Genetic and molecular risk factors within the newly identified primate-specific exon of the SAP97/DLG1 gene in the 3q29 schizophrenia-associated locus
Abstract
The synapse-associated protein 97/discs, large homolog 1 of Drosophila (DLG1) gene encodes synaptic scaffold PDZ proteins interacting with ionotropic glutamate receptors including the N-methyl-D-aspartate type glutamate receptor (NMDAR) that is presumed to be hypoactive in brains of patients with schizophrenia. The DLG1 gene resides in the chromosomal position 3q29, the microdeletion of which confers a 40-fold increase in the risk for schizophrenia. In the present study, we performed genetic association analyses for DLG1 gene using a Japanese cohort with 1808 schizophrenia patients and 2170 controls. We detected an association which remained significant after multiple comparison testing between schizophrenia and the single nucleotide polymorphism (SNP) rs3915512 that is located within the newly identified primate-specific exon (exon 3b) of the DLG1 gene and constitutes the exonic splicing enhancer sequence. When stratified by onset age, although it did not survive multiple comparisons, the association was observed in non-early onset schizophrenia, whose onset-age selectivity is consistent with our recent postmortem study demonstrating a decrease in the expression of the DLG1 variant in early-onset schizophrenia. Although the present study did not demonstrate the previously reported association of the SNP rs9843659 by itself, a meta-analysis revealed a significant association between DLG1 gene and schizophrenia. These findings provide a valuable clue for molecular mechanisms on how genetic variations in the primate-specific exon of the gene in the schizophrenia-associated 3q29 locus affect its regulation in the glutamate system and lead to the disease onset around a specific stage of brain development.
1 INTRODUCTION
A body of evidence has supported the view that dysfunction of the N-methyl-D-aspartate type glutamate receptor (NMDAR) may be implicated in the pathophysiology of schizophrenia (Coyle, 2012), a severe and very frequent psychiatric disorder with a prevalence of 0.87% (Perala et al., 2007). Accordingly, the single or repeated use of NMDAR antagonists, such as phencyclidine (PCP) and ketamine, stereoselectively cause symptoms indistinguishable from those of schizophrenia (Javitt & Zukin, 1991; Lahti, Weiler, Tamara Michaelidis, Parwani, & Tamminga, 2001). Moreover, patients with schizophrenia display a greater sensitivity to the schizophrenomimetics than healthy controls (Domino and Luby, 2012; Lahti et al., 2001). It should also be noted that a schizophrenia-like psychotic state has often been reported in patients with anti-NMDAR antibodies (Titulaer et al., 2013). The presumed NMDAR hypofunction appears to be linked to the mechanisms underlying the onset of schizophrenia, because the onset of schizophrenia and the psychotomimetic effects of the NMDAR antagonists and anti-NMDAR antibodies usually occur after adolescence in humans, or at a critical period of postnatal development in experimental animals (Ito et al., 2007; Reich & Silvay, 1989; Sato, Umino, Kaneda, Takigawa, & Nishikawa, 1997; Scalzo & Burge, 1994; Schwartz & Einhorn, 1986; Takebayashi, Yamamoto, Umino, & Nishikawa, 2009; Welch & Correa, 1980). These observations suggest that schizophrenia and the schizophrenomimetic effects of the drugs and antibodies depend on the functional maturation of a specific brain system during a critical stage of postnatal development. This hypothesis predicts that such a system in humans and its homologue in animals should consist of molecular cascades that differentially respond to NMDA antagonists across the critical period.
In this context, we have identified several genes from rat brain tissues that are upregulated by PCP only after the above critical period at around three postnatal weeks (Hiraoka, Kajii, Kuroda, Umino, & Nishikawa, 2010). These included Sap97 (synapse-associated protein 97)/Dlg1 (discs, large homolog one of Drosophila), which encodes synaptic scaffold PDZ proteins interacting with ionotropic glutamate receptors, including the NMDAR, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), and kainate subtypes. The human DLG1 gene resides in the chromosomal position 3q29, where multiple genome-wide analyses on copy number variations (CNVs) found an excess of microdeletions in schizophrenia (Kirov et al., 2012; Kushima et al., 2017; Levinson et al., 2011; Marshall et al., ; Mulle et al., 2010; Szatkiewicz et al., 2014). A meta-analysis demonstrated the 3q29 deletion confers a 40-fold increase in the risk for schizophrenia (Mulle, 2015). Among 22 protein-coding genes affected by the deletion, DLG1 draws the most attention because of its role in the NMDAR postsynaptic density complex and an enrichment of mutations in patients with schizophrenia (Purcell et al., 2014; Rutkowski et al., 2017). We previously demonstrated a genetic association between the DLG1 gene and schizophrenia in our two independent cohort studies (Sato, Shimazu, Yamamoto, & Nishikawa, 2008; Uezato et al., 2012), although there has been no replication study conducted by other groups to our knowledge. Consistently, the expression levels of DLG1 proteins or mRNAs were previously described to be changed in the postmortem brains of patients with schizophrenia by two independent research groups (Hammond, McCullumsmith, Funk, Haroutunian, & Meador-Woodruff, 2010; Toyooka et al., 2002).
We recently discovered an unreported splicing variant of DLG1 (see the Deposition of Accession Numbers section in the Methods) that is transcribed from the genome regions containing the newly identified primate-specific exon. We revealed that the expression of this primate-specific variant is largely dependent on a single nucleotide polymorphism (SNP) that constitutes a sequence of the exonic splicing enhancer (ESE) in the exon (Uezato et al., 2015). Furthermore, we revealed reduced mRNA expression of this variant in the postmortem prefrontal tissues of patients with an early-onset subgroup of schizophrenia, but not in patients with bipolar disorder. This might indicate that this variant constitutes molecular cascades for the functional maturation of a specific brain system hypothesized above, affecting the timing of the disease onset.
These convergent lines of evidence strongly indicate the involvement of DLG1 in the pathophysiology of schizophrenia. Specifically, the timing of onset of the disease is associated with the newly identified primate-specific DLG1 variant whose expression is dependent on a SNP. Therefore, we performed a genetic association analysis on a larger cohort taking into account the disease onset age and focusing on this SNP and others that are associated with schizophrenia.
2 METHODS
2.1 Subjects
We used a Japanese case-control sample consisting of 1808 schizophrenia patients (992 males, 816 females; mean age 49.8 ± 13.2 years) and 2170 controls (889 males, 1281 females; mean age 42.4 ± 14.2 years) that was independent from our two previous cohort studies (Sato et al., 2008; Uezato et al., 2012). All participants were recruited from the Hondo area. A genome-wide study of the Japanese population has shown that the Hondo area forms a distinct population cluster that is clearly separated from Han-Chinese and another Japanese cluster, Ryukyu (Hattori et al., 2009; Yamaguchi-Kabata et al., 2008). In addition, recruitment was mostly restricted to the Kanto district of the Hondo area, which includes Tokyo and its surrounding areas. Therefore, population stratification should be negligible. Patients with a consensual diagnosis of schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria, from at least two experienced psychiatrists, were included in the study. Patients with psychotic symptoms associated with substance use, head injuries, learning disabilities, and other symptomatic etiologies were excluded. Although we have no precise information of somatic illnesses, patients with conditions that potentially affect their mental status were excluded from the study. The control subjects were recruited from hospital staff and volunteers. From brief interviews, an expert psychiatrist confirmed that they showed no present or past evidence of psychoses. Subjects with psychiatric diagnosis or inappropriate use of drugs or alcohol were excluded. The current study was approved by the ethics committees of the Tokyo Medical and Dental University and RIKEN. All participants gave written informed consent to participate in the study.
2.2 Onset age-stratification
To investigate the effect of onset age, patients were stratified by onset ages of <18 years (early-onset schizophrenia, EOS) or 18 years or older (non-early-onset schizophrenia, non-EOS), according to the generally accepted threshold for EOS (Clemmensen, Vernal, & Steinhausen, 2012). Age of onset was determined by retrospective chart review, where it was defined as the age when patients meet the diagnostic criteria for schizophrenia. Patients with unconfirmed onset age data were excluded from the analysis.
2.3 SNP selection and genotyping
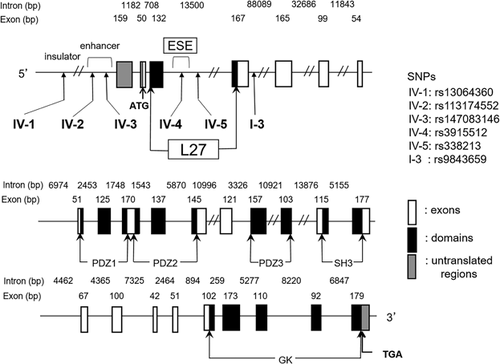
We selected a total of six SNPs (designated as SNP IV-1, -2, -3, -4, and -5 and SNP I-3) for the association analysis (Figure 1). SNP I-3 (rs9843659) was associated with male patients with schizophrenia in our previous studies (Sato et al., 2008; Uezato et al., 2012). Due to its proximity to SNP I-3 and location within the L27 domain of DLG1, SNP IV-4 (rs3915512) was selected because it determines the consensus of the exonic splicing enhancer (ESE) identified by ESEfinder, FAS-ESS, and RESCUE-ESE (http://genes.mit.edu/burgelab/rescue-ese/). We recently found that the primate-specific insertion of a 95 bp sequence (designated as exon 3b) including this SNP yields a new splicing variant of the DLG1 gene (Uezato et al., 2015). The SNP IV-5 (rs338213) was selected from between SNP IV-4 and I-3, within the L27 domain, and with high minor allele frequencies. Furthermore, to include SNPs that might affect the gene expression, the SNPs within the enhancer and insulator regions in the 10 kb up-stream of the DLG1 gene were searched using the Searching Transcription Factor Binding Sites ver 1.3 (http://www.cbrc.jp/research/db/TFSEARCH.html) and a CTCF binding site database for characterization of vertebrate genomic insulators (http://insulatordb.uthsc.edu/home.php). SNP IV-1 (rs13064360) was selected from the insulator region, and SNP IV-2 (rs113174552) and SNP IV-3 (rs147083146) from the enhancer region (Figure 1).
SNP genotyping was performed using the TaqMan system (Applied Biosystems) according to the manufacturer's instructions. PCR was performed using an ABI 9700 thermocycler and fluorescent signals were analyzed using an ABI 7900 sequence detector single point measurement and SDS v2.4 software (Applied Biosystems). To confirm the fidelity of the Taqman SNP analysis at SNP IV-4, we further performed a melt curve genotyping method called the High Resolution Melting system (HRM) with HybProbe Probes (LightCycler 480 Genotyping Master kit) and a LightCycler 480 Instrument II (Roche Diagnostics, Manheim, Germany) according to the manufacturer's protocol.
While our two previous association studies on the DLG1 gene dealt with the nucleobases on the antisense strand (Sato et al., 2008; Uezato et al., 2012), the present study investigated these on the sense strand to help uncover their functions in different sequences.
2.4 Statistical analyses
The Hardy-Weinberg equilibrium (HWE) was evaluated by Fisher's exact test using the PLINK v1.07 program (http://pngu.mgh.harvard.edu/∼ purcell/plink/) (Purcell et al., 2007). To estimate the degree of LD, two LD parameters, the standardized disequilibrium coefficient (D′) and r2, were calculated using Haploview v4.2 (http://www.broad.mit.edu/mpg/haploview/) (Barrett, Fry, Maller, & Daly, 2005). LD blocks were defined based on the D#x2032;, according to the method of Gabriel et al. (2002). The genotypic and allelic counts of the cases and controls were tested for association by Fisher's exact test using the PLINK v1.07 program.
Haplotypic association analysis was performed for the common haplotypes (frequency ≥0.05), and the individual and global haplotypic p-values were calculated using UNPHASED 3.1 (https://sites.google.com/site/fdudbridge/software/unphased-3-1) (Dudbridge, 2008). Permutation analysis was performed using the PLINK v1.07 program (10,000 permutations) for deriving the empirical significance.
For SNP I-3, a meta-analysis was performed using the Case-control and TDT Meta-Analysis Package for R-software (http://www.r-project.org/) (Nicodemus, 2008). The analysis included the case-control cohorts from two previous studies (Sato et al., 2008; Uezato et al., 2012), the current study, and case-parent trios comprising 408 parents and 204 affected children.
Moreover, we performed an association analysis between the DLG1 gene and the disease in a stratified manner according to the onset age with Fisher's exact test using the PLINK v1.07 program.
Power calculation was performed with the Genetic Association Study (GAS) Power Calculator (http://csg.sph.umich.edu/abecasis/CaTS/gas_power_calculator/index.html).
2.5 Deposition of accession numbers
The 3b(+) variants were designated Homo sapiens discs, large homolog 1 (Drosophila) (DLG1), transcript variants 6-v1 (A allele or Lys allele), and 6-v2 (T allele or Ile allele), and were deposited as AB855790 and AB855791, respectively, in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp/index-e.html).
3 RESULTS
3.1 Genotypic and allelic associations
The genomic structure of the DLG1 and SNP locations are shown in Figure 1. Preliminary genotyping of these six SNPs revealed that SNPs IV-2 and IV-3 are singleton, therefore they were excluded from the association analyses in the present study.
The genotypic and allelic distributions of each SNP in the schizophrenia patients and controls are shown in Table 1. The genotypic distribution of the SNPs showed no significant deviations from the HWE in any of the four sites in the controls. In the schizophrenia patients, significant deviations from the HWE were detected in the genotypic distributions for SNPs IV-4 and I-3. We observed genotypic (p = 0.005) and allelic associations (odds ratio (OR) = 1.15, 95% confidence interval (CI) = 1.05–1.26, p = 0.003) between SNP IV-4 and schizophrenia. The p-value remained statistically significant after permutation testing (permutation p = 0.016 and 0.009, respectively).
| HWE | genotypic count | Genotypic p (permutation) | Allelic count | MAF | OR (95%CI) | Allelic p (permutation) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP IV-1 | rs13064360 | n | A/A | A/G | G/G | A | G | |||||
| Control | 2170 | 1.000 | 0 | 29 | 2141 | 29 | 4311 | 0.007 | ||||
| SCZ-total | 1808 | 1.000 | 0 | 36 | 1772 | 0.131 (0.379) | 36 | 3580 | 0.010 | 1.49 (0.91–2.44) | 0.133 (0.349) | |
| EOS | 264 | 1.000 | 0 | 4 | 260 | 0.777 (0.994) | 4 | 524 | 0.008 | 1.13 (0.40–3.24) | 0.777 (0.993) | |
| non-EOS | 1426 | 1.000 | 0 | 32 | 1394 | 0.047 (0.157) | 32 | 2820 | 0.011 | 1.69 (1.02–2.79) | 0.048 (0.140) | |
| SNP IV-4 | rs3915512 | n | T/T | T/A | A/A | T | A | |||||
| Control | 2164 | 0.388 | 239 | 988 | 937 | 1466 | 2862 | 0.339 | ||||
| SCZ-total | 1806 | 0.010 | 223 | 893 | 690 | 0.005 (0.016) | 1339 | 2273 | 0.371 | 1.15 (1.05–1.26) | 0.003 (0.009) | |
| EOS | 263 | 0.414 | 28 | 126 | 109 | 0.802 (0.996) | 182 | 344 | 0.346 | 1.03 (0.85–1.25) | 0.733 (0.979) | |
| non-EOS | 1425 | 0.040 | 175 | 700 | 550 | 0.019 (0.064) | 1050 | 1800 | 0.368 | 1.14 (1.03–1.26) | 0.011 (0.030) | |
| SNP IV-5 | rs338213 | n | T/T | T/C | C/C | T | C | |||||
| Control | 2098 | 0.752 | 100 | 730 | 1268 | 930 | 3266 | 0.222 | ||||
| SCZ-total | 1743 | 0.075 | 85 | 660 | 998 | 0.129 (0.349) | 830 | 2656 | 0.238 | 1.10 (0.99–1.22) | 0.091 (0.241) | |
| EOS | 252 | 0.355 | 9 | 92 | 151 | 0.680 (0.971) | 110 | 394 | 0.218 | 0.98 (0.78–1.23) | 0.910 (0.999) | |
| non-EOS | 1378 | 0.182 | 69 | 519 | 790 | 0.184 (0.471) | 657 | 2099 | 0.238 | 1.10 (0.98–1.23) | 0.108 (0.277) | |
| SNP I-3 | rs9843659 | n | G/G | G/A | A/A | G | A | |||||
| Control | 2158 | 0.929 | 352 | 1042 | 764 | 1746 | 2570 | 0.405 | ||||
| SCZ-total | 1805 | 0.010 | 293 | 934 | 578 | 0.058 (0.174) | 1520 | 2090 | 0.421 | 1.07 (0.98–1.17) | 0.143 (0.365) | |
| EOS | 263 | 1.000 | 43 | 126 | 94 | 0.996 (1.000) | 212 | 314 | 0.403 | 0.99 (0.83–1.20) | 0.963 (1.000) | |
| non-EOS | 1424 | 0.014 | 226 | 739 | 459 | 0.088 (0.271) | 1191 | 1657 | 0.418 | 1.06 (0.96–1.16) | 0.259 (0.591) | |
- The results of association analysis comparing control samples with the total amount of patients (SCZ-total) and with onset-age stratified patients (EOS and non-EOS) are shown together. HWE, Hardy–Weinberg equilibrium; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval; SNP, single nucleotide polymorphism; SCZ, schizophrenia; EOS, early-onset schizophrenia (patients with an onset age of <18years); non-EOS, non-early-onset schizophrenia (patients with an onset age of 18 years or older).
- Statistically significant values are shown in bold. Note that while our two previous association studies on the DLG1 gene dealt with nucleobases on the antisense strand (Sato et al., 2008;Uezato et al., 2012), the present study investigated those on the sense strand.
There was no significant association between SNP I-3 and schizophrenia in this set of case-control samples. However, the meta-analysis for SNP I-3 using the case-control cohorts from two previous studies (Sato et al., 2008; Uezato et al., 2012), the current study, and the case-parent trios, revealed a significant association with schizophrenia (OR = 1.09, 95%CI = 1.01–1.18, p = 0.033) (Table 2).
| genotypic count | Allelic count | MAF | ||||||
|---|---|---|---|---|---|---|---|---|
| Study (SNP I-3) | rs9843659 | n | G/G | G/A | A/A | G | A | |
| Sato et al. | Control | 214 | 37 | 93 | 84 | 167 | 261 | 0.390 |
| SCZ | 229 | 49 | 111 | 69 | 209 | 249 | 0.456 | |
| Uezato et al. | Control | 393 | 65 | 172 | 156 | 302 | 484 | 0.384 |
| SCZ | 393 | 73 | 187 | 133 | 333 | 453 | 0.424 | |
| Case-parent triosa | Control | 401 | 73 | 177 | 151 | 323 | 479 | 0.403 |
| SCZ | 211 | 27 | 118 | 65 | 172 | 248 | 0.410 | |
| Current | Control | 2158 | 352 | 1042 | 764 | 1746 | 2570 | 0.405 |
| SCZ | 1805 | 293 | 934 | 578 | 1520 | 2090 | 0.421 | |
| Meta-analysis | ||||||||
| Q statistic | Statistics | OR (95%CI) | P | |||||
| 0.377 | Fixed-effects | 1.09 (1.01–1.18) | 0.033 | |||||
- Minor allele frequency; SCZ, schizophrenia; OR, odds ratio; CI, confidence interval.
- Statistically significant values are shown in bold. Note that while our two previous association studies on the DLG1 gene dealt with nucleobases on the antisense strand (Sato et al., 2008;Uezato et al., 2012), the present study investigated those on the sense strand.
- a Transmission disequilibrium test using this sample revealed allele G did not demonstrate transmission distortion (chi square = 0.049, p = 0.825, unpublished data).
3.2 Onset age-stratified analysis
Of the 1808 cases, the age of onset was not able to be determined for 118 cases because of lack of information. In the onset age-stratified analysis, SNPs IV-1 (OR = 1.69, 95%CI = 1.02–2.79, p = 0.048) and IV-4 (OR = 1.14, 95%CI = 1.03–1.26, p = 0.011) showed nominal allelic associations in the non-EOS group, and SNP IV-4 survived the multiple testing (permutation p = 0.030) (Table 1). These SNPs also showed nominal genotypic associations (p = 0.047 and 0.019, respectively). However, the significant permutation p-value for non-EOS on SNP IV-4 became non-significant if a Bonferroni correction adjusted for three tests was applied. In the EOS group, we detected no association.
Power analysis was performed for an additive model under the following parameter assumptions: prevalence of disease = 0.0087, risk allele frequency = 0.35, and ɑ = 0.05. When the genotype relative risk was set at 1.35, the power was greater than 80% to detect an association in the SCZ-total, EOS, or non-EOS. However, the power dropped to 58% for EOS when the genotype relative risk was set at 1.25.
3.3 LD block structures
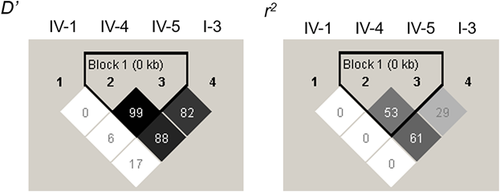
The LD block structures are shown in Figure 2. SNPs IV-4 and IV-5 were in a strong LD with each other (D′ = 0.99, r2 = 0.53). The two-SNP-based haplotype analysis is shown in Table 3. SNPs IV-1-IV-4 and SNPs IV-4-IV-5 displayed a significant global association with schizophrenia (global p = 0.017 and 0.003, respectively). SNPs IV-4-I-3, which demonstrated a high r2 (0.61) in the LD block, also displayed a significant global association (global p = 0.009). Each p-value remained statistically significant after permutation testing (permutation p = 0.007, 0.016, and 0.010, respectively).
| allele | OR (95% CI) | p | Global p | Permutation p | |
|---|---|---|---|---|---|
| IV-1 | IV-4 | ||||
| G | T | 1.13 (1.02–1.24) | 0.013 | 0.017 | 0.007 |
| G | A | 0.88 (0.80–0.97) | 0.006 | ||
| IV-4 | IV-5 | ||||
| T | T | 1.08 (0.97–1.22) | 0.160 | ||
| T | C | 1.16 (1.00–1.34) | 0.047 | 0.003 | 0.016 |
| A | C | 0.87 (0.79-.096) | 0.007 | ||
| IV-5 | I-3 | ||||
| T | G | 1.08 (0.96–1.22) | 0.163 | ||
| C | G | 1.01 (0.90–1.14) | 0.862 | 0.415 | 0.305 |
| C | A | 0.93 (0.84–1.02) | 0.146 | ||
| IV-4 | I-3 | ||||
| T | G | 1.15 (1.05–1.26) | 0.004 | ||
| A | G | 0.83 (0.70–0.97) | 0.023 | 0.009 | 0.010 |
| A | A | 0.93 (0.85–1.02) | 0.105 |
- SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval.
- The analysis was performed for common haplotypes (frequency ≥ 0.05).
- Statistically significant values are shown in bold. Note that while our two previous association studies on the DLG1 gene dealt with nucleobases on the antisense strand (Sato et al., 2008;Uezato et al., 2012), the present study investigated those on the sense strand.
4 DISCUSSION
Although the present study alone did not demonstrate the previously shown association for SNP I-3, the meta-analysis including the previous studies for this SNP revealed a significant association between the DLG1 gene and schizophrenia. Notably, the present study demonstrated that SNP IV-4 in the newly identified primate-specific exon 3b of the DLG1 gene associates with schizophrenia in the genotypic, allelic, and haplotype analyses, as well as with non-EOS in the onset age-stratified analysis.
In view of the dysregulation of the DLG1 gene function, our present data appears to be in line with the previously reported changes in the expression of the DLG1 proteins (Toyooka et al., 2002) and/or mRNAs (Hammond et al., 2010) in the postmortem brains of patients with schizophrenia. Furthermore, in agreement with the above hypothesis, we have very recently found a significant reduction in the expressional levels of the 3b(+) variant in the postmortem prefrontal cortex of patients with early-onset schizophrenia (Uezato et al., 2015).
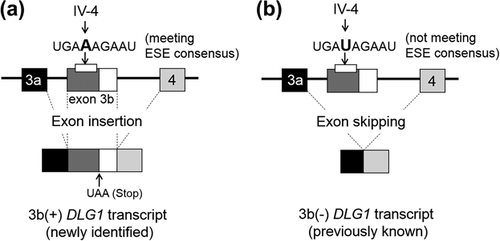
The significant deviation from the HWE observed in the genotypic distributions for SNP IV-4 is likely to be related to susceptibility and/or pathophysiology in terms of the localization of SNP IV-4 in the ESE of exon 3b, which may play an important role in controlling the expressional levels of the newly identified 3b(+) DLG1 transcript. Thus, the allele A for SNP IV-4 meets the consensus sequence for ESE (TGAAAGAAT) and should enhance the insertion of exon 3b between exons 3 and 4, which code the L27 domain (Figure 1 and Figure 3a). In contrast, the allele T for SNP IV-4 had a sequence (TGATAGAAT) that fails to fit to the ESE consensus, resulting in the preferable production of the previously known 3b(−) DLG1 transcripts by exon 3b skipping (Figure 3b). Genotype-dependent expression of 3b(+) mRNA has indeed been observed in the prefrontal cortical tissues of postmortem human brains (Uezato et al., 2015). A recent study has reported a similar association between Genome-wide association study (GWAS)-identified schizophrenia risk SNPs across the 10q24.32 locus and the expression of a novel human-specific isoform of the AS3MT gene and its potential specific molecular mechanisms (Li et al., 2016). Although the DLG1 locus 3q29 was not highlighted in the latest mega-GWAS by the Psychiatric Genomics Consortium (PGC) (Schizophrenia Working Group of the Psychiatric Genomics, 2014), the previous genome-wide CNVs studies found a significantly increased burden of rare deleterious mutations in the 3q29 locus (Kirov et al., 2012; Kushima et al., 2017; Marshall et al., ; Purcell et al., 2014; Sullivan, Daly, & O'Donovan, 2012; Szatkiewicz et al., 2014). Among the genes included in this locus, DLG1 was especially highlighted because of its role in glutamatergic postsynaptic complexes (Kirov et al., 2012; Marshall et al., ; Purcell et al., 2014; Rutkowski et al., 2017). In another study by the PGC, pathway analysis revealed that CNV burden was enriched for gene sets including DLG1 (Marshall et al., ). In this context, a recent study revealed an association of a rare mutation in DLG1 with schizophrenia, and attempted to infer a potential role of the protein with altered structure due to the mutation (Xing et al., 2016). Our study adopted a candidate gene approach. Now that accumulating evidence derived from genome-wide CNV studies have narrowed down the associated genomic region to appoint DLG1, the recursive target gene approach attempting to elucidate the effect of genomic change on its function is a reasonable methodology. Moreover, while the locus including the SRR (serine rasemase) gene in chromosome 17 is one of the 108 schizophrenia-associated loci identified by the PGC-GWAS (Schizophrenia Working Group of the Psychiatric Genomics, 2014), candidate-gene association studies have failed to demonstrate association between SRR and schizophrenia, suggesting the limitation of the traditional approach of genetic studies for multifactorial disorders including schizophrenia.
It is speculated that the presence of an in-frame stop codon in the variant 3b(+) might lead to the translation of a putative truncated DLG1 protein with an incomplete L27 domain and none of the PDZ, SH3, and GK domains. As DLG1 proteins assemble a scaffold molecular complex by interacting with themselves or other proteins through the L27 domain for regulation of ionotropic glutamate receptors (Nakagawa et al., 2004), the partial form of L27 translated from 3b(+) mRNA could play a role in modulating the assembly. For example, it is known that one of the transactivated transcriptional peptides (Aarts et al., 2002), TAT2A, works as a small interfering peptide and uncouples the NR2A subunit of NMDAR and postsynaptic density PSD95, resulting in eliminating the functional influence of NR2A on the NMDAR (Gardoni et al., 2012). In a study using rodent models with early-life stress, rats treated with TAT2A during early adolescence were protected from stress-induced loss of palvalbumin-positive interneurons and exhibited less anxious behavior (Ganguly, Holland, & Brenhouse, 2015). In a similar interfering manner, the expressed protein products of the 3b(+) might change the composition of the PSD in a cell, circuit, or brain region and affect schizophrenia onset or its timing.
Alternatively, no deduced complete domains from the splicing variant 3b(+) suggest that it might act as a non-coding RNA that may participate in the control of the transcription of 3b(−) DLG1 mRNAs. Based on our recent findings that there is a large variation in the expressional levels of 3b(+) in the postmortem prefrontal cortical tissues of individuals with psychiatric disorders and non-neuropsychiatric controls (Uezato et al., 2015), it is also possible that a regulatory nonsense-mediated mRNA decay (NMD) pathway (Nguyen, Wilkinson, & Gecz, 2014) could preserve the normal homeostasis of 3b(+) transcript expression. Consequently, the genotypes of SNP IV-4 and/or adjacent structural variations of the genome DNA could affect this homeostasis and eventually influence the composition of the PSD.
It cannot be excluded that the distinct SNP compositions indicated by the two-SNP-based haplotype analyses (Table 3) might lead to differences in certain higher order conformations of the genomic DLG1 DNA between the schizophrenia patients and control groups. The assumed structural modification in schizophrenia could cause aberrant expressions and/or functions of the DLG1 transcripts in the disorder.
GWAS failing to take into account the heterogeneity of schizophrenia might have missed loci that would be associated with a certain group of the disease, for example, early-onset schizophrenia. By an onset-stratified approach for genetic studies of schizophrenia, a sequestered group of them would show an association with a certain set of genes, as were demonstrated in early-onset Alzheimer's disease (Naj & Schellenberg, 2017) or bipolar disorder (Jamain et al., 2014; Priebe et al., 2012). A good example of the usefulness of an onset-stratified approach in the search for the genetic etiology is translational studies for Parkinson's disease (Farlow, Pankratz, Wojcieszek, & Foroud, 1993; Hatano, Kubo, Sato, & Hattori, 2009). To date, more than 10 genes have been identified as causative genes for familial Parkinson's disease. It is known that each gene is responsible for relatively distinct groups of patients differentiated by age of onset. For example, ATP13A2 is associated with patients with an onset age of 11-16 years and SNCA with an onset age of around 40 years (Hatano et al., 2009). In the onset age-stratified analysis of the present study, the non-EOS group showed a genetic association with SNP IV-4 of DLG1. The onset-age selective association is interesting to note because DLG1 mRNA expression is upregulated in the neocortex of the rats treated with a schizophrenomimetic PCP only after the critical period for the rat PCP model of schizophrenia (Hiraoka et al., 2010), indicating the involvement of DLG1 in the onset process of the disease around a specific stage of brain development. As for SNP IV-4 in our Japanese cohort, patients with the allele A that meets the ESE consensus and may facilitate the expression of mRNA with the exon 3b insertion (3b[+]) were significantly less frequent in the non-EOS group than in the control group. This assumption seems to be inconsistent with our above-mentioned observations in postmortem schizophrenia brains (Uezato et al., 2015), which demonstrated decreased 3b(+) mRNA expression in EOS, but not in non-EOS. However, several lines of evidence indicate that an ethnic population-specific variant in a specific gene has been associated with schizophrenia or other disorders (Fu, Festen, & Wijmenga, 2011; Takata et al., 2013). Indeed, according to the database of NCBI 1000 Genomes Browser Phase 3 (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/), a distinct difference in the allele frequencies for SNP IV-4 is present between the East Asian populations whose major allele is A and other ethnic populations whose major allele is T. Therefore, since our cohort for the association study comprises Japanese samples, while that for the postmortem study comprised Caucasian samples, the inconsistency might reflect the differences in the ethnic groups. Investigating postmortem samples of a Japanese cohort would help elucidate further genetic or molecular mechanisms underlying these discrepancies. While a stratified analysis with a different definition of onset age might produce other meaningful results, the present study did not examine it since there were no underpinning data.
A limitation is the uncertainty of the appropriateness of the definition of EOS. Although we defined EOS as meeting the criteria of schizophrenia before age 18 years, because this is the most commonly accepted threshold, it is not necessarily biologically driven. Furthermore, the clear discrimination between EOS and non-EOS may be limited in our cohort because retrospective determination of the onset age accompanies ambiguity confounded by prodromal symptoms. Another limitation might be a lack of power as the significant permutation p-value for non-EOS on SNP IV-4 became non-significant if a Bonferroni correction adjusted for three tests was applied (controls versus SCZ-total, EOS, and non-EOS). To estimate the number of cases needed to detect this effect, we performed a simulation with the assumptions that it was the same as that of the controls (n = 2164) and the ratio of 84.4% of the non-EOS was preserved (n = 1827), and that the minor allele frequency of 0.368 of the non-EOS was preserved. The allelic permutation p-value was calculated to be 0.018, which almost reached the significance level of the Bonferroni correction.
In conclusion, we have revealed that SNPs in the exon contributing to the differential expressions of a newly identified primate-specific variant of DLG1 displays a significant association with schizophrenia in an onset-age selective manner. Together with the localization of the DLG1 gene in a schizophrenia-associated CNV region, chromosome 3q29, and its functional link to synaptic glutamate receptors, our present findings provide a valuable clue for molecular mechanisms on how genetic variations in the DLG1 gene affect its regulation in the glutamate system and lead to the disease onset around a specific stage of brain development. Further investigation is needed in larger samples of different ethnic populations to validate this association.
ACKNOWLEDGMENTS
We thank all subjects who participated in this study. We also thank Drs. Kazuo Yamada and Eiji Hattori for recruitment of the samples. The present study is the result of “Development of biomarker candidates for social behavior” carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This manuscript was proofread by the Medical English Service. This work was supported by the grant from the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
CONFLICTS OF INTEREST
All authors declare no conflicts of interest that might be perceived as influencing authors’ objectivity in the current work.