Cloacal exstrophy: An epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research†‡§
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Presented at the 37th meeting of the International Clearinghouse Meeting November 1, 2010, Buenos Aires, Argentina and the 51st Teratology Society Meeting, San Diego, California, June 28, 2011.
How to Cite this Article: Feldkamp ML, Botto LD, Amar E, Bakker MK, Bermejo-Sánchez E, Bianca S, Canfield MA, Castilla EE, Clementi M, Csaky-Szunyogh M, Leoncini E, Li Z, Lowry RB, Mastroiacovo P, Merlob P, Morgan M, Mutchinick OM, Rissmann A, Ritvanen A, Siffel C, Carey JC. 2011. Cloacal exstrophy: An epidemiologic study from the International Clearinghouse of Birth Defects Surveillance and Research. Am J Med Genet Part C Semin Med Genet 157: 333–343.
Abstract
Cloacal exstrophy presents as a complex abdominal wall defect thought to result from a mesodermal abnormality. Anatomically, its main components are Omphalocele, bladder Exstrophy and Imperforate anus. Other associated malformations include renal malformations and Spine defects (OEIS complex). Historically, the prevalence ranges from 1 in 200,000 to 400,000 births, with higher rates in females. Cloacal exstrophy is likely etiologically heterogeneous as suggested by its recurrence in families and occurrence in monozygotic twins. The defect has been described in infants with limb-body wall, with trisomy 18, and in one pregnancy exposed to Dilantin and diazepam. Due to its rarity, the use of a nonspecific diagnostic code for case identification, and lack of validation of the clinical findings, cloacal exstrophy remains an epidemiologic challenge. The purpose of this study was to describe the prevalence, associated anomalies and maternal characteristics among infants born with cloacal exstrophy. We used data from the International Clearinghouse for Birth Defects Surveillance and Research submitted from 18 birth defect surveillance programs representing 24 countries. Cases were clinically evaluated locally and reviewed centrally by two authors. Cases of persistent cloaca were excluded. A total of 186 cases of cloacal exstrophy were identified. Overall prevalence was 1 in 131,579 births: ranging from 1 in 44,444 births in Wales to 1 in 269,464 births in South America. Live birth prevalence was 1 in 184,195 births. Prevalence ratios did not vary by maternal age. Forty-two (22.6%) cases met the criteria for the OEIS complex, whereas 60 (32.3%) were classified as OEI and 18 (9.7%) as EIS (one with suspected VATER (0.5%)). Other findings included two cases with trisomy 13 (one without a karyotype confirmation), one with mosaic trisomy 12 (0.5%), one with mosaic 45,X (0.5%) and one classified as having amnion band sequence (0.5%). Twenty-seven (14.5%) infants had other anomalies unrelated to cloacal exstrophy. Cloacal exstrophy is a rare anomaly with variability in prevalence by geographic location. The proportion of cases classified as OEIS complex was lower in this study than previously reported. Among all cases, 54.8% were reported to have an omphalocele. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Cloacal exstrophy is one of the rarest and most complex abdominal wall defects occurring in humans. Historically, the prevalence for cloacal exstrophy ranges between 1 in 200,000 to 400,000 births [Soper and Kilger, 1964; Tank and Lindenauer, 1970; Hurwitz et al., 1987; Martínez-Frías et al., 2001]. Recently, a higher live birth prevalence (1 in 158,730 births) was reported for New York State [Caton et al., 2007]. The clinical presentation of cloacal exstrophy is unique compared with that of other abdominal wall defects (i.e., omphalocele, gastroschisis, bladder exstrophy), and its etiology and pathogenesis remain poorly understood. Anatomically, the cardinal findings of cloacal exstrophy include exstrophy of the hemibladders with hindgut extrusion and imperforate anus. The hemibladders flank the openings of the small intestine and blind-ending large intestine and contain the orifices of the ureters and vasa deferentia in males and the uterovaginal canal in females (Fig. 1) [Van der Putte et al., 2008]. Two recent studies both showed a frequency of 64% of infants with omphalocele [Keppler-Noreuil, 2001; Martínez-Frías et al., 2001]. This complex condition has been referred to by many names or acronyms over the last several decades [Soper and Kilger, 1964; Spencer, 1965; Magnus, 1969] with the most recent by Carey et al. [1978] who proposed the term OEIS complex (Omphalocele, bladder Exstrophy, Imperforate anus, and Spinal defects) to simplify the common components observed in infants with cloacal exstrophy. Other malformations reported to occur commonly with cloacal exstrophy include renal malformations [Keppler-Noreuil, 2001; Martínez-Frías et al., 2001], single umbilical artery [Hartwig et al., 1991; Martínez-Frías et al., 2001; Bohring, 2002; Van der Putte et al., 2008], and limb defects [Evans and Chudley, 1999; Keppler-Noreuil, 2001; Jain and Weaver, 2004], while esophageal atresia with tracheoesophageal fistula [Bohring, 2002], duodenal atresia [McHoney et al., 2001], and Chiari I malformation [Tubbs et al., 2003] occur occasionally.
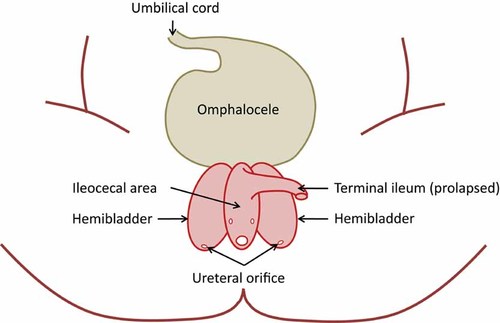
Clinical presentation of cloacal exstrophy in a newborn. Adapted from Nyberg et al. (ed.) [2002]. Diagnostic imaging of fetal anomalies, 2nd edition. Lippincott Williams & Wilkins. Illustration drawn by Sergey Krikov, MS.
Cloacal exstrophy remains an epidemiologic challenge due to its rarity, to its designation by a nonspecific code in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and to inadequate validation of its component clinical findings. Using data from the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR), we aimed to describe the overall and maternal age-specific prevalence, the associated malformations, and the maternal and infant characteristics among babies diagnosed with cloacal exstrophy.
LITERATURE REVIEW
Historical Aspects
Cloacal exstrophy was first described by Littre in 1709 [Lund and Hendren, 2001]. While some consider cloacal exstrophy to have a different embryologic origin from bladder exstrophy [Carey et al., 1978; Mildenberger et al., 1988], others consider it the most severe end of a spectrum of malformations referred to as bladder exstrophy-epispadias complex (BEEC) or exstrophy-epispadias complex (EEC)
While some consider cloacal exstrophy to have a different embryologic origin from bladder exstrophy, others consider it the most severe end of a spectrum of malformations referred to as bladder exstrophy-epispadias complex (BEEC) or exstrophy-epispadias complex (EEC).
[Beaudoin et al., 1997; Martínez-Frías et al., 2001; Vauthay et al., 2007; Gambhir et al., 2008; Ebert et al., 2009]. The hypothesis supporting the (B)EEC complex is based on the observation that the specific malformations seen may be related to the timing of rupture of the cloacal membrane during gestation. However, whether cloacal exstrophy has a different pathogenetic mechanism from bladder exstrophy remains an unresolved question. Recent data from both animal and human studies suggest that the cloacal membrane is not involved in the pathogenesis [Langer et al., 1992; Bruch et al., 1996; Nievelstein et al., 1998; Manner and Kluth, 2005] bringing that notion (i.e., cloacal membrane rupture as the mechanism) into question.
In addition to the question of the BEEC spectrum, other investigators have posited that cloacal exstrophy is part of a different continuum. Heyroth-Griffis et al. [2007] suggest that cloacal exstrophy is on a continuum with limb-body wall and urorectal septum malformation sequence (aka, persistent cloaca). The investigators speculate that there is a common etiology or pathogenetic mechanism that interferes with sequential development of the thoraco-abdominal and pelvic regions. Whether there is a continuum, as suggested, remains controversial. This question will not be easily answered and will require both embryologic and histologic data from both animals and humans.
Due to its rarity and constraints in case definition, cloacal exstrophy poses epidemiologic challenges. Because the prevalence of this congenital anomaly is so rare, it is difficult to ascertain a large enough case group from any one population-based congenital anomaly surveillance program for effective study, and therefore requires case ascertainment from multiple surveillance programs in order to have a reasonable case group to evaluate. The second challenge involves compiling a case series that is homogeneous and reflects true cloacal exstrophy malformations and not overlapping conditions, such as persistent cloaca. Creating a clinically well-defined case group from multiple sources becomes difficult without the ability to review photographs or perform a physical exam on each infant, and review the surgical or autopsy reports to make certain that each case truly represents exstrophy of the cloaca.
EMBRYOLOGY
Normal Development
The human embryo transitions during the third post-conception week from a bilaminar (epiblast, hypoblast) to trilaminar (ectoderm, mesoderm, endoderm) disc and the neural tube begins to fold [Vermeij-Keers et al., 1996; Sadler, 2006]. In the trilaminar stage, the embryo is a relatively flat disc and the cloacal membrane lies cephalic to the primordial abdominal wall and caudal to the abdominal wall which is the transition zone that will become the future umbilical ring [Hartwig et al., 1991; Vermeij-Keers et al., 1996]. The umbilical ring represents the transition from amnion to skin, or surface ectoderm [Hartwig et al., 1991]. Ectodermal cell deposition into the mesodermal compartment of the umbilical ring is critical for formation of the ventral body wall and closure of the abdominal cavity [Hartwig et al., 1991]. Also during this third week, epiblastic cells invaginate the dorsal primitive streak, migrate laterally and caudally, and contribute to the mesoderm and endoderm of the embryo [Sadler, 2006]. As rapid growth occurs during the fourth post-conception week the embryo begins to curve cephalocaudal [Moore and Persaud, 2003] resulting in the primordial structures of the lower abdominal wall, rectum, anus, urogenital sinus and caudal end of the neural tube, all closely related spatially [Hartwig et al., 1991]. The cloacal membrane, located in the curved anterior caudal end of the embryo, consists of two layers of tissue, ectoderm and endoderm, which contributes to its eventual demise. At the end of the first month, the cloaca, urogenital sinus, and primitive anorectum are present without septation [Paidas et al., 1999; Moore and Persaud, 2003]. The proximal vitelline/yolk stalk and allantois are incorporated into the body cavity and their extraembryonic mesoderm fuse to form the urorectal septum (Fig. 2) [Vermeij-Keers et al., 1996; Nievelstein et al., 1998; Paidas et al., 1999]. At approximately 29 days post-conception the mesodermally derived urorectal septum begins its growth caudally as the embryo increases in size, passively separating the primordial urogenital sinus from the anorectum [Paidas et al., 1999]. During the fifth week, the urorectal septum continues its growth caudally and during the sixth week, the four ventral folds (cephalic, two lateral, and caudal) meet in the midline to close the abdominal wall, the apex of which is the umbilical ring [Duhamel, 1963]. By the end of the seventh week (Fig. 3), the urorectal septum completes its migration in front of the hindgut, toward the cloacal membrane [Sadler, 2006]. As the embryo grows and unfolds, the distance between the urorectal septum and the cloacal membrane decreases [Nievelstein et al., 1998; Sadler, 2006]. Based on the examination of human embryos, the urorectal septum comes in close proximity but does not fuse with the cloacal membrane [Nievelstein et al., 1998]. The cloacal membrane disintegrates by apoptotic cell death by 49 days post-conception exposing two openings, the urogenital groove and the anal orifice [Nievelstein et al., 1998; Sadler, 2006]. The tip of the urorectal septum becomes the perineal body (Fig. 3) [Nievelstein et al., 1998; Sadler, 2006]. Though progress has been made in our understanding of normal caudal development in humans, the detailed mechanisms, cell–cell signaling and the genes involved still remain largely unknown [Paidas et al., 1999].
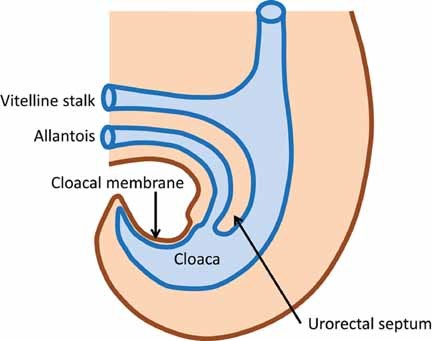
Sagittal view of the cloaca and its surrounding structures in an approximately 28 day post-conception embryo. Illustration drawn by Jeri Fowles, RN.
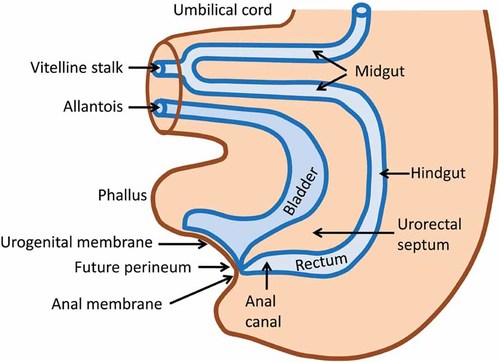
Sagittal view of the normal structures of the lower abdomen in a 7 week (post-conception) embryo prior to rupture of the cloacal membrane. Illustration drawn by Jeri Fowles, RN.
Abnormal Development-Pathogenesis of Cloacal Exstrophy
- (1)
Failure of mesodermal tissue to migrate to the lateral folds of the infraumbilical abdominal wall and rupture of the enlarged cloacal membrane before complete descent of the urorectal septum [Marshall and Muecke, 1962; Gray and Skandalakis, 1972];
- (2)
Failure of formation of the caudal fold due to failure of the splanchnic and somatic layers to form resulting in absence of the hypogastric abdominal wall in front of the allantois [Duhamel, 1963];
- (3)
Insufficient cell deposition at the body wall placode impairs normal body wall development at the site of the umbilical ring and between the umbilical ring and the cloacal membrane, hindering normal displacement of the cloacal membrane to its final position, normal septation of the cloaca, and normal external genitalia development [Hartwig et al., 1991];
- (4)
The body wall between the umbilical ring and the cloacal membrane does not develop which causes the umbilical ring to border the cloacal membrane [Vermeij-Keers et al., 1996]; and
- (5)
Malfunctioning of the umbilical ectodermal placode and primitive streak/caudal eminence [Van der Putte et al., 2008].
Based on histopathologic studies in human embryos, cloacal exstrophy is most likely the result of a very early defect involving the caudal eminence [Nievelstein et al., 1998; Van der Putte et al., 2008] as opposed to an abnormality related to premature rupture of the cloacal membrane.
Based on histopathologic studies in human embryos, cloacal exstrophy is most likely the result of a very early defect involving the caudal eminence as opposed to an abnormality related to premature rupture of the cloacal membrane.
Findings from prenatally diagnosed cases of cloacal exstrophy with an intact cloacal membrane which ruptures later in pregnancy suggest that the membrane is unrelated to its pathogenesis [Langer et al., 1992; Bruch et al., 1996]. Covered variants of cloacal exstrophies have also been reported in humans which supports this reasoning [Sahoo et al., 1997; Borwankar et al., 1998]. Since the urorectal septum does not fuse with the cloacal membrane, even premature rupture of the membrane would not be responsible for imperforate anus in cloacal exstrophy. Early rupture of the cloacal membrane as the mechanism for cloacal exstrophy is also not supported by recent studies in chickens [Manner and Kluth, 2005]. Additional evidence that points to cloacal exstrophy occurring very early during organogenesis is that the omphalocele in infants with cloacal exstrophy is caudally displaced [Carey et al., 1978]. Caudal displacement of the body stalk may result from reduced cell deposition at the primordial abdominal wall during the trilaminar state.
GENETICS
In humans, little is known about the genes involved in caudal development or those that might be involved in abnormal development leading to cloacal exstrophy. The etiology of cloacal exstrophy is thought to be heterogeneous as suggested by reports of recurrence in families [Smith et al., 1992; Keppler-Noreuil, 2001]; increased occurrence among conjoined [Goldfischer et al., 1997; Casale et al., 2004; Tihtonen et al., 2009] and monozygotic twins [Redman et al., 1981; McLaughlin et al., 1984; Lee et al., 1999; Siebert et al., 2005]; concordant conjoined twins [Métneki and Czeizel, 1989]; discordant dizyogtic twins (one with omphalocele and the other with cloacal exstrophy) [Bruch et al., 1996]; and documented genetic abnormalities among affected infants including trisomy 18 [Carey et al., 1978], 9q34.1-qter deletion [Thauvin-Robinet et al., 2004], del(3)(q2.2q13.2) [Kosaki, 2005], a 1p36 deletion [El-Hattab et al., 2010], and a mitochondrial 12SrRNA mutation [Nye et al., 2000]. Discordant dizygotic twins may provide evidence of an environmental influence, but the MZ twin data and the familial cases support some genetic basis of cloacal exstrophy. Noteworthy is the lack of occurrence of cloacal exstrophy, neither occasionally nor frequently, as a component of known syndromes [Jones, 2006].
EPIDEMIOLOGY
Two recent studies (one hospital- and one population-based) provide data on the prevalence of cloacal exstrophy, both with slightly higher frequencies than that generally reported in the literature (1 in 200,000 to 400,000 births) (Table I). This disparity in prevalence from earlier reports supports the need for comprehensive population-based congenital anomaly surveillance programs to monitor all pregnancy outcomes when evaluating prevalence. In these two studies, similar patterns are observed for sex ratios with females affected more often than males. Based on data from the Spanish hospital-based surveillance program, Martínez-Frías et al. [2001] reported a mean maternal age of 27.09 and paternal age 29.91 with twins occurring in 36.4% of the cases. Gambhir et al. [2008] reported a similar average maternal age (27.9 years) but paternal age was slightly higher (31.4 years) in a convenience sample from five urology clinics in Europe. Neither study used a non-malformed comparison group to assess whether the mean maternal or paternal age varied from the underlying population.
| Author, year of publication | Study period | Number of cases | Prevalencea | 1 in births | Male:female ratio | |
|---|---|---|---|---|---|---|
|
Martínez-Frías et al. [2001 ] |
1976–1999 | 11 | Totalb | 0.68 | 146,534 | 0.5:1 |
| 8 | Live birth | 0.50 | 200,233 | |||
|
Caton et al. [2007 ] |
1983–1999 | 29 | Live birth | 0.63 | 158,730 | 0.4:1 |
- a Prevalence per 105.
- b Prevalence among live births and stillbirths.
Studies investigating environmental risk factors for cloacal exstrophy are scarce. Among live born infants in New York State, Caton et al. [2007] reported multiple births and residence outside of New York City as factors statistically significantly associated with an increased risk for cloacal exstrophy. Other associated factors that did not reach statistical significance in that study included Hispanic ethnicity, maternal education less than 12 years, conception during the spring, younger (less than 20 years of age) and older (35 or older) maternal age, and having had three or more previous pregnancies. No increasing trends in prevalence were observed during the 17-year study period. A recent investigation by Reefhuis et al. [2011] using data from the population-based case-control National Birth Defects Prevention Study reported a five-fold increase in the risk for cloacal exstrophy with maternal use of clomiphene citrate between two months before conception through the first month of pregnancy. Whereas, in a prospective study of 67 women who conceived while taking clomiphene citrate none had a pregnancy affected by cloacal exstrophy [Bánhidy et al., 2008]. Case reports or case series have suggested an association of clocal exstrophy with in-vitro fertilization (IVF) [Wood et al., 2003; Gambhir et al., 2008], smoking [Gambhir et al., 2008] and use of the medication, diazepam [Lizcano-Gil et al., 1995].
PROGNOSIS, TREATMENT, SURVIVAL
Until 1960 when the first surgery was performed in a baby with cloacal exstrophy [Rickman, 1960], infant mortality was 100% [Soffer et al., 2000]. With advances in neonatal intensive care units, corrective surgery, nutrition, and antibiotics, survival increased to 90% by the 1980s [Zderic et al., 2002]. This dramatic improvement in survival has led to a paradigm shift in treating the patient and family and improvement in the quality of life [Marvin, 2007]. Co-morbidities as a result of cloacal exstrophy include issues related to urinary [Hendren, 1998; Boldec et al., 2002] and bowel function [Hendren, 1998; Shimotake et al., 2006; Sawaya et al., 2010] and, perhaps most challenging, the assignment of gender [Diamond et al., 2006; Mukherjee et al., 2007].
METHODS
The ICBDSR collects data from 46 member birth defect surveillance programs worldwide, representing 37 countries [ICBDSR Annual Report, 2009]. Seven countries have two or more participating birth defect surveillance programs, and one covers ten countries. Each surveillance program submits data annually in a specified format to the ICBDSR for compilation of the annual report which includes detailed descriptions of each member surveillance program [ICBDSR Annual Report, 2009].
For this study, we used both population- and hospital-based data from 18 surveillance programs, representing 24 countries. Surveillance programs included all cases of cloacal exstrophy that were live born (LB), stillborn (SB) and, when collected, those that resulted in elective terminations of pregnancy for a fetal anomaly (ETOPFA). Prior to submission of data to the ICBDSR, each surveillance program's case information was reviewed locally by a clinician involved in the birth defect surveillance program [Castilla and Mastroiacovo, 2011]. Following data submission, each surveillance program's case information was reviewed by a dysmorphologist (PM) at the ICBDSR to determine if the case met eligibility criteria for cloacal exstrophy. Next, the surveillance programs were asked to provide any additional clinical information (e.g., autopsy, surgical report, physical examination) to confirm their cases' status for this study. Based on this additional clinical information from each surveillance program, we used the following criteria for inclusion: presence of clearly defined cloacal exstrophy with imperforate anus, with or without omphalocele or spina bifida. This allowed two of the study's authors (PM and MLF) to exclude 70 cases that were either confirmed to be, or clinically suggestive of persistent cloaca, rectovaginal fistula, isolated bladder exstrophy, or limb body wall complex. Persistent cloaca was defined as a cloacal malformation with imperforate anus that did not include exstrophy. Particular attention was paid to cases submitted as bladder exstrophy to determine if the clinical descriptions were more likely to represent cloacal exstrophy (i.e., bladder exstrophy with imperforate anus) [Siffel et al., 2011]. Finally, using strict criteria, cloacal exstrophy cases were classified by the presence of omphalocele with spina bifida (OEIS), only omphalocele (OEI), only spina bifida (EIS), or cloacal exstrophy alone. Those infants with other spinal abnormalities (e.g., spina bifida occulta, vertebral anomalies) were not considered to meet the criteria for the OEIS complex or EIS spectrum and were included in the study as cloacal exstrophy alone.
The total prevalence estimates of cloacal extrophy were computed for each program (LB + SB + ETOPFA cases/LB + SB births) with its 95% confidence interval (CI) according to the Poisson distribution. Comparison among programs of the total prevalence estimates of cloacal exstrophy was evaluated computing the expected number of cases in each program under the hypothesis of homogeneity among all programs and the exact Poisson probability of observing N or more cases [p(N ≥ x)] in each program. Statistical significance was set to P < 0.05 with Bonferroni correction for multiple testing (α/18 = 0.028, where 18 is the number of programs). Marginally statistically significant differences with P < 0.05 without Bonferroni correction were also noted.
Prevalence ratios for maternal age groups relative to the reference age group of 25–29 years with corresponding 95% CI were also calculated.
RESULTS
There were 186 cases of eligible cloacal exstrophy registered from the 18 participating surveillance programs out of a total of 24,497,955 births (Table II). Among all participating surveillance programs, the overall total prevalence of cloacal exstrophy was 1 in 131,579 births (0.76 per 100,000 births), with the lowest reported total prevalence from South America Estudio Colaborativo Latino Americano de Malformaciones Congénitas (ECLAMC) program (1 in 270,270 births; 0.37 per 100,000 births; CI 0.22–0.60; P = 0.0007) and the highest from Wales (1 in 44,444 births; 2.25 per 100,000 births; CI 0.73–5.25; P = 0.029) (Fig. 4). A marginally statistical significant total prevalence was lower in Hungary (1 in 215,271; 0.46 per 100,000 births, CI 0.25–0.78; P = 0.032) and higher in Canada Alberta (1 in 66,405 births; 1.51 per 100,000 births; P = 0.0088). The majority of cases were live born (n = 133; 71.5%) with the remainder resulting in either ETOPFA (n = 24; 12.9%) or stillbirth (n = 29; 15.6%). The overall total prevalence among live births only (n = 133) was 1 in 184,195 births (0.54 per 100,000 live births). Excluding ETOPFA (n = 24 cases) the overall prevalence of cloacal exstrophy was reduced by 12.9%.
| Surveillance programa | Period | Births | Total cases | % of ETOPFA on total cases | Total prevalence (per 100,000 births) | 95% CI |
|---|---|---|---|---|---|---|
| Canada Alberta | 1980–2005 | 1,062,483 | 16 | 12.5 | 1.51 | 0.86–2.45 |
| USA Utah | 1997–2004 | 380,706 | 3 | 33.3 | 0.79 | 0.16–2.30 |
| USA Atlanta | 1968–2004 | 1,283,999 | 8 | 0 | 0.62 | 0.27–1.23 |
| USA Texas | 1996–2002 | 2,054,788 | 24 | 4.2 | 1.17 | 0.75–1.74 |
| Mexico RYVEMCE | 1978–2005 | 1,058,885 | 10 | NP | 0.94 | 0.45–1.74 |
| South America ECLAMC | 1982–2006 | 4,556,173 | 17 | NP | 0.37 | 0.22–0.60 |
| Finland | 1993–2004 | 713,494 | 8 | 25.0 | 1.12 | 0.48–2.21 |
| Wales | 1998–2004 | 222,309 | 5 | 20.0 | 2.25 | 0.73–5.25 |
| Northern Netherlands | 1981–2003 | 369,658 | 4 | 0 | 1.08 | 0.29–2.77 |
| Germany Saxony Anhalt | 1980–2004 | 355,184 | 3 | 66.7 | 0.84 | 0.17–2.47 |
| Hungary | 1980–2005 | 3,022,194 | 14 | 0 | 0.46 | 0.25–0.78 |
| France Central East | 1979–2004 | 2,500,214 | 15 | 40.0 | 0.60 | 0.34–0.99 |
| Italy North East | 1981–2004 | 1,186,497 | 13 | 38.5 | 1.10 | 0.58–1.87 |
| Italy Sicily | 1991–2002 | 216,257 | 2 | 0 | 0.92 | 0.11–3.34 |
| Spain ECEMC | 1980–2004 | 2,045,751 | 17 | NR | 0.83 | 0.48–1.33 |
| Israel | 1975–2005 | 151,562 | 3 | 33.3 | 1.98 | 0.41–5.78 |
| China Beijing | 1992–2005 | 1,927,622 | 12 | NR | 0.62 | 0.32–1.09 |
| Australia Victoria | 1983–2004 | 1,390,179 | 12 | 25.0 | 0.86 | 0.45–1.51 |
| Total | 24,497,955 | 186 | 12.9b | 0.76 | 0.65–0.88 |
- ETOPFA, elective termination of pregnancy for fetal anomaly; CI, confidence interval; NP, not permitted; NR, not reported; RYVEMCE, Registro Y Vigilancia Epidemiológica de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino Americano de Malformaciones Congénitas; ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas.
- a Surveillance programs are ordered by geography North-South and West-East.
- b The percentage of ETOPFA computed on the 14 surveillance programs registering ETOPFA is 18.5% (n = 24/130).
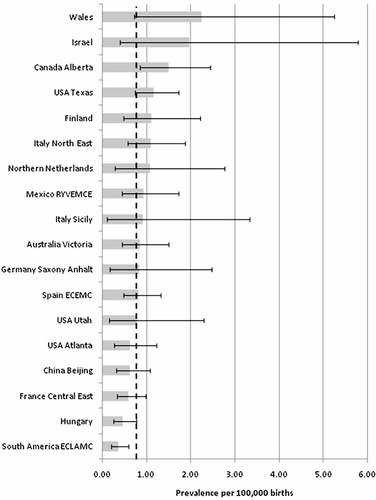
Total prevalence per 100,000 births (bar) and 95% confidence interval (line) by surveillance program and overall (dotted line) of cloacal exstrophy in 18 surveillance programs, members of the International Clearinghouse for Birth Defects Surveillance and Research.
The average age for case mothers was 26.6 years (SD 5.6, median 26.0, range 14–44) and for fathers was 28.8 years (SD 5.7, median 29, range 18–45). However, it must be noted that age was missing for 7.0% (n = 13) of mothers and 44% (n = 82) of fathers. The prevalence ratio for 5-year maternal age groups relative to the reference group of 25–29 years did not demonstrate an association of cloacal exstrophy with maternal age (Fig. 5).
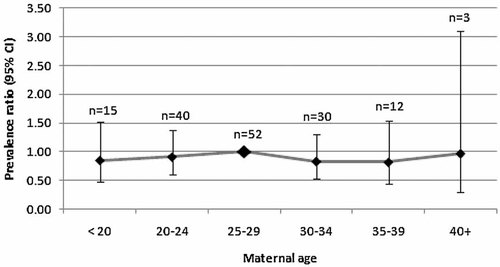
Prevalence ratios for maternal age groups relative to the reference age group of 25–29 years with corresponding 95% confidence interval (CI) for cloacal exstrophy.
Among live born infants, 52 (39.1%) were ≥37 weeks gestation at birth, 68 (51.1%) were less than 37 weeks gestation, and for 13 (9.8%) the length of gestation was not known. Mean birth weight among live born infants was 2,383 g (1 SD 600.49 g, range 1,035–3,720 g). For those infants with known sex (submitted by the surveillance program or confirmed by karyotype), females (n = 74; 53.2%) were more frequent than males (n = 65; 46.8%) resulting in a male:female sex ratio of 1:1.14; however, for 25% of the infants, sex was not determined but listed as either unknown (n = 12; 6.5%) or indeterminate (n = 35; 18.8%). Among the 170 cases of cloacal exstrophy with known plurality, 152 (89.4%) were singleton births, 17 (10.0%) were part of a twin pregnancy, and one (0.6%) was part of a triplet pregnancy. Among the cases identified as twins, three were known to be dizygotic (opposite-sex) and one was part of a same-sex twin pair. The same-sex twins were reported to be discordant for cloacal exstrophy but both infants had an omphalocele with intestinal atresia and double cervix.
There were 42 (22.6%) cases of cloacal exstrophy with the full OEIS complex, 60 (32.3%) with OEI, and 18 (9.7%) with EIS. Omphalocele was reported in 102 (85%) of these 120 cases and in 186 (54.8%) of all cases of cloacal exstrophy. Four infants with cloacal exstrophy had chromosomal anomalies: two with trisomy 13 (one without karyotype confirmation) and one each with mosaic trisomy 12 and mosaic 45,X. One infant had a balanced translocation, 46,XX,t(14:22)(q32:q11.2). One infant classified as EIS was suspected of having the VATER association (vertebral anomalies, anal atresia, tracheoesophageal fistula, esophageal atresia, and renal anomalies). One infant classified as OEI was listed as having amnion band sequence.
Twenty-seven (14.5%) infants had other congenital anomalies that were not considered part of the constellation of findings associated with cloacal exstrophy. Table III lists these unrelated anomalies, stratified by the OEIS, OEI, EIS, and cloacal exstrophy only groups.
| OEIS (n = 8) | OEI (n = 7) | EIS (n = 4) | CE alone (n = 8) |
|---|---|---|---|
| Rib anomaly | Absent ribs | VSD | ASD, VSD |
| Stenosis of the bronchial root | Amniotic bands | Thoracic cage anomaly | VSD |
| Diaphragmatic hernia | Hydrocephaly | CHD NOS | ASD, VSD |
| VSD | Ectrodactyly | CHD, encephalocele | Absent ribs |
| VSD, absent ribs | Arthrogryposis, VSD | Anencephaly | |
| Abnormal ears, absent ribs, finger anomaly | Microcephaly | Encephalocele | |
| Pulmonary stenovsis, PDA | ASD, VSD | TEF/EA | |
| Absent ribs, rocker bottom feet | ASD, VSD, BAV, redundant nuchal fold |
- OEIS, omphalocele, bladder exstrophy, imperforate anus, spina bifida; OEI, omphalocele, bladder exstrophy, imperforate anus; EIS, bladder exstrophy, imperforate anus, spina bifida; CE alone, bladder exstrophy, imperforate anus; ASD, atrial septal defect; BAV, bicuspid aortic valve; CHD NOS, congenital heart defect, not otherwise specified; PDA, patent ductus arteriosus; VSD, ventricular septal defect; TEF, tracheoesophageal fistula; EA, esophageal atresia.
DISCUSSION
Cloacal exstrophy is a very rare congenital anomaly with some variability in prevalence by geographic location, which may be the result of random fluctuation with small numbers. We report an overall prevalence of 1 in 131,579 births (0.76 per 100,000 births). This prevalence is slightly higher than previous estimates, which may reflect the inclusion of both stillbirths and ETOPFA. Considering only live births, the prevalence was 1 in 184,195 births (0.54 per 100,000 births) similar to previous estimates
We report an overall prevalence of 1 in 131,579 births (0.76 per 100,000 births). This prevalence is slightly higher than previous estimates, which may reflect the inclusion of both stillbirths and ETOPFA. Considering only live births, the prevalence was 1 in 184,195 births (0.54 per 100,000 births) similar to previous estimates.
[Martínez-Frías et al., 2001; Caton et al., 2007]. An advantage of this study is the inclusion of cloacal exstrophy cases that were either stillborn or resulted in ETOPFA; excluding the ETOPFA cases, we observed a reduction in the overall prevalence by 12.9%. The lower prevalences reported by some programs may be related in part to no registration (e.g., Spain ECEMC), or under-registration (e.g., Hungary), of pregnancy terminations among those fetuses prenatally diagnosed. In addition, there is the possibility that some cases of cloacal exstrophy were missed completely, and therefore never registered, by some surveillance programs. Considering these problems we would speculate that the true prevalence of cloacal exstrophy could be as high as 1 per 100,000 births.
The proportion of cases classified as OEIS complex in this study (22.6%) was lower than that previously reported in the literature [Keppler-Noreuil, 2001; Martínez-Frías et al., 2001]. These two previous reports were based on a small number of cases from hospital-based studies: Keppler-Noreuil [2001] retrospectively selected a cohort of infants diagnosed with OEIS complex, of which 9 out of 14 (64%) had omphalocele, bladder exstrophy, imperforate anus and spine defects. Spine defects was an inclusive term for vertebral anomalies, hypogenesis or segmentation anomalies of the sacrum, and dysraphism; only two of these infants were noted to have lipomyelomeningocele with a tethered cord. Martínez-Frías et al. [2001] selected cases based on a diagnosis of cloacal exstrophy (n = 11) among live born (n = 8) and stillborn (n = 3) infants at delivery and determined the frequency of omphalocele (63.6%), spina bifida (54.6%), and spine defects (54.5%). In this study, the proportion that had cloacal exstrophy and imperforate anus with omphalocele, and spina bifida or spinal defects was 8 out of 11 (73%). The variability in the proportions for the OEIS complex and omphalocele reported between these two previous studies and our investigation likely reflects differences in the use of hospital-based vs. population-based data, the selection criteria used to identify cases of cloacal exstrophy, and the inclusion criteria used to define OEIS, particularly spinal anomalies. For our study, 16 of the surveillance programs were population-based, and we chose to apply strict criteria for use of the term OEIS complex, including the diagnosis of spina bifida.
Our study reports on the worldwide estimate of the prevalence of cloacal exstrophy and, to date, represents the largest identified case group originating from many countries. However, limitations must be considered. Cloacal exstrophy remains an epidemiologic challenge due to its rarity, the use by many surveillance programs of nonspecific codes that include persistent cloaca to identify cases, and lack of full validation of the clinical findings. These issues may likely result in misclassification within a surveillance program unless detailed clinical information is available to document the different congenital anomalies occurring in an infant suspected of having cloacal exstrophy. In this study, our use of very strict inclusion criteria and the reassessment of clinical information after initial reporting could have resulted in the exclusion of some true cases of cloacal exstrophy. Moreover, not all surveillance programs are permitted to capture data on elective terminations of pregnancy for fetal anomalies. Among the countries represented by the South America ECLAMC surveillance program and in Mexico, elective terminations are not permitted for any reason. In Spain ECEMC and China Beijing, elective terminations are not registered in their respective surveillance programs although they are permitted in these two countries. This limitation may result in an underestimation of the true prevalence of cloacal exstrophy. Because data on maternal characteristics such as education, race/ethnicity, and gravidity were either missing in a high proportion of cases or were not submitted by the programs, we were unable to report on these factors.
A strength of this study is the use of data from 18 surveillance programs representing 24 countries. In addition, all cloacal exstrophy cases underwent careful clinical review and, when necessary and to the extent possible, the surveillance programs provided additional clinical information describing the findings (e.g., autopsy, surgical report). As a result of this additional clinical data, we were able to exclude 70 infants that were originally submitted as cases of cloacal exstrophy for this study. This demonstrates the importance of adequate clinical data to properly classify cases. With the exclusion of what we considered to be false positive cases of cloacal exstrophy, we reported a live birth prevalence similar to that reported by Martínez-Frías et al. [2001] and Caton et al. [2007].
Based on the reports from each surveillance program, 27 (14.5%) of the cases had other congenital anomalies unrelated to their cloacal exstrophy. Using strict criteria, 42 (22.6%) were classified as OEIS, 60 (32.3%) as OIE and 18 (9.7%) as EIS. It should be noted that these percentages exclude those cloacal exstrophy cases that had either spina bifida occulta or vertebral anomalies. Our findings of a cloacal exstrophy case with suspected VATER and another with amniotic band sequence have been previously reported in the literature [Bohring, 2002; Curry et al., 2006]. Within our large cohort of cloacal exstrophy, four infants had chromosomal abnormalities: two with trisomy 13, one with mosaic 12, and one with mosaic 45,X. To our knowledge, these associated abnormalities have not previously been reported in the literature.
The embryologic timing for cloacal exstrophy is very early in organogenesis and the defect is likely due to an abnormality of cellular proliferation at the caudal eminence. Based on the existing animal and human evidence, cloacal exstrophy does not seem to result from the premature rupture of the cloacal membrane as previously thought. Due to missing data on cases for many of the maternal and infant characteristics, we were not able to discern whether cloacal exstrophy is part of an embryologic continuum of abnormal development (i.e., BEEC or EEC). In other words, are the distributions of the epidemiologic characteristics different between groups (i.e., cloacal exstrophy, BEEC, or EEC)?
In summary, the overall prevalence of cloacal exstrophy was higher than previously reported and varied slightly by geographic region. Only 22.6% of the cloacal exstrophy cases met our strict criteria for the OEIS complex used in this study, a lower proportion than previous reported. More than half of the cloacal exstrophy cases had an omphalocele and 14.5% had other anomalies that were not associated with the cloacal exstrophy. Documentation of true cloacal exstrophy can be challenging and we would therefore recommend that, when feasible, birth defect surveillance programs include photographs, clinical descriptions, and surgical reports on each case for confirmation of the diagnosis. Further investigation of the etiology and pathogenesis of this very rare and intriguing defect is necessary to understand how to prevent its occurrence.
Acknowledgements
The authors are grateful to each monitoring systems' staff for their work in collecting case data and submission to the ICBDSR Centre. Work conducted at the ICBDSR was supported by the Centers for Disease Control and Prevention (1U50DD000524-02). The authors are also grateful to Jeri Fowles, R.N., for the illustrations and Sergey Krikov, M.S. (University of Utah) for assisting with data analysis and illustrations. Grant sponsor for South America ECLAMC: MCT/CNPq, Brazil; Grant numbers: 573993/2008-4, 476978/2008-4, 554755/2009-2; 306750/2009-0; 402045/2010-6. In Spain, this work was supported in part by the Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) and the Fundación 1000 sobre Defectos. CIBERER is an initiative of ISCIII. Components of ECEMC's Peripheral Group are gratefully acknowledged.