FOXG1 variants can be associated with milder phenotypes than congenital Rett syndrome with unassisted walking and language development
Abstract
Since 2008, FOXG1 haploinsufficiency has been linked to a severe neurodevelopmental phenotype resembling Rett syndrome but with earlier onset. Most patients are unable to sit, walk, or speak. For years, FOXG1 sequencing was only prescribed in such severe cases, limiting insight into the full clinical spectrum associated with this gene. Next-generation sequencing (NGS) now enables unbiased diagnostics. Through the European Reference Network for Rare Malformation Syndromes, Intellectual and Other Neurodevelopmental Disorders, we gathered data from patients with heterozygous FOXG1 variants presenting a mild phenotype, defined as able to speak and walk independently. We also reviewed data from three previously reported patients meeting our criteria. We identified five new patients with pathogenic FOXG1 missense variants, primarily in the forkhead domain, showing varying nonspecific intellectual disability and developmental delay. These features are not typical of congenital Rett syndrome and were rarely associated with microcephaly and epilepsy. Our findings are consistent with a previous genotype–phenotype analysis by Mitter et al. suggesting the delineation of five different FOXG1 genotype groups. Milder phenotypes were associated with missense variants in the forkhead domain. This information may facilitate prognostic assessments in children carrying a FOXG1 variant and improve the interpretation of new variants identified with genomic sequencing.
1 INTRODUCTION
Forkhead box G1 (FOXG1) is part of the highly conserved forkhead box transcription factors superfamily. Mainly expressed in a variety of nervous system cell types and tissues, it has been shown that FOXG1 plays a major role in brain development, including telencephalon specification and patterning, neuronal differentiation and survival maintenance of mature neurons, and retina, inner ear, and olfactory bulb development (Hettige & Ernst, 2019; Kumamoto & Hanashima, 2017; Toresson et al., 1998; Xuan et al., 1995).
FOXG1 haploinsufficiency is a rare cause of early-onset neurodevelopmental disorders known as “FOXG1-related syndrome” or “FOXG1-related encephalopathy”. First described in 2008, this syndrome is also known as congenital variant of Rett syndrome because it has phenotypic similarities with typical Rett syndrome linked to MECP2 (methyl-CpG-binding protein 2) variants (Ariani et al., 2008). Typically, heterozygous pathogenic variants in FOXG1 result in severe neurodevelopmental encephalopathy whose main clinical features include severe to profound intellectual disability (ID), psychomotor delay (inability to walk and to use hands purposefully, absence of speech), drug-resistant epilepsy, and morphological brain anomalies (Kortüm et al., 2011; Wong, Singh, et al., 2019; Wong, Wu, et al., 2019). Rett syndrome almost exclusively affects females and is characterized by a similar clinical presentation but with normal growth and development for the first 6–18 months of life, followed by a slowing of development and the loss of some acquired motor and speech skills. However, unlike typical Rett syndrome, the congenital variant is not X-linked, and most patients lack some characteristic clinical features and therefore do not meet all the diagnostic criteria for Rett syndrome (Collins & Neul, 2022).
Recently, a 3-year-old boy was referred to our genetics department for the mild psychomotor delay. Exome sequencing (ES) identified a heterozygous de novo missense variant in the FOXG1 gene meeting the American College of Medical Genetics (ACMG) criteria for pathogenicity (PS2, PM1, PP3, PP5, and PM2). However, it was first classified as a variant of unknown significance because of the mismatch between the mild phenotype and what is usually described with FOXG1 variants. To reclassify the variant as likely pathogenic, a collegial exchange with international experts was required.
Although some attenuated forms of congenital Rett syndrome have been described in the literature (Khan & Kirmani, 2020; Méneret et al., 2012; Vegas et al., 2018), these cases remain rare. Currently, it may still be unclear to some physicians or biologists that FOXG1 variants can be associated with phenotypes that are milder than congenital Rett syndrome. With this study, we aim to provide a short review and data from a small cohort of patients presenting with a mild form of FOXG1 syndrome to help geneticists make an early diagnosis and improve the management of affected patients.
2 METHODS
Through international data sharing (European Reference Network for Rare Malformation Syndromes, Intellectual and Other Neurodevelopmental Disorders [ERN-ITHACA] and collaborators from the AnDDI-Rares network [Filière de Santé Anomalies du Développement avec ou sans Déficience intellectuelle de causes Rares]), we gathered the phenotypic and genetic data for five unrelated probands carrying heterozygous pathogenic or likely pathogenic (P/LP) FOXG1 variants with a mild presentation of FOXG1 syndrome, defined as a patient who acquires speech and can walk independently.
Using PubMed, we also searched the National Center for Biotechnology Information database to identify studies reporting patients with FOXG1 syndrome (case reports and prospective or retrospective cohorts). We reviewed the data from three previously described cases involving patients with a mild expression of this syndrome, according to our definition.
The procedures followed for genetic testing were in accordance with ethical standards, and appropriate informed consent was obtained from all individuals. Individuals underwent ES or multigene panel sequencing as part of their care. Variants were classified according to the ACMG guidelines (Richards et al., 2015).
3 RESULTS
Patients in our cohort were recruited from five centers in France and Italy (detailed case reports in Supporting information). We also reviewed the clinical data of three patients described in the literature as having a mild presentation (Jang et al., 2021; Mitter et al., 2018; Philippe et al., 2010). The clinical characteristics of the cohort and a comparison with the results of Mitter et al., involving 83 patients with FOXG1 syndrome, are summarized in Tables 1 and 2.
| Patient 1 (France) | Patient 2 (France) | Patient 3 (Italy) | Patient 4 (France) | Patient 5 (Italy) | Philippe et al. (2010) | Mitter et al. (2018) | Jang et al. (2021) | |
|---|---|---|---|---|---|---|---|---|
| Varianta | ||||||||
| DNA | c.713G>A | c.647C>T | c.708C>A | c.1178C>G | c.553A>G | c.1200C>G | c.1141delG | c.761A>C |
| Protein | p.(Cys238Tyr) | p.(Pro216Leu) | p.(Asn236Lys) | p.(Ser393Trp) | p.(Ser185Gly) | p.(Tyr400*) | p.(Ala381Profs*4) | p.(Tyr254Ser) |
| Demographics | ||||||||
| Sex | M | M | F | F | F | F | F | F |
| Age at last examination | 6 years | 9 years | 39 years | 12 years | 2 years 5 months | 10 years | 17 years | 5 years |
| Development | ||||||||
| ID/DD | Moderate | Moderate | Severe | Mild | Mild | Severe | Severe | Profound |
| Unassisted sitting at follow-up | + | + | + | + | + | + | + | + |
| Unassisted sitting age (months) | 17 | 9 | 10 | 7 | 10 | NA | 11 | 13 |
| Unassisted walking | + | + | + | + | + | + | + | + |
| Walking age (months) | 26 | 17 | 18 | 13 | 21 | 30 | 132 | 24 |
| Motor skills | Runs, jumps, ascends, and descends stairs by himself, plays football | Runs, ascends and descends stairs by himself, rides a bike with training wheels | Walks unassisted although with a wide base, holds and uses small objects (e.g., fork) | Walks autonomously | Walks autonomously | Walks autonomously | Walks autonomously | Walks autonomously |
| Speech at follow-up | + | + | + | + | + | + | + | + |
| Age at first words (months) | 19 | 12 | 12 | NA | 18 | NA | NA | 35 |
| Language skills | About 5 fluent words and 15 signs in sign language | Started learning to read and write | Primarily composed of words with a large vocabulary | Demonstrates correct verbal production, can read and write | Understands short sentences, produces about 10 words | Capable of saying a few words | Uses 10 spoken words | Communicates verbally |
| Regression | − | − | − | − | − | − | + | − |
| Neurologic | ||||||||
| Epilepsy | − | − | + | − | − | − | + | − |
| Abnormal muscle tone | + | + | − | − | + | + | + | + |
| Spasticity | − | − | − | − | + | NA | + | − |
| Stereotypic movements | + | − | + | + | + | + | + | − |
| Brain MRI | Mild delayed myelination and mild ventriculomegaly | Normal | NA | Simplified gyration, some cystic dilatations, white matter T2 hypersignals, corpus callosum hypoplasia (third per) | Normal | Normal | Cortical anomalies | Slightly delayed myelination, mildly enlarged lateral ventricles, hypoplastic olfactory bulbs, mild thinning of the rostrum of the corpus callosum |
| Other | ||||||||
| Microcephaly | − | − | + | − | − | + | − | + |
| Scoliosis | − | − | − | − | − | − | − | NA |
- Abbreviations: DD, developmental delay; F, female; FOXG1, forkhead box G1; ID, intellectual disability; M, male; MRI, magnetic resonance imaging; NA, not available; per, percentile.
- a Variants are named according to NM_005249.5.
| Total of the cohort and the three reviewed cases (n and % or mean/median) | Congenital variant of Rett syndrome Mitter et al. (2018) Total cohort (n = 81) (% or mean/median) | Mitter et al. (2018) Forkhead domain missenses only, including in the conserved site 1 (n = 21) (n and % or mean/median) | |||
|---|---|---|---|---|---|
| Age at last examination (months) | 150/114 | 105/78 | 87.5/NA | ||
| Development | |||||
| Intellectual disability/developmental delay | Variable | Variable | Variable | ||
| Unassisted sitting at follow-up | 8/8 | 100% | 45% | 15/17 | 88% |
| Unassisted sitting age (months) | 11/10 | 28/18 | NA | ||
| Unassisted walking at follow-up | 8/8 | 100% | 15% | 8/21 | 38% |
| Walking age (months) | 35/22 | 53/45 | NA | ||
| Speech at follow-up | 8/8 | 100% | 21% | 6/20 | 30% |
| Age at first words (months) | 19/18 | 46/33 | NA | ||
| Regression | 1/8 | 12.5% | 18% | 1/12 | 8% |
| Neurological features | |||||
| Epilepsy | 2/8 | 25% | 68% | 11/21 | 52% |
| Abnormal muscle tone | 6/8 | 75% | 95% | NA | |
| Spasticity | 2/7 | 29% | 60% | 6/15 | 40% |
| Stereotypic movements | 6/8 | 75% | 90% | 13/15 | 87% |
| Other | |||||
| Microcephaly | 3/8 | 38% | 84% | 13/18 | 72% |
| Scoliosis | 0/8 | 0% | 40% | 4/12 | 33% |
| MRI features | |||||
| Normal | 3/7 | 43% | NA | NA | |
| Corpus callosum anomalies | 2/7 | 29% | 67% | 4/14 | 28.5% |
| Delayed myelination | 2/7 | 29% | 56% | 3/10 | 30% |
| Cortical anomalies | 2/7 | 29% | 72% | 8/12 | 67% |
| Ventriculomegaly | 2/7 | 29% | NA | NA | |
- Abbreviations: MRI, magnetic resonance imaging; NA, not available.
All cases presented with nonspecific ID, ranging from mild to severe, and distinct from the profound ID exhibited in the congenital variant of Rett syndrome. They were able to walk unassisted even if they still presented coordination and praxis difficulties. Fine motor skills were affected in all patients. Similarly, speech development was delayed but present. However, some patients had poorly developed language, sometimes with only a few spoken words, but still appropriately used. Postnatal microcephaly was present in three cases and seizures were present in only two patients who both responded well to anti-epileptic medication. Brain imaging was normal or revealed anomalies that were milder than those encountered in congenital Rett syndrome (Ariani et al., 2008; Mencarelli et al., 2010; Mitter et al., 2018). Some other clinical features typically found in congenital Rett syndrome, such as stereotypies (hands, head, and/or trunk), hypotonia, and impaired social interactions, were frequently observed in our cohort but were mostly less severe.
All patients in the cohort carried P/LP heterozygous missense FOXG1 variants, identified by ES in patients 1 and 4 and by multigene panel sequencing in patients 2, 3, and 5. Four variants were de novo, while inheritance has not been tested for the fifth. Four of the variants were located in the forkhead domain (FHD), including one in the conserved site 1 of the FHD (patient 5), and the last variant was located in the JARID1B-binding domain (JBD) (Figure 1). Patient 5 had one of the mildest phenotypes in the cohort. Among the five variants reported here, there are two novel variants that were previously not reported as pathogenic in the dbSNP (https://www.ncbi.nlm.nih.gov/snp/), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and gnomAD v3.1.2 (https://gnomad.broadinstitute.org/) databases [NM_005249.5: c.553A>G, p.(Ser185Gly) and c.647C>T, p.(Pro216Leu)]. The three remaining variants were already reported in ClinVar, but detailed clinical features were not available.
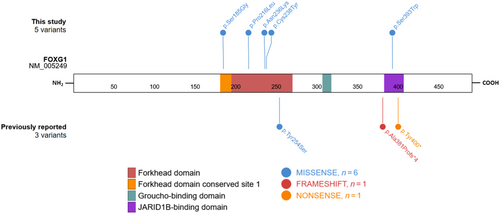
Variants previously reported in the literature and associated with a mild form of FOXG1 syndrome (Table 1) included one missense variant (p.Tyr254Ser) located in the FHD, one nonsense variant (p.Tyr400*) located in the JBD, and one frameshift variant (p.Ala381Profs*4) located just upstream from the JBD and leading to a transcription disruption at the very beginning of the sequence coding for this domain. We can therefore define, among the eight variants, two types of variations: (1) missenses in the FHD and (2) premature stop codon with preserved FHD and Groucho-binding domain but with truncated or absent JBD.
We then compared our cohort to the general cohort gathered by Mitter et al., who described different groups of patients depending on the type and position of variants (missense versus frameshift and nonsense; N-terminal versus FHD except conserved site 1 versus conserved site 1 versus C-terminal). When we compared our cohort with the patients from Mitter et al. (2018) who tended to have the least severe clinical features, that is, those carrying missense variants in the FHD (including in the conserved site 1), our cohort exhibited much milder phenotypes (Table 2).
4 DISCUSSION
Since FOXG1 was first identified as being responsible for the congenital variant of Rett syndrome in 2008 by Ariani et al., most cases have been found to present with profound psychomotor disability (Allou et al., 2012; Bahi-Buisson et al., 2010; Cellini et al., 2016; Le Guen et al., 2011; Terrone et al., 2014; Van der Aa et al., 2011). For years, targeted sequencing of FOXG1 was only prescribed in such severe cases. However, the improvement and wider use of NGS has made it possible to highlight causal genetic anomalies in patients for whom no formal diagnosis could be made from clinical evidence, which has led to the observation of milder forms of FOXG1 syndrome.
With this study, we describe a new class of patients with FOXG1 syndrome that present a phenotype that is much milder than congenital Rett syndrome. We have defined this mild phenotype as patients walking unassisted and able to communicate verbally. Though rare, several previous studies have contributed to our knowledge of mild forms of FOXG1 syndrome (De Filippis et al., 2012; Kortüm et al., 2011; Mencarelli et al., 2010). In our cohort, phenotypes and variant distribution (located within the FHD and the C-terminal part of the protein) are consistent with the observations of Mitter et al., who, in 2018, described a large cohort of patients presenting with FOXG1 syndrome and highly variable phenotype expressivity. The authors focused on the genotype–phenotype relationship observed in their cohort and suggested the delineation of the FOXG1 genotypes into five different groups. Milder phenotypes were associated with FOXG1 missense variants in the FHD, and carriers of variants in the conserved site 1 presented with the mildest phenotype. However, some patients with a missense variant in the FHD (including in the conserved site 1) still exhibited a severe phenotype, including 62% who were unable to walk unassisted and 70% who did not acquire verbal speech. Frameshift and nonsense variants located in the FHD were responsible for a more severe phenotype: none of the patients acquired walking or speech, and these variants were associated with a higher rate of brain malformations. It is worth emphasizing that the single exon structure of FOXG1 prevents abnormal mRNA containing premature stop codons from undergoing nonsense-mediated mRNA decay. Consequently, premature truncating variants in this gene most probably result in the production of truncated proteins. This mechanism likely contributes to the increased pathogenicity of nonsense and frameshift variants before or affecting the FHD, as opposed to missense variants.
C-terminal truncating variants, on the other hand, were responsible for both mild and severe presentations. Frullanti et al. (2019) also noted, in their cohort of 26 patients, that FOXG1 late truncating variants tended to result in a less severe phenotype than early truncating variants. This correlates with the review from Akol et al. (2022) in which truncating mutations in the N-terminal domain and the FHD seemingly caused more severe phenotypes than missenses and variants affecting the C-terminus. However, the authors also noted, based on the observations of Vegas et al. (2018), that some mutation hotspots were associated with highly variable features and severity, indicating a poor genotype–phenotype correlation. It is thus plausible that additional genetic and/or environmental factors modulate the clinical phenotypes observed in FOXG1-related disorders. Notably, the possibility that genetic alterations in other genes or regulatory sequences may contribute to the phenotype should be considered. This could be particularly relevant for patients who have undergone targeted sequencing of FOXG1 or a panel of genes.
Conversely, many benign or likely benign missense variants located outside the functional domains, especially in the N-terminal part of the protein, are reported in the gnomAD and ClinVar databases, indicating regions with tolerance to missenses.
Finally, our definition of the mild form of FOXG1 syndrome could have been refined, for instance regarding the number of spoken words, to reflect the variability of phenotypes encountered in this syndrome more precisely. Nevertheless, refining the definition would be challenging due to the rarity of mild forms and the fact that some patients are very young and future psychomotor progress cannot yet be established. For instance, we could not include the patient reported by Khan & Kirmani (2020), although considered by the authors as presenting a mild phenotype. The authors described a boy aged 4 years and 10 months carrying a de novo heterozygous FOXG1 variant (p.Gln86Profs*35) and presenting with postnatal microcephaly and delayed developmental milestones, but he had a neurological phenotype that was considerably less severe than usual in patients with congenital Rett syndrome. At publication, the boy could not yet walk unassisted and remained nonverbal. This example highlights the difficulties that clinicians may have in agreeing on the definition of milder forms, especially regarding severe neurodevelopmental disorders. Prospective studies of FOXG1 patients or the description of a larger cohort would be necessary to overcome these biases.
In conclusion, some patients with FOXG1 P/LP variants can walk unassisted and acquire speech, particularly in association with missense variants located in the FHD. Other determinants, such as genetic background, epigenetic landscapes, or environmental factors may also play a role and warrant further exploration. This information could be used to refine prognosis after the identification of FOXG1 variants in young children and to facilitate the interpretation of novel FOXG1 variants identified after exome/genome sequencing.
AUTHOR CONTRIBUTIONS
Benoit Mazel wrote the manuscript and was responsible for data collection. Julian Delanne, Aurore Garde, Caroline Racine, Ange-Line Bruel, Yannis Duffourd, Diego Lopergolo, Filippo Maria Santorelli, Viviana Marchi, Anna Maria Pinto, Maria Antonietta Mencarelli, Roberto Canitano, Floriana Valentino, Filomena Tiziana Papa, Chiara Fallerini, Francesca Mari, Alessandra Renieri, Arnold Munnich, Tanguy Niclass, Gwenaël Le Guyader, Christel Thauvin-Robinet, and Christophe Philippe contributed to the clinical and/or biological data collection. Laurence Faivre contributed to the clinical data collection, writing, review, and editing of the manuscript. All authors reviewed and approved the latest version of the manuscript.
ACKNOWLEDGMENTS
We thank the patients and their families for taking part in the study. Several authors are part of the European Reference Network ITHACA. The authors wish to thank Suzanne Rankin (Dijon Bourgogne University Hospital) for reviewing the English manuscript. We also thank Ricerca Corrente 5X1000 (to F.M.S.).
FUNDING INFORMATION
This work was supported by grants from Dijon University Hospital and the European Union through the FEDER programs.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




