointestinal tract manifestations. The low body weight was due to severe wasting of the skeletal muscles. All these patients had the common leaky IVS splice site mutation.
The Cardio-Cerebrovascular Pattern
Besides muscular weakness, a variety of cardiac alterations were noted in our population of late onset patients. Twenty-nine percent (13/44) of our patients showed different types of cardiac arrhythmias, some with more common but unspecific sinus arrhythmias while others presented with more specific arrhythmias including sick sinus syndrome, Lown IVb ventricular arrhythmia, and especially Wolff–Parkinson–White syndrome. Onset of the cardiac involvement was between the ages of 18 and 62 years (mean 38 years). We did not identify any additional conditions or risk factors in this group of patients.
Furthermore, 11% (5/44) presented with right ventricular hypertrophy on cardiac ultrasound. There was no association with pulmonary hypertension, sleep apnea, or other respiratory problems. Vascular abnormalities were found in three (7%, 3/44) patients: (i) A 52-year-old female with an aortic arch elongation with aneurysm of the aortic arch; (ii) a 30-year-old female with a megadolicho basilar artery combined with an aneurysm of the medial cerebral artery; (iii) a 53-year-old female presenting with a transient brachiofacial hemiparesis, and anterior cerebral artery stroke. In these three female patients, no other reason for vascular alterations or risk factors such as hypertension, diabetes, hypercholesterolemia, or coagulopathies were evident (Table II, Fig. 1).
Laboratory Parameters
The mean CK level at the time of diagnosis was elevated in 73% (32/44) (mean 511 U/L; range normal to 2,051 U/L; normal < 180 U/L). A rise in muscle enzymes (AST, ALT) was present in 22 (50%) patients. In six patients, AST and ALT elevation without CK level elevation was found (Table II). No distinct laboratory parameter constellation was bound to any specific pattern of LOPD manifestations.
DISCUSSION
Variability in LOPD and Overview of Common Patterns of Presentations
This report describes clinical findings of the Munich cohort of 44 patients with LOPD who were diagnosed during the past 26 years. There is a growing line of evidence, that structural myofiber modification, lysosomal dysfunction, and autophagic mechanisms modify disease phenotype and course [Fukuda et al., 2006; Raben et al., 2007, 2010a, b; Schoser et al., 2007; DeRuisseau et al., 2009]. This background may explain the variability in the phenotype spectrum, severity, and distribution of the disease. Almost all studies investigating genotype–phenotype correlations in LOPD did not produce reliable correlations [Laforet et al., 2000; Ausems et al., 2001; Hermans et al., 2004; Kroos et al., 2007; Joshi et al., 2008; Kroos et al., 2008]. A comparison of the clinical progression of Chinese late onset Pompe patients with European Pompe patients revealed that none of the Chinese patients had the common leaky splicing c.-32-13T > G GAA gene mutation. However, the more rapid progression in the Chinese patients might be determined by hetero- or homozygous mutations, and by position and type of the GAA gene mutation [Yang et al., 2011]. In cases of very low GAA enzyme activity, there seems to be no common GAA gene mutation and phenotype related, however, none of the patients in this cohort had the common leaky splice site mutation [Bali et al., 2011]. At present, there is no clear understanding of the mechanisms involved in the variable clinical presentation and outcome in the LOPD population.
At present, there is no clear understanding of the mechanisms involved in the variable clinical presentation and outcome in the LOPD population.
The Limb Girdle and Diaphragmatic Weakness Pattern
The most common symptom pattern found in 77% (34/44) of our patients is lower limb girdle and truncal muscular weakness combined with exercise intolerance. Respiratory muscular insufficiency in combination with limb girdle weakness is found in 55% (24/44) of our patients. Respiratory failure can precede the onset of overt muscular weakness. Presently, 36% (16/44) of our patients need non-invasive ventilator assistance at least 12 hr per day, whereas 22% (10/44) are under invasive ventilation 24 hr per day. These data correlate well with given numbers in the literature [Wokke et al., 2008; Byrne et al., 2011; Gungor et al., 2011; van der Beek et al., 2011].
The RSS, Scoliosis, and Low Body Weight Pattern of LOPD
Scoliosis is defined as an abnormal >10° deviation with a rotational deformity curve of the spine. Most cases are referred as idiopathic; however, progressive scoliosis is a frequent spine alteration in neuromuscular diseases related to primary or secondary weakness and atrophy of truncal muscles. Depending on the rotation and deformation of the trunk, scoliosis can severely compromise respiration, which in due course can cause respiratory failure and eventually premature death. In a recently published article with data from the International Pompe Registry, scoliosis was found in 24.8% (87/351) of LOPD patients. Scoliosis was reported more frequently among wheelchair users for all age groups and 44% (48/109) of patients with scoliosis required respiratory support [Roberts et al., 2011]. Scoliosis in LOPD may be related to asymmetrical weakness of paravertebral muscles, rapid growth during adolescence, and the deforming force of gravity on the vertebral column [Roberts et al., 2011]. The underestimated, yet more frequently described severe low body weight presentation in LOPD [Slonim et al., 2006; Slonim et al., 2007; Ravaglia et al., 2010; Papadimas et al., 2011] seems to be more related to a lack of adequate caloric intake. At this time, a systematic look at swallowing difficulties and dysphagia leaves the cause for low body weight unknown.
RSS is considered as a progressive limitation of flexion of the neck and trunk leading to postural anomalies. The neck may be held in hyperextension and the trunk is tilted forward. Body flexion is often possible only at the hips. Onset of RSS commonly occurs during childhood. Association with several distinct rare degenerative myopathies have been reported during the past years [Fadic et al., 1997; Kostera-Pruszczyk et al., 2006; Müller-Felber et al., 2007; Laforet et al., 2010]. As truncal muscles are also frequently involved in LOPD, especially in relation to scoliosis, we suggest RSS is under-recognized in LOPD. All of our patients had the common IVS splice site mutation on one allele, thus no evidence of any clear-cut genotypically differences could be found.
The Cardio-Cerebrovascular Pattern of LOPD
Most reported symptoms involve changes in aortic and cerebral vessels leading to stiffness and dilatation of ascending thoracic aorta or strokes [Nemes et al., 2007; El-Gharbawy et al., 2011]. There is a reported increase in incidence of vascular smooth muscle changes in patients with LOPD in contrast to normal population (2.7% vs. 1.7%) [Winkel et al., 2005]. An increasing number of reports describe central nervous system (CNS) symptoms like unilateral ptosis, migraine, and diplopia [Kamphoven et al., 2004; Anneser et al., 2005; Laforet et al., 2008; Refai et al., 2008b; Sacconi et al., 2010; Yanovitch et al., 2010]. Although the absolute number of articles concerning CNS symptoms and the number of herein described patients is low, these new clinical details are of important interest. The well-outlined LOPD phenotype should be complemented by a new cardio-cerebrovascular pattern. The cardio-cerebrovascular pattern is caused by changes in the smooth muscle cells by accumulating glycogen which leads to aortic stiffness, dilatation of thoracic aortic, and dolichoectasias in cerebral arteries, including dolichoectasia of basilar artery and ectasia of internal carotids [Miyamoto et al., 1985; Braunsdorf, 1987; Matsuoka et al., 1988; Kretzschmar et al., 1990; Toda et al., 2001; Anneser et al., 2005; Laforet et al., 2008; Refai et al., 2008a, b; Sacconi et al., 2010; El-Gharbawy et al., 2011]. Acid alpha-glucosidase normally clears glycogen from any type of smooth muscle cells, nevertheless, structural long-term changes of the vasculature before introduction of interventions such as enzyme replacement therapy may hamper reversibility in adults [Kretzschmar et al., 1990; Anneser et al., 2005; Schoser et al., 2007; El-Gharbawy et al., 2011].
IMPLICATIONS FOR MANAGEMENT
LOPD is a multisystem disorder that requires multidisciplinary care, including genetic counseling for affected patients and their families. This article emphasizes that beyond the well-recognized muscular and respiratory symptoms, there exist other manifestation pattern; thus, patients who may have been followed as idiopathic scoliosis, RSS, or cardio-cerebrovascular disease of unknown cause as primary or part of the presentation should be evaluated for LOPD given the availability of rapid blood based tests (GAA assays and molecular analysis).
This article emphasizes that beyond the well-recognized muscular and respiratory symptoms, there exist other manifestation pattern; thus, patients who may have been followed as idiopathic scoliosis, RSS or cardio-cerebrovascular disease of unknown cause as primary or part of the presentation should be evaluated for LOPD given the availability of rapid blood based tests (GAA assays and molecular analysis).
Furthermore, patients with LOPD should be carefully monitored for some of these features that are being increasingly recognized. Skeletal evaluation is essential because of the various degrees of scoliosis, hyperlordosis, and rigid spine. For curves less than 15–20°, spinal radiographs should be done annually or with a significant change in curve. For more severe curves (>20°), spinal radiographs should be obtained every 6 months. Less frequent radiographs increase the risk of missing progression of the scoliosis [Bushby et al., 2010]. A curve length >8 vertebrae, a non-S-form curve, and a lower-end vertebrae located at L4 can be indicative of neuromuscular scoliosis and may help identify patients for further radiological investigations [Abul-Kasim and Ohlin, 2010; Roberts et al., 2011].
As in other neuromuscular conditions such as congenital myopathies, muscular dystrophies, and amyotrophic lateral sclerosis, nutritional monitoring is vital. For instance, weight loss is a common independent prognostic factor in amyotrophic lateral sclerosis [Chiò et al., 2009]. A low-carbohydrate intake may reduce both intra- and extralysosomal glycogen accumulation, whereas protein and alanine supplementation increase muscle protein synthesis and reduce muscle degradation [Slonim et al., 2007]. A fair number of small studies could provide some evidence that a high-protein diet produced clinical improvement in LOPD. Up to 25% of patients showed improvement in muscle or respiratory function on a high-protein diet [Isaacs et al., 1986; Margolis and Hill, 1986; Mobarhan et al., 1990; Bodamer et al., 1997].
Given the known smooth muscle involvement and the potential risk, cardiovascular work-up should encompass evaluation of the heart and large vessels via imaging modalities such as ultrasound. In selected cases, an MRI of the brain with MR angiography of the brain and/or aorta is needed. An abdominal ultrasound may also be warranted given the frequency of uncertain liver and spleen anomalies seen in the Munich cohort. Further study is needed in this area as the irregularities may be related to other factors than Pompe disease (e.g., alcohol, nutrition). The interfamilial variability of LOPD appears significant and warrants molecular testing of families for more accurate counseling. The results of this study indicate the complexity of multisystem involvement in LOPD and the continuum of emerging phenotypes that prompt more specific investigations to further delineate the frequency of these evolving phenotypes.
CONCLUSIONS
Our data mirror the wide phenotypic spectrum of LOPD. In an attempt to deconstruct the common LOPD phenotype, we found more subtle clinical features that tend to segregate together in patients with LOPD. This evidence of multisystem involvement supports former reports of autoptic LOPD cases. Our findings help further delineate the complexity of multisystem involvement in LOPD, thereby enhancing clinicians' awareness of these subtle symptom patterns that may otherwise not be recognized. Whether patients presenting with the different symptom patterns respond differently to enzyme replacement therapy remains a key question for future research.
Acknowledgements
We thank all individuals, their families, and our medical colleagues for supporting our research. A special recognition and thanks is extended to our technicians Mrs. Birgit Konkol and Juliane Zaiska, who continuously take care of all the Pompe patients receiving enzyme replacement therapy in our institute.




