Predictive testing of minors for Huntington's disease: The UK and Netherlands experiences
Abstract
A consistent feature of predictive testing guidelines for Huntington's disease (HD) is the recommendation not to undertake predictive tests on those < 18 years. Exceptions are made but the extent of, and reasons for, deviation from the guidelines are unknown. The UK Huntington's Prediction Consortium has collected data annually on predictive tests undertaken from the 23 UK genetic centers. DNA analysis for HD in the Netherlands is centralized in the Laboratory for Diagnostic Genome Analysis in Leiden. In the UK, 60 tests were performed on minors between 1994 and 2015 representing 0.63% of the total number of tests performed. In the Netherlands, 23 tests were performed on minors between 1997 and 2016. The majority of the tests were performed on those aged 16 and 17 years for both countries (23% and 57% for the UK, and 26% and 57% for the Netherlands). Data on the reasons for testing were identified for 36 UK and 22 Netherlands cases and included: close to the age of 18 years, pregnancy, currently in local authority care and likely to have less support available after 18 years, person never having the capacity to consent and other miscellaneous reasons. This study documents the extent of HD testing of minors in the UK and the Netherlands and suggests that, in general, the recommendation is being followed. We provide some empirical evidence as to reasons why clinicians have departed from the recommendation. We do not advise changing the recommendation but suggest that testing of minors continues to be monitored.
1 INTRODUCTION
Huntington's disease (HD) is a well-known neurodegenerative disorder with age dependent penetrance (Walker, 2007). Predictive testing for HD became available in 1986 (Meissen et al., 1988) and was initially undertaken as part of research projects; however, guidelines for predictive testing were developed and first published in 1989 and 1990 in neurology and genetics journals (Went, 1990; World Federation of Neurology: Research Group on Huntington's Chorea, 1989) and have since been updated twice (International Huntington Association (IHA) and The World Federation of Neurology (WFN) Research Group on Huntington's Chorea, 1994a,1994b; MacLeod et al., 2013). In the earlier versions the guidance was not to test before the age of majority but the latest update specifically states that “ … the minimum age of testing be 18 years. Minors at risk requesting the test should have access to genetic counseling, support and information including discussion of all their options for dealing with being at risk.” Elsewhere in that document there is a comment that the recommendations are “not intended as rigid rules but rather recommendations to guide and inform practice, based on current evidence and expertise” (MacLeod et al., 2013). The debate and recommendation not to test minors are based on theoretical concepts and long-standing clinical practice with limited empirical evidence available (Binedell, Soldan, Scourfield, & Harper, 1996; Borry, Stultiens, Nys, Cassiman, & Dierickx, 2006; Clarke, 2012; Duncan et al., 2007; Mand, Gillam, Delatycki, & Duncan, 2012; Mand, Gillam, Duncan, & Delatycki, 2013). In this report we document the extent of predictive testing for minors in the UK and the Netherlands, and discuss some of the reasons why clinicians have departed from the guidelines/recommendations.
2 METHODS
2.1 Data collection in the UK
In the UK, a Huntington's Prediction Consortium (UKHPC) was launched in 1989 to collect annually anonymized data on predictive tests for HD performed in the UK. This database has been described previously and has been used to present data on the uptake of predictive testing in the UK (Baig et al., 2016). We analyzed data from 1994 because that was the first full year for which predictive testing was available based on measurement of the CAG repeat length. Given the strong and consistent recommendation not to test those < 18 years, the few cases where this had occurred were not included in that report. We have now used the UKHPC database to report on the extent of predictive testing of minors in the UK. Some reasons for predictive testing were noted on the database but, in addition, the UK clinical genetic centers were asked to give more detail on the reasons why a test was undertaken on a minor. This request resulted in two additional cases being reported.
2.2 Data collection in the Netherlands
DNA analysis for HD in the Netherlands is centralized in the Laboratory for Diagnostic Genome Analysis (LDGA), Department of Clinical Genetics, Leiden Medical University Center, Leiden, The Netherlands. All requests for pre-symptomatic testing of minors were identified from patients who had undergone DNA analysis for HD between January 1997 and December 2016. The referring clinical geneticists were asked to give more detail on the reasons for testing.
3 RESULTS
There are 23 genetic centers in the UK but not all centers reported data for every year. In the period 1994–2015 there should have been 506 center level annual reports (22 years × 23 centers) but in fact 476 (95%) were made. Testing of minors was reported from 20 of the 23 UK genetic centers. There were 9,616 tests recorded on the database with 9,466 having an age of testing recorded. 63 tests were reported on patients < 18 years but subsequently two entries were questioned and one entry was marked as already symptomatic. The extent of predictive testing of those < 18 years in the UK is 60/9, 463 = 0.63%. An equivalent figure was not available for the Netherlands data. The male/female ratio for the UK data was 21:39. The ratio of normal to abnormal results was 38:22. Neither ratio was significantly different from that seen in the UK adults having predictive tests (Baig et al., 2016). The prior probability was 50% in 53 cases and 25% in five cases; in one case the prior probability was low because it was a re-test to confirm a previous pre-natal test result based on a linkage analysis; in a further case, the prior probability was difficult to determine from the UKHPC database.
In the data from the Netherlands, the male/female ratio was 9:14; the ratio of normal to abnormal results was 5:18. The former ratio was the same as seen in a series of adults from the Netherlands having predictive tests from 2005 to 2011 (102:141, unpublished data). The latter ratio of normal:abnormal results was significantly different from the data in adults: in a group of 107 individuals aged 18–40 years having predictive tests the ratio was 55:52 (Chi-square with Yates continuity correction = 5.56; p-value = 0.018). The prior probability of minors being tested was 50% in 21 cases and 25% in one case. In one case, the prior probability was low because of an intermediate allele in the mother.
In the Netherlands series, not only were results skewed to abnormal, but in addition, half of expanded CAG repeat lengths were in the high range (48–56 repeats), associated with younger age at onset. Similar data are not available from the UK because the actual CAG repeat length was not recorded on the UKHPC database.
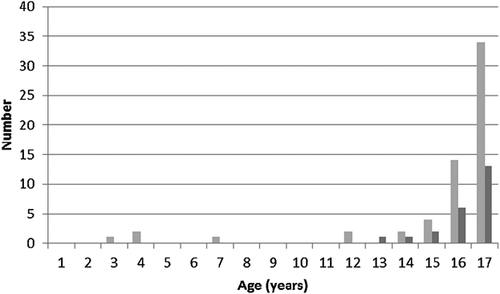
Some comments on the reasons for testing were recorded on the database but centers were also contacted to provide more detail on the reasons for the predictive test. This resulted in the description of two cases not recorded on the database. Reasons for testing 36 cases from the UK and 22 from the Netherlands are summarized in Table 1. The age structure of predictive tests undertaken on minors in the UK and the Netherlands is shown in Figure 1; it can be seen that most tests of minors were performed on those aged 16 and 17 years (23% and 57% respectively for the UK, and 26% and 57% for the Netherlands).
| Reasons for testing | Number UK n = 36 | Number the Netherlands n = 22 | Number UK and Netherlands combined |
|---|---|---|---|
| Close to age 18 years | 13 | 12 | 25 |
| Pregnancy | 5 | 1 | 6 |
| Currently in care and more support available < 18 years | 4 | 0 | 4 |
| Person never having the capacity to consent | 2 | 0 | 2 |
| Siblings in the family with young onset HD | 3 | 0 | 3 |
| Persistent requests age 15 and 16 years | 2 | 2 | 4 |
| Mother had an intermediate result | 1 | 1 | 2 |
| ? Psychiatric symptoms | 1 | 2 | 3 |
| Confirm a prenatal test result based on linkage analysis | 1 | 0 | 1 |
| Estranged father with HD, mother had mental health problems and the young person had been told he/she would die young | 1 | 0 | 1 |
| Tested posthumously because mother wanted to make sense of the death which was due to another illness | 1 | 0 | 1 |
| Tested at the same time as mother | 1 | 0 | 1 |
| Diagnostic test but result was normal | 1 | 0 | 1 |
| Tested together with siblings > 18 years (family wanted to go through the process as one) | 0 | 3 | 3 |
| Testing procedure not according to protocol (persistent request from minor was granted by the child neurologist, results given by clinical geneticist) | 0 | 1 | 1 |
One of the additional cases reported from the UK was a boy with a maternal family history of HD and paternal family history of seizures. He had an unremarkable birth history but developed neonatal seizures at 5 days. By the age of 4 years he had evidence of developmental delay and intention tremor. An MRI scan showed normal basal ganglia but a small cerebellum was questioned. He had brisk lower limb reflexes and by the age of 5 years juvenile onset HD was considered. He had a diagnostic test for HD and the result was normal.
4 DISCUSSION
The current recommendation advising against predictive tests of minors (under 18 years) for HD is based on theoretical concerns, long-standing clinical practice, and expert opinion of experienced practitioners (MacLeod et al., 2013). We have provided some empirical evidence for the extent of predictive testing of minors in the UK. In both the UK and the Netherlands, the most common reason for testing a minor was that the young person was close to 18 years and counseling sessions had been undertaken.
Testing was also performed in a small number of cases in relation to a pregnancy. Discussion of options when a person at 50% risk of HD (or his partner) presents already pregnant is challenging; perhaps more so if the person is under 18 years. One issue for discussion is attitudes toward pre-natal testing and termination of pregnancy. If predictive testing is considered prior to pre-natal testing then the time-scale for the counseling process is reduced and this recognized as an exception in the current guidelines (MacLeod et al., 2013). If the person is under 18 years then this departs from the guidelines in two respects. There are other options for managing a pregnancy including exclusion testing or direct pre-natal testing in the full knowledge that there is a one in four chance that the at-risk person and fetus have inherited the HD mutation. Difficulties can arise if individuals change their minds after a predictive test and decide not to have a pre-natal test, or if they decide not to go ahead with a termination of pregnancy after having a pre-natal test with an adverse result. However, these points are outside the scope of this paper.
One small category of cases relates to a young person in foster care, or some other form of institutional care, when more support may be available to them as a minor rather than waiting until after 18 years when there might be less support available. The counselor has to be as confident as possible that the request is coming from the young person and that the implications for the future are understood. It has to be acknowledged that it is impossible to know if the young person would still request predictive testing after having left the “care” environment. We also noted cases where the young person had another condition (Down syndrome or severe autism) which meant that the ability to consent was impaired, and was expected to remain so irrespective of the age of testing; therefore, the basis for the testing was an assessment of the best interests of the young person in terms of decisions to be made regarding planning for future care. In both cases the mother gave consent. An unusual scenario which occurred more than once was that juvenile HD had occurred in the family and testing of a sibling was undertaken < 18 years. Each such case has to be assessed individually.
Another scenario which occurred more than once was that all siblings in a sibship requested to be tested pre-symptomatically at the same time, including one or two minors. Repeated attempts to exclude the minors from such “group based” testing were made but this led to strong feelings of isolation and not being part of the family dynamics. For the sake of the family system, predictive testing was undertaken in those cases after a careful counseling procedure. Testing sibships in these circumstances is controversial and we would not want this to set a precedent.
We have reported one diagnostic test performed because the possibility of juvenile HD was raised. It was not reported to the database because it had been performed as a potentially diagnostic test but it illustrates that challenging scenarios occasionally arise in at-risk patients with neurological or behavioral problems. Such a “diagnostic” test could be predictive, and difficult to interpret, if the CAG repeat expansion was not in the range clearly associated with juvenile onset disease. For this reason, there are recommendations that if a child has exclusively behavioral problems, cognitive (and imaging) studies should be performed and repeated to assess whether there has been decline in function (Nance, 2009; Quarrell et al., 2013).
In several files from the Netherlands cases it was noted that the parent was young when he or she developed symptoms of HD and this may, in part, explain the reason for the higher CAG repeat lengths of the abnormal results. Given the early age of onset in their parent, offspring may feel a greater urge to know their own status at a younger age than offspring of a parent whose onset was in their mid-forties or later. They (or their caregivers) may also experience subtle changes in behavior and executive functions at a younger age, and consequently question whether such changes represent the onset of symptoms at a younger age. This was the case in two minors in the Netherlands series, who presented behavioral problems at the time of their request.
In one case the testing procedure departed from the protocol as the request came from a paediatric neurologist. The laboratory noticed this only after DNA analysis had been completed. After consultation, the results were sent to a clinical geneticist, who reported the results at the first contact with the family. In order to prevent recurrence of this difficult situation, the Netherlands laboratory now keeps strictly to the rule that pre-symptomatic testing can only be ordered by a clinical geneticist and the reason for testing is checked with the referring clinician for all requests regarding minors (including apparently diagnostic ones).
The relatively small number of tests which have been undertaken, and the fact that the majority were performed on those aged 17 years, suggests that the guideline is generally being observed. The current recommendation acknowledges that there was controversy surrounding the age cut-off at 18 years with some authors opting for a less restrictive recommendation. It was deemed necessary in the absence of evidence of harms or benefits of testing minors (MacLeod et al., 2013). In these circumstances, one may always argue that a person aged 17.5 years may be as mature as someone aged 18 years. Some have argued that when a young person presents for a predictive test there should be an assessment of the young person's maturity (Binedell et al., 1996). Unless the recommendation is changed, testing at 17 years should still be considered an exception. It should be noted that both in the UK and the Netherlands a young person at age 16 years can be presumed to have the capacity to consent (Artikel 447 Wet Geneeskundige Behandelingsovereenkomst (WGBO), 1995; General Medical Council, 2007). A significant weakness of this study is that we have no information on the longer term outcomes of the testing. Recording of cases by the UKHPC is voluntary, with a high reporting rate, but we cannot be sure that all cases in the UK are submitted. This information was extracted from a large data set and the accompanying notes on the reasons for testing are frequently brief; there are no notes to indicate why the very young children were tested. Some of the reasons for testing a minor which have been reported here may be more controversial than others; for example, testing the sibling of a case of juvenile HD. The controversy being that the young person should be deciding for themselves; but without more detail, it is difficult to comment.
In conclusion, we do not advocate that the guidelines are changed but rather that the practice of testing minors continues to be assessed carefully by genetic counselors and clinical geneticists on a case by case basis. The reasons for testing outside the recommendation should be documented carefully and we suggest that a decision to deviate from the recommendation is not left with a counselor and/or a clinician but should, where possible, be discussed more widely with other professionals or in a multidisciplinary team. Wherever possible, counselors and clinicians should follow up such cases to assess whether harm has been done so that more empirical evidence may be gathered. The practice of laboratories accepting referrals for predictive tests and apparently diagnostic tests for HD on minors only in conjunction with clinical geneticists or genetic counsellors appears reasonable.