Auditory function and hearing loss in children and adults with Williams syndrome: Cochlear impairment in individuals with otherwise normal hearing†
How to cite this article: Marler JA, Sitcovsky JL, Mervis CB, Kistler DJ, Wightman FL. 2010. Auditory function and hearing loss in children and adults with Williams syndrome: Cochlear impairment in individuals with otherwise normal hearing. Am J Med Genet Part C Semin Med Genet 154C:249–265.
Abstract
Hearing loss is common in school-age individuals with Williams syndrome (WS) and extensive in adults. Prior studies with relatively small sample sizes suggest that hearing loss in WS has an early onset and may be progressive, yet the auditory phenotype and the scope of the hearing loss have not been adequately characterized. We used standard audiometric tools: Otoscopy, tympanometry, air-conduction (bone conduction when available) behavioral testing, and distortion product otoacoustic emissions (DPOAEs) to measure hearing sensitivity and outer hair cell function. We tested 81 individuals with WS aged 5.33–59.50 years. Sixty-three percent of the school-age and 92% of the adult participants had mild to moderately-severe hearing loss. The hearing loss in at least 50% was sensorineural. DPOAE testing corroborated behavioral results. Strikingly, 12 of 14 participants with hearing within normal limits bilaterally had 4,000-Hz DPOAE input/output (DPOAE IO) functions indicative of outer hair cell damage and impaired cochlear compression. Our results indicate that hearing loss is very common in WS. Furthermore, individuals with WS who have “normal” hearing as defined by behavioral thresholds may actually have sub-clinical impairments or undetected cochlear pathology. Our findings suggest outer hair cell dysfunction in otherwise normal hearing individuals. The DPOAE IO in this same group revealed growth functions typically seen in groups with noise-induced damage. Given this pattern of findings, individuals with WS may be at increased risk of noise-induced hearing loss. Recommendations regarding audiological testing for individuals with WS and accommodations for these individuals in both academic and nonacademic settings are provided. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Considerable success has been achieved in identifying the genetic causes of deafness; however, much less is known about the contribution of genetic factors to milder forms of hearing loss. This is unfortunate, given that for every child born with profound hearing loss, one or two are born with less severe but clinically significant hearing deficits [Nance, 2003]. Hearing losses described as mild can have serious consequences for typically developing children with regard to acquisition of critical vocabulary comprehension and syntax skills [Davis et al., 1986; Norbury et al., 2001], understanding speech in noisy environments [Davis et al., 1986], and development of effective attention behaviors [Bess et al., 1998]. The cognitive and educational consequences of mild hearing loss for school-age individuals with neurodevelopmental disabilities, which would be expected to be more severe, have only rarely been addressed [Evenhuis et al., 2001; Laws, 2004; Van Naarden Braun et al., 2005]. Studies of hearing disability associated with genetic disorders provide an opportunity to better understand the genetic, biological, and therapeutic issues associated with hearing loss [Tekin et al., 2001].
Studies of hearing disability associated with genetic disorders provide an opportunity to better understand the genetic, biological, and therapeutic issues associated with hearing loss.
Williams syndrome (WS) is a rare neurogenetic developmental disorder with a prevalence of 1 in 7,500 live births [Strømme et al., 2002]. WS is caused by a hemizygous 1.5 Mb deletion on chromosome 7q11.23 [Ewart et al., 1993]; so far, ∼25 genes have been mapped to the deleted region [Hillier et al., 2003]. Approximately 95% of individuals with WS have the same set of genes deleted [Bayes et al., 2003]; most of the remaining individuals have additional genes deleted. WS is characterized by specific facial features (craniofacial dysmorphology), growth deficiency, developmental delay, mild to moderate intellectual disability, characteristic cognitive and personality profiles, connective tissue abnormalities, and vascular obstructive disease, most frequently supravalvar aortic stenosis (SVAS).
The results of recent studies from several independent laboratories suggest that WS is associated with an increase in auditory pathology [Cherniske et al., 2004; Levitin et al., 2005; Marler et al., 2005, 2008; Gothelf et al., 2006]. Chronic otitis media occurs in 50% of children with WS [American Academy of Pediatrics Committee on Genetics, 2001; Mervis and Morris, 2007] compared to 41% of children in the general population [Auinger et al., 2003]. In the general population, the occurrence of otitis media frequently diminishes in older school-age children, only to increase again in middle age [Rudin et al., 1985; Kim et al., 1993; Daly et al., 1998]. Due to the genetic disruptions in the middle-ear system in WS, otitis media and the conductive hearing loss that frequently accompanies it may persist in adulthood [Cherniske et al., 2004; Marler et al., 2008]. High-frequency sensorineural hearing loss (SNHL) or mixed hearing loss in the mild to moderate range has been reported in 60–70% of school-aged children with WS [Marler et al., 2005, 2008; Gothelf et al., 2006] compared to 7% of the general school-age population [Bess et al., 1998; Niskar et al., 1998]. Gothelf et al. 2006 hypothesized that individuals with WS are hypersensitive to noise-induced hearing loss. Finally, even after controlling for middle-ear pathology and medical conditions or ototoxic mediations known to impair middle-ear health, individuals with WS may evidence surprisingly poor cochlear outer hair cell function relative to expectations for their hearing thresholds [Marler et al., 2005, 2008].
The purpose of the present study was to further characterize auditory functioning in individuals with WS. We provide data on the incidence of hearing loss in the largest sample of individuals with WS examined to date. Of considerable clinical relevance is our finding that a large proportion of the school-aged participants with WS had previously undiagnosed hearing loss (conductive, sensorineural, or mixed). In addition, we focused on the question of whether individuals with WS who had “normal” hearing nevertheless evidenced cochlear pathology. For this purpose, we studied otoacoustic emissions. Below we briefly describe these emissions, how they are measured, and what can be inferred from these measurements, before presenting the study methods and results. We report data indicating that individuals with WS who have normal behavioral hearing sensitivity may not have normal auditory function; these individuals may have a subclinical auditory dysfunction characterized by impaired cochlear compression.1
Otoacoustic emissions are very low-level sounds that can be measured in the ear canal when test stimuli are delivered via an in-the-ear probe. They are part of a standard test battery to evaluate cochlear function. Otoacoustic emissions are not a direct measure of perceptual hearing sensitivity, but a noninvasive, objective test of the integrity of the cochlear outer hair cells. The outer hair cells are part of the cochlear amplifier system, largely responsible for the tremendous dynamic range of hearing [Ruggero et al., 1997; Robles and Ruggero, 2001; Kemp, 2002; Bacon, 2004]. The outer hair cells are thought to have a motor capability allowing them to change their shape according to the frequency characteristics of an acoustic stimulus [Holley, 1996; Bacon, 2004]. These changes in electromotility result in an amplification or increased vibration of the basilar membrane. The amplification gain can be as much as 50–80 dB, especially for low-to-mid intensity sounds [Ruggero et al., 1997].
Another phenomenon associated with the large dynamic range and frequency selectivity of hearing is cochlear compression.1 The mechanical responses of the cochlea to stimulus changes of low-intensity levels are linear, including those generated by the outer hair cell system. As intensity levels increase, the responses become compressed (nonlinear), with high-intensity stimuli again eliciting linear responses [Robles and Ruggero, 2001]. It is generally accepted that the outer hair cells play a primary role in modulating cochlear compression [Robles and Ruggero, 2001; Oxenham and Bacon, 2003]. Cochlear hearing losses caused by damage to the outer hair cells result in elevated behavioral thresholds, loudness recruitment (a disproportionate growth of perceptual loudness in the hearing-impaired listener), difficulty perceiving shorter-duration sounds (temporal integration), and difficulty understanding speech in a noisy environment [Oxenham and Bacon, 2003].
One clinical method of testing outer hair cell function is distortion product otoacoustic emissions (DPOAEs2). DPOAEs are produced in the cochlea when two pure-tone stimuli [frequency 1 (f1) and frequency 2 (f2)] cause the generation of non-linear intermodulation tones. The intensity of one of the nonlinear distortion tones (2f1 − f2) is a common clinical metric to evaluate cochlear integrity [Kemp, 2002]. DPOAEs are especially helpful clinically for predicting auditory sensitivity and cochlear health in individuals with hearing thresholds ≤50 dB HL [Gorga et al., 1997, 2000] and are useful in corroborating behavioral measures in individuals who have intellectual disabilities [Neuman et al., 2006; Hild et al., 2008]. DPOAE recordings are stable and reliable for individuals 7 years and older [Kon et al., 2000].
Historically, the detectability rather than the strength of DPOAE amplitudes has been considered clinically relevant [Kemp, 2002]. However, in a large-scale clinical study, Gorga et al. 1997, 2000, 2005 showed that when normal middle-ear status was demonstrated (e.g., by acoustic immittance testing or tympanometry), very low-amplitude DPOAEs could be interpreted as suggesting cochlear pathology. DPOAEs are of primary importance to the present study as low-amplitude DPOAE levels in individuals with “normal” hearing sensitivity have also been hypothesized to reveal subclinical noise-induced damage [Zheng et al., 1997; Lucertini et al., 2002; Mills et al., 2007; Sisto et al., 2007; Marshall et al., 2009]. Furthermore, when DPOAEs are measured to single pairs of pure tones, with a sweep from high to low stimulus intensities, the resulting DPOAE input/output (DPOAE IO) function gives insight into the compressive, nonlinear sound processing of the cochlea as well as providing further demonstrations of a loss of cochlear compression and cochlear impairment in individuals with known sensorineural hearing loss [Dorn et al., 2001; Johannesen and Lopez-Poveda, 2008]. Thus, while DPOAEs have historically been used to identify mid-to-high-frequency hearing loss [Gorga et al., 1993], recent research indicates that they may also be an important first round of defense in attempts to identifying subclinical sensorineural hearing loss and physiological susceptibility to noise-induced hearing damage [Lucertini et al., 2002; Seixas et al., 2004; Sisto et al., 2007; Job et al., 2009; Marshall et al., 2009].
MATERIALS AND METHODS
The research protocol was approved by the James Madison University Institutional Review Board. Written consent to participate was obtained from parents of all children, from parents of all adults who had legal guardians, and from all adult participants who did not have legal guardians. Written assent was obtained from all children aged 7 years or older and from all adult participants who had legal guardians. Each participant received a $20 gift card for participation, and parents were sent a written report summarizing the results of the evaluation of hearing sensitivity and auditory function.
Participants
This study included two groups of participants. The primary group was composed of 81 individuals with genetically confirmed WS ranging in age from 5.33 to 59.50 years. Portions of the data from 27 of these participants were previously reported [Marler et al., 2005]. Participants were recruited through the national Williams Syndrome Association (WSA), from an ongoing study of the development of children with WS conducted by C.B.M., and through referrals from geneticists and private physicians. Participants with WS were divided into two groups. The School Age group (n = 43, 17 males, 26 females) ranged in age from 5.33 to 17.92 years [mean CA (chronological age): 12.08 years, SD: 3.33 years, median CA: 11.67 years]. The Adult group (n = 38, 20 males, 18 females) ranged in age from 18.00 to 59.50 years (mean CA: 31.83 years, SD: 11.75 years, median CA: 28.25 years).
To test the hypothesis that individuals with WS who have normal behavioral hearing may actually have subclinical auditory impairments, a group of 14 typically developing individuals who had hearing within normal limits bilaterally (TDNH; 7 males, 7 females) aged 7.76–32.5 years (mean CA: 13.86 years, SD: 7.37 years, median CA: 10.67 years) was matched for gender and CA to 14 individuals with WS who also had hearing within normal limits bilaterally (WSNH; mean CA: 14.25 years, SD: 7.67 years, median: 11.08 years).
Equipment
We evaluated auditory function using two different methods in two separate settings. Most of the participants with WS (n = 69) were tested in quiet locations outside of clinic settings, typically at one of the WSA conventions. In the “convention protocol,” instrumentation consisted of a Grason-Stadler GSI-38 Auto Tympanometer, allowing the evaluation of both tympanometry and pure-tone, air-conduction behavioral responses (TDH-39 headphones). When we were able to evaluate individuals with WS in a standard clinic setting (“clinic protocol,” n = 12), hearing was tested with a Grason-Stadler GSI-61 audiometer (ER-3A insert earphones) and a Grason-Stadler Tympstar. DPOAEs were elicited in both protocols using an Intelligent Hearing Systems (IHS) SmartOAE system with insert earphone (10D-OAE probe).
Procedures
Medical and hearing background
Parents of all participants with WS were interviewed about the participant's medical and hearing background. The questions focused on the risk factors associated with hearing loss, specifically history of exposure to noise, occurrence and frequency of otitis media, other diseases of childhood associated with hearing loss, and medications known to be ototoxic.
Middle ear function
The possible presence of middle ear disorders was evaluated with otoscopic observation of the external ear canal for any tympanic membrane abnormalities and with acoustic immittance (tympanometry). Otoscopy was determined unremarkable when the tympanic membrane color was normal and the position was not retracted or bulging. Acoustic immittance measures used to determine middle ear function were obtained using a commercially available middle ear analyzer (Grason Stadler, Model GSI-38). These tympanometric measures were recorded using a 226-Hz probe tone and judged within normal limits when static compliance (a measure of tympanic membrane mobility) was between 0.3 and 1.2 cm3 and peak pressure in the middle ear was ±100 daPa3 [Alaerts et al., 2007]. In the convention protocol, participants with minimal response levels >25 dB HL4 at 500 kHz in either ear (even in the presence of acceptable tympanometric results) were assumed to be showing a detrimental middle ear contribution to the hearing responses and therefore were not included in further hearing or DPOAE analyses. In the clinic protocol, bone-conduction testing was used to further evaluate middle-ear contributions and the presence of air-bone gaps.
Behavioral hearing sensitivity
In the clinic protocol, participants were tested in a noise-controlled environment (double-walled booth) with ambient noise levels sufficiently low to permit diagnostic assessment of hearing thresholds [ANSI, 1996]. Ambient noise levels in the convention protocol precluded the measurement or reporting of absolute thresholds; however, one of the evaluators (J.A.M. or J.L.S.) checked the stimulus levels daily to ensure that 0 dB HL stimuli across test frequencies between 500 and 8,000 Hz were audible. It is important to note that even in the convention protocol, some participants responded to test signals at 0 dB HL across all test frequencies, further demonstrating that stimuli were audible even at very low intensity levels and that the convention protocol environments were sufficiently quiet. An additional demonstration of the integrity of the data is that four individuals seen during the WSA conventions were subsequently seen by independent, licensed audiologists for full audiometric evaluations. The audiological reports for these individuals indicated that their diagnostic threshold responses were within 5 dB of the responses measured in our convention-protocol environment. This is considered well within clinical measurement error. For these reasons, we have collapsed participant responses across the two protocols and have reported behavioral hearing sensitivity data as “minimum response levels” (rather than suggesting that all are estimations of hearing threshold).
In both protocols, a standard bracketing method was used in which test tones were first presented at an a priori estimated comfortable suprathreshold level (30 dB HL). If there was no response, the tone level was increased until the participant responded. At that point the tone was decreased in 10 dB steps until the participant failed to respond, at which point the tone level was raised in 5 dB steps until a response occurred. This process was continued until at least two ascents and two descents had been completed.
Hearing levels for both ears were determined at frequencies 500, 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hz for air conduction in both protocols. In the clinic protocol, bone-conduction was used, when necessary, at octave frequencies between 500 and 4,000 Hz. For the School Age group, a response >20 dB HL at any single frequency in a single ear measurement was considered a “fail.” For the adult group, any response at a level higher (poorer) than the normative data reported by the International Organization for Standardization [ISO, 1984] for the participant's age was considered a “fail.” This criterion takes into account normal age-related changes in hearing sensitivity [Cruickshanks et al., 1998].
Frequency-sweep distortion product otoacoustic emissions (DPOAEs)
The frequency-sweep DPOAEs were measured to pairs of primary tones, with f1 and f2 frequencies at a fixed f2/f1 ratio of 1.22. The elicited f2 frequencies ranged from 1 to 8 kHz, in 1/8th-octave steps. The intensity levels of the primary-tone pairs were fixed across all frequencies, with L1 (intensity level of f1) = 65 dB SPL5 and L2 (of f2) = 55 dB SPL. These intensity levels were chosen because of previous reports that they best discriminate between normal and impaired ears [Gorga et al., 1997]. Due to some reports of decreased reliability of the DPOAEs at very low and high frequencies [Roede et al., 1993; Siegel and Hirohata, 1994; Marshall et al., 2009], analyses were restricted to f2 DPOAE frequencies between 1,500 and 6,000 Hz. To decrease the likelihood of an error resulting from a single-frequency outlier, for each frequency of interest (1,500, 2,000, 3,000, 4,000, and 6,000 Hz), we averaged the DPOAE level immediately preceding and immediately following the frequency of interest (e.g., DPOAE f2 levels reported for 1,500 Hz were derived by averaging the DPOAE f2 levels at 1,418, 1,550, and 1,687 Hz). Further analyses were then performed on these averaged frequency-sweep DPOAEs to make direct comparisons to normative data [Knight and Kemp, 2000].
A DPOAE “fail” was any single averaged DPOAE amplitude (dB SPL) within the 1,500- to 6,000-Hz range falling at or below the 5th percentile of normal-ear distributions as reported by the Gorga et al. 1997 normative study. When one or more DPOAE amplitude(s) fell at or below the 5th percentile, it was interpreted as predictive of impaired cochlear function.
Cochlear compression as measured by DPOAEs
Finally, to test the theory that hearing loss in WS may result from a physiological vulnerability to noise levels present in a typical environment, we tested the 4,000-Hz DPOAE IO function with the 14 participants with WS who had hearing within normal limits (WSNH; clinical protocol, n = 6; convention protocol, n = 8) and 14 age- and gender-matched typically developing individuals who had hearing within normal limits (TDNH; clinical protocol, n = 11; convention protocol, n = 3). Dorn et al. 2001 found that at 4,000 Hz, there was a significantly reduced range of stimulus intensity levels over which cochlear compression is clearly demonstrated. DPOAE IO functions were performed at the ear with the best (lowest) high-frequency, pure-tone average minimal response levels measured at 4,000, 6,000, and 8,000 Hz. We predicted that we would elicit the strongest DPOAE amplitudes if we used the best-ear data, thereby making it more difficult to find a significant group difference. As previously reported in the frequency-sweep DPOAE section, the DPOAE IO function was measured to pairs of primary tones (f1 and f2) with a fixed f2/f1 ratio at 1.22, and with the f2 frequency set to 4,000 Hz. The DPOAE IO was elicited with L1 levels ranging from 75 to 35 dB SPL and L2 levels ranging from 65 to 25 dB SPL.
Statistical Analyses
Repeated measures ANOVAs, paired comparisons, and t tests were performed using the Statistical Package for the Social Sciences version 17.0 (SPSS 17.0) with α = 0.05. In order to assess cochlear compression in the WSNH group, DPOAE IO functions were fit using multilevel modeling [Moskowtiz and Hershberger, 2002; Raudenbush and Bryk, 2002]. Multilevel modeling is particularly well suited to analyses of these data, as it permits simultaneous fits of group (WSNH vs. TDNH) and individual DPOAE IO functions, as well as providing a measure of the extent to which functions for individuals deviate from group or average data. We used HLM 6.0 [Raudenbush et al., 2004] to estimate second-order polynomial functions. Non-linear functions (e.g., cubic polynomials) have been fit to either average or individual data previously [Dorn et al., 2001; Williams and Bacon, 2005; Johannesen and Lopez-Poveda, 2008].
RESULTS
Middle Ear
Routine otoscopic examination revealed that the tympanic membrane was either wholly or partially visualized in 74/81 participants. Of the seven participants with significant cerumen buildup, two had tympanometric measurements within normal limits and were included in hearing and DPOAE analyses. Our observations confirm previous reports of excessive cerumen build up (ear wax) in both children and adults with WS [Cherniske et al., 2004; Marler et al., 2005]. Due to a growing awareness in the WS community of the prevalence of hearing loss and the importance of cerumen management, several of the participants' families reported having excess cerumen removed prior to study participation.
To evaluate whether certain effects of otitis media might persist into later childhood or adulthood, we analyzed middle ear compliance and pressure. It is necessary to determine that middle ear function is within normal limits prior to interpreting the DPOAE amplitudes. Tympanometry could not be tested in 4 of 81 participants. One adult and two school-age participants reported being afraid of the sensation caused by the tympanometry stimulus. In one other adult (age = 53 years) we could not achieve a seal for either ear. This participant's audiogram (clinic protocol) showed hearing loss in the lower and higher frequencies but a threshold within normal limits at 2,000 Hz. This pattern is often reported in otosclerosis and therefore is a strong indicator of middle ear dysfunction. In 11 participants (7 School Age and 4 Adult), a seal could be maintained sufficient for measurement in only one ear; for these individuals, only unilateral tympanometric results were available. As we did not have bilateral tympanometric measurements for these 15 participants and it is possible that the unmeasured ear was not within normal limits, the middle ear measurements from these participants were not included in any of our analyses. The results for the individuals for whom bilateral tympanometric measurements were obtained are presented in Table I. In the School Age group with bilateral data, 34/34 children had tympanometric measurements falling within normal limits. Of the Adult participants for whom bilateral data were obtained, 7 of 32 (22%) had tympanometric measurements for one or both ears falling outside the acceptable range for a healthy Type A tympanogram, indicating abnormal middle ear function (5 with Type Ad tympanograms indicating normal middle ear pressure but high compliance and 2 with Type C tympanograms indicating normal compliance but significant negative pressure). These data suggest a larger percentage of adults with WS may have middle-ear pathology than the approximately 17% expected in the general adult population [Rudin et al., 1985; Kim et al., 1993].
These data suggest a larger percentage of adults with WS may have middle-ear pathology than the approximately 17% expected in the general adult population.
. The pure-tone and DPOAE measurements from these seven Adult participants were not included in the later hearing or DPOAE analyses.
| Group | n | Ear | Mean (cm3) | WS sample (90% range) | Norms* (mean, cm3) | Norms* (90% range) |
|---|---|---|---|---|---|---|
| Right | 0.63 | 0.33–0.93 | ||||
| WS—School Age | 34 | Left | 0.52 | 0.36–0.68 | ||
| Average | 0.58 | 0.36–0.80 | 0.5 | 0.3–1.11 | ||
| Right | 0.77 | 0.46–1.08 | ||||
| WS—Adult | 32 | Left | 0.68 | 0.51–0.85 | ||
| Average | 0.73 | 0.50–0.96 | 0.72 | 0.27–1.38 |
To investigate acoustic immittance of the middle ear system, two 2 (Group: School Age, Adult) × 2 (Ear: Right, Left) mixed-model ANOVAs were performed on compliance (cm3) and pressure (daPa). As stated above, analyses were only performed on participants who had normal bilateral tympanometry results. Sixty-six participants were included in the compliance measures (34 School Age, 32 Adult). The Group effect was not significant [F(1,64) = 0.597, P = 0.442, 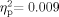 ]; the main effect for Ear [F(1,64) = 1.465, P = 0.231,
]; the main effect for Ear [F(1,64) = 1.465, P = 0.231, 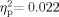 ], and the Group × Ear interaction [F(1,64) = 0.029, P = 0.866,
], and the Group × Ear interaction [F(1,64) = 0.029, P = 0.866, 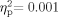 ] also were not significant. Sixty-five participants were included in the pressure measures [33 School Age (pressure data were missing for one school-age participant), 32 adult]. Neither the group effect [F(1,63) = 0.030, P = 0.863,
] also were not significant. Sixty-five participants were included in the pressure measures [33 School Age (pressure data were missing for one school-age participant), 32 adult]. Neither the group effect [F(1,63) = 0.030, P = 0.863, 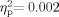 ] nor the Ear effect [F(1,63) = 879, P = 0.352,
] nor the Ear effect [F(1,63) = 879, P = 0.352, 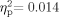 ] was significant. There was a significant Group × Ear interaction [F(1,63) = 4.640, P = 0.035,
] was significant. There was a significant Group × Ear interaction [F(1,63) = 4.640, P = 0.035, 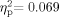 ]. In the School Age group, there was greater middle ear negative pressure in the left ear than in the right. This relation was reversed in the Adult group. However, both the confidence interval and the negligible effect size suggest that the Group × Ear interaction is not clinically meaningful (see Table I). The lack of a significant Group effect is interesting because one might have expected decreased compliance and increased negative middle-ear pressure to be more prevalent in the School Age group, becoming less pronounced in adults, as has been reported in the general population [Daly et al., 1998; Coyte et al., 1999]. The failure to find a main effect for Ear may be an artifact of the analysis method, as we included only data from individuals with bilaterally normal tympanometry results.
]. In the School Age group, there was greater middle ear negative pressure in the left ear than in the right. This relation was reversed in the Adult group. However, both the confidence interval and the negligible effect size suggest that the Group × Ear interaction is not clinically meaningful (see Table I). The lack of a significant Group effect is interesting because one might have expected decreased compliance and increased negative middle-ear pressure to be more prevalent in the School Age group, becoming less pronounced in adults, as has been reported in the general population [Daly et al., 1998; Coyte et al., 1999]. The failure to find a main effect for Ear may be an artifact of the analysis method, as we included only data from individuals with bilaterally normal tympanometry results.
Auditory Function
Behavioral hearing sensitivity
Traditional behavioral audiometry could not be obtained from two School Age participants (one in each protocol). A modified play-audiometry protocol was used with these children, measuring participant response to 20 dB HL tones at octave frequencies between 500 and 8,000 Hz in a free-field environment. Both participants passed, indicating pure-tone responses sufficient for speech, but as their responses were to single “screening” frequencies rather than being presented in the standard stair-step method, their data were not included in the hearing analyses.
To explore possible differences in hearing sensitivity as a function of age, the hearing data were submitted to a 2 (Group: School Age, Adult) × 2 (Ear: Left, Right) × 5 (Frequency: 0.5-, 1-, 2-, 4-, 8-Hz) mixed-model ANOVA. Some School Age participants' attention to task was insufficient for the length of time required to measure mid-octave frequencies, so 3,000- and 6,000-Hz data were not obtained in some cases. All participants who had tympanometric data indicating middle ear pathology or who lacked tympanometry results for both ears were excluded from the hearing sensitivity analyses. Accordingly, 59 participants (34 School Age, 25 Adult) were included in these analyses. A significant Group × Frequency interaction was obtained [F(4,54) = 4.243, P = 0.005, 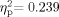 ]. In addition, the main effects for Group [F(1,57) = 12.785, P = 0.001,
]. In addition, the main effects for Group [F(1,57) = 12.785, P = 0.001, 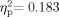 ] and Frequency [F(4,54) = 24.351, P < .001,
] and Frequency [F(4,54) = 24.351, P < .001, 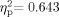 ] were significant. The main effect for Ear (P = 0.06), the Group × Ear interaction (P = 0.10) and the Group × Ear × Frequency interaction (P = 0.80) were not significant.
] were significant. The main effect for Ear (P = 0.06), the Group × Ear interaction (P = 0.10) and the Group × Ear × Frequency interaction (P = 0.80) were not significant.
Post hoc paired comparisons were performed to investigate the Group × Frequency interaction. A Bonferroni adjustment was calculated to correct for multiple comparisons (0.05/10 with P = 0.005). Results indicated that the Adult group had significantly higher (worse) minimal response levels at 4,000 and 8,000 Hz (the two highest frequencies tested) than the School-Age group. These cross-sectional analyses support previous reports that hearing loss in WS may be progressive [Marler et al., 2005, 2008].
As most of the hearing measures (minimal response levels) were performed in the convention protocol, we have not presented the group data in a standard audiogram format. A large proportion of the participants across both age groups had higher-than-expected minimal response levels, indicating hearing loss [School Age: 20 of 34 (59%), Adult: 21 of 25 (84%)]. Furthermore, in 9 of 20 School Age (45%) and 6 of 21 Adult (29%) participants, the hearing loss was unilateral. It has been shown that typically developing children with unilateral hearing loss are at increased risk for academic failure and learning disabilities [Bess et al., 1998]. We plotted worst-ear data, as that appears to be a more accurate indicator of performance in academic settings and functional, everyday environments [Bovo et al., 1988; Bess et al., 1998]. We made the worst-ear determination using a high-frequency pure-tone average of frequencies 4,000 and 8,000 Hz. Figure 1 presents the 1,000-, 4,000-, and 8,000 Hz worst-ear data for all participants with middle-ear function within normal limits. The data are plotted in comparison to the 90th percentile for the normative data for children as reported by the Third National Health and Nutrition Examination Survey [Holmes et al., 2004] and the 90th percentile for otologically normal males as reported by the ISO [1984]. The male results from the survey are plotted since those thresholds were equal to or greater than the female thresholds.
Pure-tone minimal response levels for worst ear as a function of age for 36 children and 21 adults with WS. For comparison, worst-ear minimal response levels at frequencies 1,000, 4,000, and 8,000 Hz are plotted against the 90th percentile for children [based on Holmes et al., 2004] and the 90th percentile for male adults (based on ISO [1984]). The small vertical lines identify overlapping data points.
The hearing threshold data from all three frequencies, but especially 4,000 and 8,000 Hz, indicate that the incidence of hearing loss in individuals with WS is considerably greater than that reported for the general population: 7% of school-age children [Bess et al., 1998; Niskar et al., 1998] and 46% of adults between the ages of 46 and 80 years [Cruickshanks et al., 1998]. The lack of bone-conduction measurements in the convention protocol precludes ruling out a conductive contribution in the group minimal response levels, particularly in the lower frequencies (≤4,000 Hz). Recall that all the above analyses included only participants with Type A (normal) tympanograms. Therefore, the extent of hearing loss in the higher frequencies supports the interpretation of at least a mixed hearing loss (i.e., both conductive and sensorineural components) in these individuals. When all participants with minimal response level data are factored in (including those with unilateral middle ear pathology), the incidence of hearing loss increases [School Age: 24 of 38 (63%), Adult: 33 of 36 (92%)].
We were able to evaluate 12 individuals with WS in the clinic protocol that included bone conduction measures. Of those 12 participants, 10 were school-aged (7–18 years). The thresholds of this subset of the School Age group are plotted as an audiogram in Figure 2. Of the 10 participants, 5 had mild high-frequency SNHL and 1 had moderate high-frequency SNHL. Three of the six participants with SNHL had unilateral loss. Three of the six participants with SNHL had lower right-ear high-frequency pure-tone averages (averaged at 4,000, 6,000, and 8,000 Hz) and three had lower left-ear high-frequency pure-tone averages. It is important to note that four of the six participants with high-frequency SNHL had thresholds ≤20 dB HL in frequency regions between 1,000 and 4,000 Hz. Therefore, these four participants would have passed a standard hearing screening as performed in school settings.

Thresholds for best ear and worse ear are plotted in a standard audiogram format for 10 school-age participants with WS evaluated in the clinic protocol. Five of the 10 participants had mild highfrequency SNHL and 1 of the 10 had moderate high-frequency SNHL. The hearing loss for three of the six participants with SNHL was unilateral. Three of the six participants with SNHL had lower right-ear high-frequency pure-tone averages (averaged at 4,000, 6,000, and 8,000 Hz) and three had lower left-ear high-frequency pure-tone averages. Flag = 1 SEM.
Cochlear outer-hair cell integrity
Best-ear, frequency-sweep DPOAEs
Best-ear frequency-sweep DPOAE amplitudes were analyzed from the 49 participants who had [1] normal bilateral tympanometry, [2] minimal response levels ≤20 dB HL at 500 and 1,000 Hz, and [3] minimal response levels ≤40 dB HL at 2,000, 3,000, 4,000 and 6,000 Hz in the best ear (or minimal response levels ≤40 dB HL at 2,000 and 4,000 Hz if hearing data for 3,000 and 6,000 Hz were not available). These criteria were chosen because DPOAEs are most appropriate for detecting mild to mild-to-moderate hearing loss [Gorga et al., 1997, 2005] and to further minimize possible detrimental middle ear contributions to DPOAE amplitudes. Based on these criteria 10 participants (6 School Age, 4 Adult) were excluded, leaving 49 (28 School Age, 21 Adult) for the analysis. Data at 2,000, 3,000, 4,000, and 6,000 Hz were available for all 49 participants.
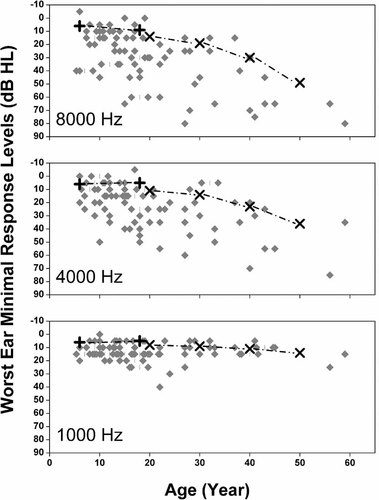
To explore the predicted DPOAE differences as a function of age, best-ear average DPOAE amplitudes were submitted to a 2 (Group: School Age, Adult) × 5 (Frequency: 1,500-, 2,000-, 3,000-, 4,000-, 6,000 Hz) mixed-model ANOVA. Neither the Group effect [F(1,47) = 0.053, P = 0.819, 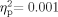 ] nor the Group × Frequency interaction [F(4,44) = 0.955, P = 0.442,
] nor the Group × Frequency interaction [F(4,44) = 0.955, P = 0.442, 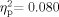 ] was significant. The majority of the participants in the School Age group had best-ear minimal response levels ≤25 dB HL across all frequencies, whereas 60% of the Adult group had minimal response levels ≥35 dB HL for at least two frequencies. On this basis, one might have expected DPOAE amplitudes to be lower (poorer) in the Adult Group. However, it is possible that DPOAE levels were already depressed in many of the School Age participants, resulting in a significant reduction in the overall range of outer hair cell activity in both “normal” and mild-hearing loss ears in individuals with WS. This hypothesis is supported by the results of the DPOAE IO function data reported later.
] was significant. The majority of the participants in the School Age group had best-ear minimal response levels ≤25 dB HL across all frequencies, whereas 60% of the Adult group had minimal response levels ≥35 dB HL for at least two frequencies. On this basis, one might have expected DPOAE amplitudes to be lower (poorer) in the Adult Group. However, it is possible that DPOAE levels were already depressed in many of the School Age participants, resulting in a significant reduction in the overall range of outer hair cell activity in both “normal” and mild-hearing loss ears in individuals with WS. This hypothesis is supported by the results of the DPOAE IO function data reported later.
The main effect of Frequency was significant [F(4,44) = 27.750, P < 0.001, 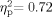 ]. Post hoc paired comparisons with a Bonferroni adjustment (0.05/5 with P = 0.01) indicated that for both groups the averaged DPOAE f2 amplitudes were lowest (poorest) at 4,000 Hz, those at 3,000 and 6,000 Hz did not differ significantly, and that the 3,000-, 4,000-, and 6,000-Hz averaged DPOAE f2 amplitudes were lower (poorer) than those at 1,000 and 2,000 Hz. Figure 3 plots the DPOAE f2 amplitudes as a function of frequency for the participants with WS who had normal bilateral tympanometric results against the Gorga et al. 1997 normative data. As can be seen from Figure 3, a significant number of averaged DPOAE f2 amplitudes at 4,000 Hz were lower than the 5th percentile criterion of the Gorga et al. 1997 data. These data corroborate the behavioral results and support the presence of impaired cochlear function in the majority of participants with WS. This is an important corroboration given the intellectual disability associated with WS and possible difficulty these individuals might have with threshold estimation, an attentionally demanding task. The absence of a significant Group effect in the DPOAE amplitude levels suggests that outer hair cell function in the School Age group was not significantly better than in the Adult group, despite the significantly better hearing sensitivity in the School Age group (see the hearing analyses above).
]. Post hoc paired comparisons with a Bonferroni adjustment (0.05/5 with P = 0.01) indicated that for both groups the averaged DPOAE f2 amplitudes were lowest (poorest) at 4,000 Hz, those at 3,000 and 6,000 Hz did not differ significantly, and that the 3,000-, 4,000-, and 6,000-Hz averaged DPOAE f2 amplitudes were lower (poorer) than those at 1,000 and 2,000 Hz. Figure 3 plots the DPOAE f2 amplitudes as a function of frequency for the participants with WS who had normal bilateral tympanometric results against the Gorga et al. 1997 normative data. As can be seen from Figure 3, a significant number of averaged DPOAE f2 amplitudes at 4,000 Hz were lower than the 5th percentile criterion of the Gorga et al. 1997 data. These data corroborate the behavioral results and support the presence of impaired cochlear function in the majority of participants with WS. This is an important corroboration given the intellectual disability associated with WS and possible difficulty these individuals might have with threshold estimation, an attentionally demanding task. The absence of a significant Group effect in the DPOAE amplitude levels suggests that outer hair cell function in the School Age group was not significantly better than in the Adult group, despite the significantly better hearing sensitivity in the School Age group (see the hearing analyses above).
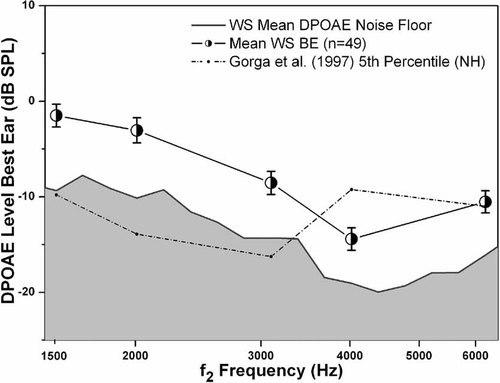
Best-ear averaged DPOAE amplitudes as a function of f2 frequencies for participants with WS who had normal tympanometric results. Any 4,000-Hz DPOAE f2 amplitudes ≤−9.23 dB SPL [the 5th percentile for normal ears as reported by Gorga et al., 1997] are predictive of impaired cochlear function. The majority of participants with WS have impaired cochlear outer hair cell function, providing further support for the behavioral hearing data. Flag = 1 SEM.
4,000-Hz DPOAE input/output function
To evaluate the presence of cochlear compression in individuals with WS who have hearing within normal limits (WSNH), we compared DPOAE IO functions at 4,000 Hz in 14 WS participants (12 School Age, 2 Adults) with those of 14 age- and gender-matched typically developing (TDNH) participants. For all of these individuals, response levels were ≤20 dB across all test frequencies. We chose to evaluate 4,000 Hz based on previous reports [Dorn et al., 2001] that the slope of the DPOAE I/O function at this frequency was most sensitive to the effects of hearing-loss or sub-clinical cochlear pathology (a steeper slope indicating diminished compression due to cochlear damage).
To confirm that the minimal response levels for best ear did not differ significantly across groups, we first performed a 2 (Group: WSNH, TDNH) × 6 (Frequency: 1,000-, 2,000-, 3,000-, 4,000-, 6,000-, 8,000 Hz) mixed-model ANOVA on the best-ear hearing data. The Group [F(1,26) = 2.857, P = 0.103, 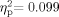 ] and the Group × Frequency interaction [F(5,22) = 1.294, P = 0.162,
] and the Group × Frequency interaction [F(5,22) = 1.294, P = 0.162, 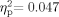 ] effects were not significant. The main effect for Frequency was significant [F(5,22) = 2.417, P = 0.04,
] effects were not significant. The main effect for Frequency was significant [F(5,22) = 2.417, P = 0.04, 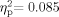 ]. Post hoc paired comparisons with a Bonferroni adjustment (P = 0.05/6 = 0.008) indicated that minimal response levels at 4,000 Hz were lower than those for 3,000 Hz but other frequencies did not differ significantly (P ≥ 0.144).
]. Post hoc paired comparisons with a Bonferroni adjustment (P = 0.05/6 = 0.008) indicated that minimal response levels at 4,000 Hz were lower than those for 3,000 Hz but other frequencies did not differ significantly (P ≥ 0.144).
Post hoc review of individual 4,000-Hz DPOAE IO function data revealed that the lowest intensity for which all participants from both groups had responses ≥3 dB above the noise floor was at DPOAE f2 levels of 50 dB SPL. To determine if there was a difference in OHC response as a function of stimulus intensity, multilevel modeling was used to describe the relation between DPOAE IO response amplitude and f2 level (75, 70, 65, 60, 55, 50 dB SPL).
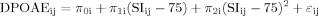
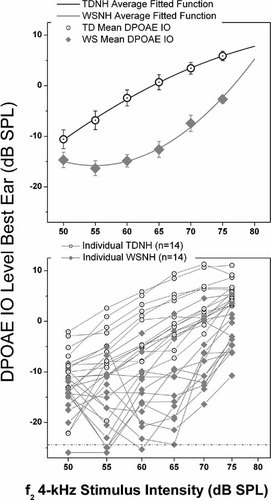
DPOAE IO function, a physiological correlate of normal behavioral dynamic range and cochlear compression. The top graph shows the averaged fitted function for each group (TDNH, typically developing with normal hearing; WSNH, Williams syndrome with normal hearing). Group mean amplitudes are plotted as a function of DPOAE f2. Flags = 1 SEM. The bottom graph shows individual participant data for both groups, demonstrating the small degree of group overlap at the higher-intensity DPOAE f2 amplitudes. The WSNH group had lower DPOAE levels with a precipitous drop in level as stimulus intensity decreased. Multilevel model analyses showed a significant difference between the curvilinear functions of the two groups. This difference suggested a loss of cochlear compression in the WSNH group, all of whom had minimal response levels within normal limits bilaterally across all frequencies.
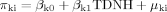
The results of the multilevel model are summarized in Table II. The average DPOAE IO functions are significantly different for the two groups. The average intercept (e.g., the DPOAE level at 75 dB SPL) for the WSNH group, β00, is −2.3 dB, which is 8.2 dB lower than that of the WSTD group, which has an estimated intercept of 5.9 dB (i.e., β00 + β01). The average linear coefficient, β10, is 1.34 or 0.9 dB greater for the WSNH group than the estimated value of 0.43 for the TDNH group. This coefficient reflects the greater initial change in WSNH DPOAE levels as signal intensity decreases from 75 dB SPL. The average quadratic coefficients also differ for the WSNH (0.03) and TDNH (−0.009) groups, reflecting differences in the curvature of the DPOAE IO functions. The variance components for the three parameters indicate significant variation in the individual functions, independent of group status.
| Parameter | Estimate | Standard error | t | Approx. df | P |
|---|---|---|---|---|---|
| Average effects | |||||
| Intercept, β00 | −2.295 | 1.271 | −1.806 | 26 | 0.082 |
| TDNH, β01 | 8.183 | 1.437 | 5.695 | 26 | <0.0001 |
| Linear, β10 | 1.345 | 0.260 | 5.166 | 26 | <0.0001 |
| TDNH, β11 | −0.915 | 0.303 | −3.024 | 26 | 0.006 |
| Quadratic, β20 | 0.033 | 0.0109 | 3.078 | 26 | 0.005 |
| TDNH, β21 | −0.0429 | 0.0125 | −3.422 | 26 | 0.002 |
| Parameter | Standard deviation | Variance | χ2 | df | P |
|---|---|---|---|---|---|
| Variance components | |||||
| Intercept, σµ0 | 2.894 | 8.377 | 56.306 | 26 | 0.001 |
| Linear, σµ1 | 0.660 | 0.436 | 70.543 | 26 | 0.0001 |
| Quadratic, σµ2 | 0.028 | 0.001 | 82.070 | 26 | 0.0001 |
| Level 1, σε | 2.958 | 8.749 | |||
- The intercept, linear and quadratic terms are estimates for the WSNH group. For each term, TDNH is the estimated difference in the coefficient for the TDNH group.
The group functions estimated from the multilevel model are plotted in the top panel of Figure 4 along with the means at each signal intensity. The average data in Figure 4 (top panel) indicate a DPOAE IO function for the TDNH group as would be expected from a healthy cochlea. In contrast, the slope of the DPOAE IO function for the WSNH group shows a precipitous drop in level as signal intensity decreases from 75 to 65 dB SPL. The slope of the WSNH DPOAE IO function is what would be predicted from a cochlea with moderate SNHL. It is unlikely that the significantly greater change in WSNH DPOAE levels as signal intensity decreases from 75 dB SPL is due to a conductive hearing loss, as such a loss would cause an overall decrease in the DPOAE amplitudes, but the curvilinear function of the DPOAE IO would remain intact [Gehr et al., 2004]. The results from this analysis provide further support for the hypothesis of cochlear outer hair cell dysfunction in individuals with WS who have otherwise “normal” hearing.
DISCUSSION
To our knowledge, the present study is the first to describe sub-clinical impairments or previously undetected cochlear damage in children with WS who have otherwise “normal” middle ear function and behavioral hearing responses. In the present study, a large number of individuals with WS had behavioral responses indicative of high-frequency hearing loss of a cochlear origin; numerous others had a mixed hearing loss.
In the present study, a large number of individuals with WS had behavioral responses indicative of high-frequency hearing loss of a cochlear origin; numerous others had a mixed hearing loss.
These behavioral findings were corroborated by DPOAE measures. In no case did an individual with WS present with behavioral hearing loss while having DPOAEs within normal limits. And when the data from individuals with middle ear pathology and individuals with probable SNHL were combined (i.e., to include individuals with probable mixed-hearing loss), the prevalence of hearing loss in WS was strikingly high: 63% of school-age children and 92% of adults. Furthermore, 22% of adults with WS had middle-ear pathology; this figure is slightly higher than the ∼17% expected in the general adult population [Rudin et al., 1985; Kim et al., 1993]. Our findings are consistent with earlier smaller-sample studies reporting hearing loss in WS [Cherniske et al., 2004; Marler et al., 2005, 2008; Gothelf et al., 2006]. Furthermore, results of our cross-sectional analyses suggested a progressive pattern to hearing loss in WS. Strikingly, very few of the parents were aware that their family member with WS had hearing loss prior to participation in this study. The difficulty parents have identifying mild hearing loss in their children has been previously reported [Rosenfeld et al., 1998; Brody et al., 1999]. Below we discuss possible genetic factors contributing to auditory dysfunction in WS and address some of the hypotheses regarding the basis of auditory dysfunction proposed in earlier studies of hearing in WS. We conclude with clinical suggestions for strengthening the audiometric evaluation of individuals with WS and with therapy recommendations.
Genetic Contributions to Auditory Dysfunction in WS
Middle ear system
Individuals with WS have a multiorgan and muticognitive systems phenotype resulting from a hemizygous microdeletion of ∼25 genes on chromosome 7q11.23 [Urban et al., 1999; Osborne and Mervis, 2007], including the elastin gene (ELN). The ELN gene's protein, elastin, is the most abundant element in elastic fibers, directly influencing smooth muscle cell development, adhesion, and proliferation [Urban et al., 2001, 2002; Kielty, 2006]. Elastin is also found in the ligaments, skin, cartilages of the ear, the epiglottis, larynx, and the muscles of the vocal folds [Keith, 1980; Hahn et al., 2006; Kusuhara et al., 2009; Watts et al., in press]. Elastic fibers play an important role in craniofacial development. In middle-ear structures, elastin contributes to normal development and function in the tympanic membrane [Yan et al., 1988; Ruah et al., 1992], the Eustachian tube [Matsune et al., 1992; Sando et al., 1994], and tendons supporting the ossicular chain [e.g., stapedius tendon; Franz et al., 1993]. The middle layer of the round window membrane contains elastin [Schachern et al., 1982; Sone, 1998], and this layer is sometimes in physical contact with the perilymphatic space of the inner ear [Goycoolea and Lundman, 1997].
In middle-ear structures, elastin contributes to normal development and function in the tympanic membrane, the Eustachian tube, and tendons supporting the ossicular chain (e.g., stapedius tendon). The middle layer of the round window membrane contains elastin, and this layer is sometimes in physical contact with the perilymphatic space of the inner ear.
The results of the few studies investigating the possible presence of elastin in the inner ear have been contradictory [Osborne and Comis, 1990; Mikuni et al., 1994; Katori et al., 1996; Kielty et al., 2002; Marler et al., 2006].
Elastin haploinsufficiency may have a negative impact on auditory function in WS. WS is associated with craniofacial anomalies that in other genetic conditions contribute to middle ear dysfunction and conductive hearing loss (e.g., Crouzon, Down, Treacher Collins, and velocardiofacial syndromes). One middle-ear structural disruption negatively impacting auditory function is the structure and orientation of the Eustachian tube and consequent disruptions of middle ear mechanics. The elastin-rich portion of the Eustachian tube is important for the evacuation of heavier fluid from the middle-ear space [Sando et al., 1994; Matsune et al., 1996]. This function could be further compromised in WS by the hypotonia that is universally present [C.A. Morris, personal communication]. A “floppier” Eustachian tube contributes to decreased dilation necessary for evacuation and further difficulties in opening effectively when the tensor veli palatine muscles contract [Bluestone, 2005]. Middle ear pathology (e.g., otitis media) also causes a chronic or fluctuating mild conductive hearing loss. The resulting anatomical disorganization and mechanical inefficiencies in the middle-ear system could contribute to the middle ear findings in WS. Furthermore, elastic fibers in the Eustachian tube are significantly less dense in children than in adults, suggesting that it is not simply the more horizontal orientation that contributes to increased otitis media in children [Matsune et al., 1996]. The functional obstruction of the Eustachian tube in infants and young children with WS would contribute to a failure to open and evacuate. The increase in otitis media in adults with WS may be related to ELN haploinsufficiency effects, that is, less dense elastin in the hinge portion of the Eustachian tube [Bluestone, 2005]. Such a disruption might explain the higher-than-expected occurrence of middle-ear pathology in adults with WS.
Disruption of the elastic fibers contributing to the mechanical function of the tympanic membrane, the Eustachian tube, and the tendons supporting the ossicular chain might also have a cascade effect on the middle ear system and any consequent retrograde transmission of DPOAEs (impacting the f1 and f2 amplitude levels as they pass out through the middle ear). At the level of the tympanic membrane, less flexibility might appear as greater stiffness. Any interruption of the mesh-like elastin structure in the Eustachian tube could decrease its mechanical ability to equalize middle ear pressure. The disruption of middle ear mechanics would result in a conductive hearing loss, decreasing the overall amplitude of DPOAE levels.
Finally, if elastin haploinsufficiency results in a stiffer stapedius tendon, the acoustic reflex6 may only occur at intensities greater than 110 dB HL, which would be intolerable to individuals with decreased loudness discomfort levels or auditory hypersensitivity, as reported in approximately 95% of individuals with WS [Levitin et al., 2005]. Some researchers have hypothesized that the impaired acoustic reflexes reported in WS might contribute to their hearing loss [Attias et al., 20087]. However, it has been consistently shown that the acoustic reflex is neither sufficiently fast nor sufficient in its dampening ability to have any protective function in high-intensity environments [Gelfand, 2009]. Further investigation of the possible role of ELN in general auditory function is important. However, it is highly unlikely that the degree of high-frequency hearing loss we report could be explained solely by elastin disruptions in middle ear mechanics. Changes due to increased stiffening would contribute to a conductive hearing loss, affecting frequencies >4,000 Hz, and the DPOAE levels of the IO function would be shifted down (lower), but would maintain their curvilinear function.
Accelerated aging effects
Another phenomenon that has been reported in WS is systemic accelerated aging [Cherniske et al., 2004]. It has been previously reported that a natural process of middle-ear aging in the general population is increased loosening of the middle ear system, which might result in an increase in static admittance and a decrease in DPOAE amplitudes in the 2,000- to 4,000-Hz range [Marshall et al., 1983]. While this DPOAE range is fundamental to our report of cochlear pathology in WS, it is unlikely that a premature aging process or middle ear “looseness” was a significant contributor. Although a decrease in the natural stiffness would cause a downward shift of DPOAE amplitudes as measured by an IO function, the curvilinear shape of this function would be expected to remain intact [Gehr et al., 2004]. A loosened middle ear system would continue to reflect cochlear nonlinearity if the cochlear system was healthy. The DPOAE IO findings shown in Figure 4 are not consistent with either a stiffening or accelerated aging effect (loosening).
Cochlear function as reflected by DPOAEs
There are now multiple controlled investigations identifying behavioral hearing loss in WS [Cherniske et al., 2004; Marler et al., 2005, 2008; Gothelf et al., 2006]. Our data provide corroborative otoacoustic emission measures indicating at least a cochlear contribution to the hearing loss. The DPOAE IO findings of this study suggest subclinical cochlear pathology even in children with WS who have normal behavioral hearing thresholds. In particular, 12 of 14 (85%) individuals with WS who had hearing within normal limits also showed a clear, steep slope to the higher-intensity stimuli in their DPOAE IO functions—a strong indication of a loss of cochlear compression and cochlear pathology.
The DPOAE IO findings of this study suggest subclinical cochlear pathology even in children with WS who have normal behavioral hearing thresholds. In particular, 12 of 14 (85%) individuals with WS who had hearing within normal limits also showed a clear, steep slope to the higher-intensity stimuli in their DPOAE IO functions—a strong indication of a loss of cochlear compression and cochlear pathology.
The 4,000-Hz region is particularly important when considering possible noise-related cochlear damage. The loss of cochlear compression is a common phenomenon in noise-induced hearing loss and is reflected in the DPOAE IO [Dorn et al., 2001]. The absence or loss of cochlear compression in 12 of 14 participants with WS who had healthy middle-ear systems and hearing within normal limits raises the question of whether individuals with WS might be predisposed to noise-induced damage, even in typical everyday environments.
Clinical Ramifications
Audiometric evaluation of individuals with WS
It is possible that single-frequency tympanometry using a 226-probe tone is insufficient to identify middle-ear pathology in individuals with WS, especially as it has limited power in identifying small amounts of fluid in the middle ear [Shanks and Shohet, 2009]. Elastin haploinsufficiency might cause important mass differences in the middle-ear system, causing inefficient ventilation or clearance of fluid from the middle-ear space. One of the middle ear consequences of elastin disruption in WS would be varying degrees of middle-ear effusion, ranging from severe (clinical otitis media) to mild (subclinical otitis media). Small amounts of fluid in the middle ear would result in poorer transmission of sound at higher frequencies. Multiple-frequency tympanometry using both the standard 226-Hz and a 678-Hz higher-frequency probe would assist in differentiating dysfunction of middle-ear transmission due either to stiffness characteristics (negative pressure, middle-ear effusion, otosclerosis or other stiffness factors) or the effects of mass [small amounts of fluid behind the tympanic membrane; Margolis et al., 1985; Abou-Elhamd et al., 2005; Shanks and Shohet, 2009].
The DPOAE conditions performed in this study were conducted with equipment readily available to otolaryngologists and clinical audiologists. We suggest that the 4,000-Hz DPOAE IO be incorporated into the audiological battery of tests for individuals with WS, particularly children who show normal behavioral hearing. Further investigations need to be performed to evaluate whether a loss of cochlear compression as measured by the DPOAE IO might actually precede measurable hearing loss in WS or whether measures of residual compression can be determined [Johannesen and Lopez-Poveda, 2008]. It is important to emphasize that across both children with WS and adults with WS, the frequency-sweep DPOAEs were significantly lower than would be expected given the behavioral hearing responses of the participants. As the cross-sectional pattern of results in this study continues to support the hypothesis that hearing loss in WS is progressive, we recommend that individuals with WS have annual hearing evaluations. It is also important to note that while our experience is that many individuals with WS give reliable responses during extended and attentionally taxing threshold testing, most benefit from frequent adjustments to the order of testing conditions in a standard audiometric battery. This is particularly true for children. Examples of procedural adjustments made in J.A.M.'s lab are (1) inclusion of multi-frequency tympanometry and measuring tympanometry toward the end of the evaluation to minimize fear responses to probe tips and tympanometry stimuli (2) frequent alternation between air-conduction, bone-conduction, and speech testing, as these children better tolerate the pure-tone testing when interspersed with speech stimuli. Finally, the clinical advantages of performing acoustic reflex testing with individuals with WS should be carefully reconsidered. Current diagnostic methods that include tympanometry, pure-tone and speech testing, otoacoustic emissions, and auditory brainstem responses usually provide sufficient differential information to preclude the necessity of subjecting individuals who are significantly hypersensitive to sounds to a test procedure built upon the repeated presentation of high intensity, acoustic reflex stimuli.
Therapy recommendations
The mathematics skills of almost all children with WS are markedly below grade level [O'Hearn and Luna, 2009] and for most, reading skills also are below grade level [Mervis, 2009]. The ability to clearly understand auditory information is crucial for learning in academic, vocational, home, and social settings. When possible, children with WS should be seated in the center of the front row of their classrooms, as this position provides the optimal listening environment (binaural listening cues). For the child diagnosed with a hearing loss, even if “only” mild, a recommendation for training with a personal listening device (e.g., frequency modulated or FM system) should be seriously considered by the physician, audiologist, and members of the child's individualized education program (IEP) committee. These systems have been shown to be beneficial to speech perception in both noisy academic and nonacademic settings for school-age children and adults who have varying degrees of hearing loss [Moeller et al., 1996; Anderson and Goldstein, 2004; Boothroyd, 2004].
If individuals with WS are predisposed to noise-induced damage, then until the factors contributing to their susceptibility to noise-induced hearing loss have been clarified, any therapies incorporating acoustic training or conditioning with moderately intense sounds should be avoided. Some parents have reported to J.A.M. and C.B.M. that therapies similar to auditory enhancement therapy (AIT) have been recommended for their child with WS. The basic premise of AIT is that the individual who is hypersensitive to moderately intense sounds can be “conditioned” to learn to tolerate them via adaptive training [Miani et al., 2001]. These types of therapies are ill-advised until the possibility of hypersensitivity to noise-induced hearing loss in WS has been systematically investigated.
Finally, it is also important to counsel family members of individuals with WS about the importance of hearing conservation (the preservation of existing hearing). Parents of children with WS, regardless of whether their child currently has a hearing loss, should be educated about methods of protecting their child from exposure to sustained noise (e.g., sustained vacuuming, public events in enclosed arenas, movie theaters, use of personal listening devices such as iPods). It is equally important to maintain these protective methods of hearing conservation with adults with WS, most of whom have already developed hearing loss. Although hearing professionals understand that the presence of hearing loss is not a protection from further noise-induced damage, this fact should be clearly presented and often repeated in discussions with individuals with WS and their family members.
CONCLUSION
Hearing loss in WS has an early onset and is likely progressive. The increased prevalence of otitis media in children with WS and the persistence of middle ear issues into adulthood suggest a marked conductive contribution to hearing loss in WS. The DPOAE data strongly suggest that as individuals with WS mature, there is an increasing contribution of cochlear dysfunction to the high-frequency hearing loss found in WS. Importantly, it is likely that cochlear pathology is present prior to the onset of hearing loss. Further research is needed to determine whether the cochlear pathology present in WS could be monitored with a test that could be easily performed in a clinical setting (4,000-Hz DPOAE IO functions). Finally, even mild hearing loss, whether unilateral or bilateral, has serious developmental consequences for individuals with disabilities with regard to language acquisition, speech perception in noisy environments, and attentional regulation. Many children with WS have diagnosed or undiagnosed hearing loss and thus are at increased risk for academic and social difficulties beyond those associated with intellectual disability.
Acknowledgements
This research was supported by National Institute of Child Health and Human Development Grants R03 HD44468 (J.A.M.) and R37 HD29957 (C.B.M.), National Institute of Neurological Disorders and Stroke Grant R01 NS35102 (C.B.M.), a grant from the national Williams Syndrome Association (J.A.M.), and support from the Ohio State University New Faculty Investigator Award (J.A.M.). We thank the children and their families for their enthusiastic participation in these studies. We also thank an anonymous reviewer, Colleen Morris, Zsolt Urban, and Susan Nittrouer for their helpful suggestions on earlier versions of this manuscript. Portions of these data were presented at the 12th International Professional Conference on Williams Syndrome in Anaheim, CA, 2008.




