Association study of NDST3 gene for schizophrenia, bipolar disorder, major depressive disorder in the Han Chinese population
Abstract
The NDST3 gene at 4q26 was a functional candidate gene for mental disorders. Recently, a novel genome-wide significant risk locus at chromosome 4q26 was identified and the top single nucleotide polymorphism rs11098403 in the vicinity of NDST3 gene was reported to confer risk of schizophrenia in Caucasian. Nevertheless, association between NDST3 gene polymorphisms and schizophrenia, bipolar disorder, or major depressive disorders has not been well studied in the Han Chinese population. To further investigate whether NDST3 is a risk gene for these mental disorders, we genotyped and analyzed eight tag SNPs (rs11098403, rs10857057, rs2389521, rs4833564, rs6837896, rs7689157, rs3817274, rs609512) covering NDST3 gene in 1,248 schizophrenia cases, 1,056 major depression cases, 1,344 bipolar disorder cases, and 1,248 controls of Chinese origin. However, there was no significant difference in allelic or genotypic frequency observed between each case group and healthy controls. Accordingly, our study does not support that the NDST3 gene plays a major role in schizophrenia, bipolar disorder, and major depressive disorder in the Han Chinese population.
1 INTRODUCTION
Schizophrenia (SCZ), bipolar disorder (BPD), and major depressive disorder (MDD) are three general disorders in the Psychiatric Genomics Consortium with relatively high morbidity and heritability. Schizophrenia (SCZ) is a severe psychiatric disorder characterized by delusions, hallucinations, or cognitive dysfunctions with heritability estimated at up to 80% (Cardno and Gottesman, 2000). Bipolar disorder (BPD) is another severe mental disease and manifested with commutative mania and depressive episodes as its psychotic features (Lee et al., 2011). Major depressive disorder (MDD) is a frequently recurrent mood disorder which its major clinical symptoms are sorted to low mood, inability to concentrate, and loss of interest (Lee et al., 2012). Moreover, MDD is the primary cause resulting in suicides and has high lifetime prevalence in Han Chinese population (Lee et al., 2012). However, compared with virtually all other relatively common disorders, the pathogenesis of these mental disorders remains undetermined (Insel, 2010). Recent studies have suggested that individual gene associated with complex diseases may reveal underlying biological pathways (Purcell et al., 2009; Shi et al., 2009), and demonstrated that genetic factors play a crucial role in the disorder etiology (Tandon et al., 2008). Notably, recent genome-wide association studies have revealed a lot of common variations and rare copy-number variations contributing to the risk of mental disorders, and also uncovered overlapping genetic risk factors shared across these diseases (Berrettini, 2003; Tsuang et al., 2004).
N-deacetylase/N-sulfotransferase (heparin glucosaminyl) 3(NDST3) gene is a member of the NDST1-NDST4 family which encodes NDST isozymes (Aikawa et al., 2001; Eriksson et al., 1994; Hashimoto et al., 1992; Kusche-Gullberg et al., 1998). The glucosaminyl N-deacetylase/N-sulfotransferase (NDST) enzymes catalyze N-deacetylation and N-sulfation during HS biosynthesis and have a key role in designing the sulfation pattern (Filipek-Górniok et al., 2015). NDST is a bifunctional enzyme, which removes acetyl groups from GlcNAc residues and replaces them with sulfate groups (Filipek-Górniok et al., 2015). NDST1 and NDST2 are expressed ubiquitously in both embryonic and adult mice, whereas NDST3 and NDST4 are mostly expressed during embryonic development (Ford-Perriss et al., 2002; Grobe et al., 2002). It has been confirmed that NDST2-/- mice has abnormal mast cells without properly sulfated heparin and mast-cell proteases and disruption of NDST1 results in severe malformations in lung, brain, cranial facial, lens, vascular, skeletal, and lacrimal gland during embryonic development (Abramsson et al., 2007; Forsberg et al., 1999; Hollnagel et al., 1999). However, there are very limited reports focusing on the function of the NDST3 gene (Grobe et al., 2002). Recently, the NDST3 gene was designated as the chromosome 4q26 target which was identified as a novel genome-wide significant risk locus of schizophrenia (Lencz et al., 2013). It encodes a kind of enzyme critical to heparin sulphate metabolism, while heparan sulfate binding plays a pivotal role in neurite outgrowth, axon formation, and synaptic processes which are thought to be aberrant among these mental disorders (Lencz et al., 2013).
Lencz et al. (2013) have indicated the NDST3 rs11098403 was significantly associated with schizophrenia and bipolar disorder in Caucasians, but it failed to observe this association in Asian population. Zhang et al. (2016) has identified an association of rs11098403 with schizophrenia in Han Chinese. Nevertheless, no significant association between NDST3 and bipolar disorder was observed in the Han Chinese samples (Zhang et al., 2016). Moreover, there has been no genetic study to research whether the common variants in the NDST3 gene confer risk of another severe mental disease major depressive disorder (MDD) up to now. Considering the current situation of studies of the NDST3 gene, we performed this study to replicate the association of rs1109803 with schizophrenia in Han Chinese samples and further investigate the association between NDST3 variants and bipolar disorder, major depressive disorder as well as schizophrenia in the Han Chinese population.
2 MATERIALS AND METHODS
2.1 Subjects
The subjects in this study were Han Chinese origin samples recruited from Shanghai. Our study was reviewed and approved by the Institutional Ethical Committee of Human Genetics Resources in Shanghai Jiao Tong University. A total of 4,896 unrelated participants were collected in our sample set, including 1,248 unrelated schizophrenic patients, 1,056 unrelated major depressive disorder patients, 1,344 unrelated bipolar disorder patients, and 1,248 health controls. The sex ratio and mean age were described in detail in Table 1. Each patient has been interviewed by at least two board-certified psychiatrists before entering the group. We consulted the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition) to make the inclusion criteria. The controls were recruited from the local community residents by means of announcements on bulletin boards. All controls were in good health and randomly selected from the general Han Chinese population. Before collecting blood samples, the healthy controls were also screened by psychiatrists. Health controls with a family history of psychiatric disorders or with a severe medical illness were already excluded. The objectives and procedures in our study were described to the participants or their representatives. The informed consents were also signed.
| n | Age (years) | ||||
|---|---|---|---|---|---|
| Males | Females | In total | Mean age | Age deviation | |
| Schizophrenia | 845 | 403 | 1,248 | 36.44 | 9.00 |
| BPD | 584 | 760 | 1,344 | 34.84 | 11.44 |
| MDD | 737 | 319 | 1,056 | 34.41 | 12.09 |
| Healthy controls | 672 | 576 | 1,248 | 30.62 | 11.35 |
- BPD, bipolar disorder; MDD, major depressive disorder.
2.2 Genotyping
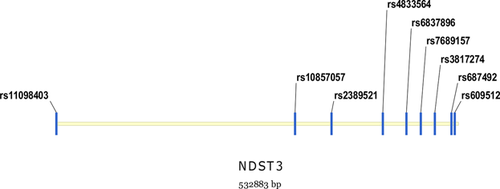
We collected subjects’ peripheral blood samples and used the Quick Gene DNA whole blood kit (Tokyo, Japan) to extract the genomic DNA. Then, 9 tag SNPs (rs11098403, rs10857057, rs2389521, rs4833564, rs6837896, rs7689157, rs3817274, rs687492, rs609512) in the NDST3 gene (532883 bp) were selected to be genotype on the DNA samples. During the SNPs selection, we referred to the genotype data of Han Chinese in Beijing, China (CHB) of the International HapMap Project (http://hapmap.ncbi.nlm.nih.gov) and set linkage disequilibrium r2 threshold at 0.5 on Haploview 4.2 software. Among the SNPs, rs11098403 is a 5′untranslated region variant and the other eight SNPs are all located in the intron region. Detailed information of the nine sites is listed in Table 2, and their relative locations are illustrated in Figure 1. The Mysequenom online software (https://www.mysequenom.com/Tools) was used to design all the probes and primers involved in the polymerase chain reaction (PCR). The genotyping procedure was completely identical to the normal method of our group as described in a previous publication (Wen et al., 2016). We selected the matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (Sequenom, San Diego, CA) to analyze the mass spectrum.
| Marker | Position | Function | Polymorphism | |
|---|---|---|---|---|
| rs11098403 | chr 4:118646907 | 5′ Near gene | A/G | |
| rs10857057 | chr 4:118963085 | Intron | A/G | |
| rs2389521 | chr 4:119011443 | Intron | C/T | |
| rs4833564 | chr 4:119079639 | Intron | A/G | |
| rs6837896 | chr 4:119110853 | Intron | A/C | |
| rs7689157 | chr 4:119129669 | Intron | G/T | |
| rs3817274 | chr 4:119148346 | Intron | C/G | |
| rs687492 | chr 4:119170256 | Intron | C/T | |
| rs609512 | chr 4:119174563 | Intron | A/T |
- SNP, single nucleotide polymorphism.
2.3 Statistical analysis
Statistical analysis was used on the SHEsisPlus online software platform (http://shesisplus.bio-x.cn/SHEsis.html). It was developed by our research group and the operation method was described in Shen et al.'s study in 2016 (Shen et al., 2016). Hardy–Weinberg equilibrium and single site association including allele and genotype frequency distributions were analyzed. Odds ratios and their 95% confidence intervals (CIs) were obtained in this study. The χ2 test for independence was adapted to check the discrepancies of allele and genotype frequency between cases and controls, and χ2 test for goodness of fit was used to test the Hardy–Weinberg equilibrium in each group. For all analyses, we set significant level at p < 0.05 and adapted two tailed p values.
2.4 Meta-analysis
Meta-analysis was conducted using Review Manager 5.3 software. The I2 test was used to estimate the percentage of variability heterogeneity across combined studies and ensure that each group of studies was suitable for meta-analysis. If the result of the heterogeneity test was p > 0.05, the ORs were pooled according to the fixed-effects model (Mantel–Haenszel methods); otherwise, the random-effects model was used. An odds ratio (OR) with 95% confidence interval (CI) represented the effect size. The Z-score test was used to generate the p values of overall OR. The significance level was set at 0.05. All p values were reported as two-tailed tests.
3 RESULTS
3.1 Single marker association analysis
Among the sites, rs687492 was excluded from further analysis because it deviated from Hardy–Weinberg (H–W) equilibrium in healthy controls, the significance threshold being p < 0.05. For the other eight SNPs, the calculated allelic and genotypic distributions showed no significant deviation from Hardy–Weinberg equilibrium in the control group (p > 0.05). The distribution of allele and genotype frequencies for the NDST3 polymorphisms in SCZ cases, BPD cases, MDD cases, and controls were summarized in Table 3. According to association study, there was no significant difference (p > 0.05 before Bonferroni correction) in the frequencies of the allele or genotype for all the eight SNPs between control subjects and schizophrenia cases. Moreover, all the eight SNPs showed no allelic or genotypic significance with bipolar disorder (p > 0.05 before Bonferroni correction). For major depressive disorder, the eight SNPs were not statistically significant in both allele and genotype distributions before Bonferroni correction.
| SNPa ID | Allele frequency | Odds ratio | 95%CIb | Allelic p-value | Genotype frequency | Genotypic p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| rs11098403 | G | GG | GA | AA | |||||
| Schizophrenia | 379 (0.153) | 1.026 | 0.879 ∼ 1.199 | 0.738 | 29 (0.023) | 321 (0.259) | 888 (0.717) | 0.588 | |
| BPD | 402 (0.150) | 1.006 | 0.863 ∼ 1.172 | 0.938 | 30 (0.022) | 342 (0.255) | 964 (0.721) | 0.635 | |
| MDD | 274 (0.133) | 0.878 | 0.742 ∼ 1.039 | 0.130 | 34 (0.033) | 278 (0.270) | 719 (0.697) | 0.287 | |
| Control | 373 (0.149) | 22 (0.017) | 329 (0.264) | 895 (0.718) | |||||
| rs10857057 | A | AA | AG | GG | |||||
| Schizophrenia | 671 (0.273) | 0.992 | 0.875 ∼ 1.124 | 0.906 | 90 (0.073) | 491 (0.399) | 647 (0.526) | 0.967 | |
| BPD | 733 (0.274) | 0.999 | 0.884 ∼ 1.129 | 0.989 | 95 (0.071) | 543 (0.406) | 697 (0.522) | 0.991 | |
| MDD | 529 (0.263) | 0.946 | 0.829 ∼ 1.081 | 0.420 | 75 (0.074) | 379 (0.378) | 548 (0.546) | 0.438 | |
| Control | 684 (0.274) | 90 (0.072) | 504 (0.404) | 651 (0.522) | |||||
| rs2389521 | T | TT | TC | CC | |||||
| SCZ | 492 (0.199) | 0.962 | 0.838 ∼ 1.105 | 0.592 | 53 (0.042) | 386 (0.312) | 795 (0.644) | 0.821 | |
| BPD | 546 (0.204) | 0.992 | 0.866 ∼ 1.135 | 0.910 | 54 (0.040) | 438 (0.327) | 845 (0.632) | 0.924 | |
| MDD | 394 (0.195) | 0.940 | 0.812 ∼ 1.089 | 0.413 | 39 (0.038) | 316 (0.313) | 652 (0.647) | 0.714 | |
| Control | 512 (0.205) | 54 (0.043) | 404 (0.324) | 788 (0.632) | |||||
| rs4833564 | A | AA | AG | GG | |||||
| SCZ | 618 (0.251) | 0.992 | 0.871 ∼ 1.129 | 0.903 | 82 (0.066) | 454 (0.368) | 695 (0.564) | 0.894 | |
| BPD | 687 (0.257) | 1.024 | 0.902 ∼ 1.162 | 0.708 | 95 (0.071) | 497 (0.372) | 744 (0.556) | 0.763 | |
| MDD | 498 (0.242) | 0.949 | 0.828 ∼ 1.089 | 0.460 | 67 (0.065) | 364 (0.355) | 594 (0.579) | 0.556 | |
| Control | 601 (0.252) | 76 (0.063) | 449 (0.377) | 665 (0.558) | |||||
| rs6837896 | C | CC | CA | AA | |||||
| SCZ | 470 (0.195) | 0.970 | 0.842 ∼ 1.116 | 0.673 | 49 (0.040) | 372 (0.310) | 779 (0.649) | 0.814 | |
| BPD | 529 (0.199) | 0.991 | 0.864 ∼ 1.137 | 0.905 | 51 (0.038) | 427 (0.321) | 849 (0.639) | 0.986 | |
| MDD | 377 (0.181) | 0.880 | 0.759 ∼ 1.022 | 0.094 | 45 (0.043) | 287 (0.275) | 709 (0.681) | 0.055 | |
| Control | 496 (0.200) | 49 (0.039) | 398 (0.322) | 789 (0.638) | |||||
| rs7689157 | G | GG | GT | TT | |||||
| SCZ | 974 (0.407) | 1.014 | 0.904 ∼ 1.137 | 0.805 | 223 (0.186) | 528 (0.441) | 445 (0.372) | 0.945 | |
| BPD | 1,136 (0.425) | 1.095 | 0.980 ∼ 1.223 | 0.108 | 269 (0.201) | 598 (0.448) | 467 (0.350) | 0.300 | |
| MDD | 827 (0.397) | 0.974 | 0.865 ∼ 1.098 | 0.674 | 173 (0.166) | 481 (0.462) | 386 (0.371) | 0.573 | |
| Control | 998 (0.403) | 224 (0.181) | 550 (0.444) | 462 (0.373) | |||||
| rs3817274 | G | GG | GC | CC | |||||
| SCZ | 112 (0.045) | 1.022 | 0.779 ∼ 1.341 | 0.872 | 2 (0.001) | 108 (0.087) | 1,124 (0.91) | 0.983 | |
| BPD | 141 (0.052) | 1.195 | 0.923 ∼ 1.547 | 0.174 | 1 (0.0007) | 139 (0.103) | 1,199 (0.895) | 0.235 | |
| MDD | 103 (0.050) | 1.137 | 0.861 ∼ 1.502 | 0.361 | 1 (0.001) | 101 (0.098) | 923 (0.900) | 0.517 | |
| Control | 106 (0.044) | 2 (0.001) | 102 (0.085) | 1,089 (0.912) | |||||
| rs609512 | A | AA | AT | TT | |||||
| SCZ | 449 (0.185) | 0.953 | 0.869 ∼ 1.159 | 1.004 | 45 (0.037) | 359 (0.296) | 807 (0.666) | 0.654 | |
| BPD | 514 (0.192) | 1.052 | 0.914 ∼ 1.209 | 0.475 | 41 (0.030)) | 432 (0.323) | 862 (0.645) | 0.657 | |
| MDD | 379 (0.189) | 1.029 | 0.885 ∼ 1.196 | 0.707 | 43 (0.042) | 293 (0.292) | 666 (0.664) | 0.296 | |
| Control | 460 (0.184) | 39 (0.031) | 382 (0.306) | 824 (0.661) | |||||
- SCZ, schizophrenia; BPD, bipolar disorder; MDD, major depressive disorder.
- a SNP, single nucleotide polymorphism.
- b CI, confidence interval.
3.2 Meta-analysis
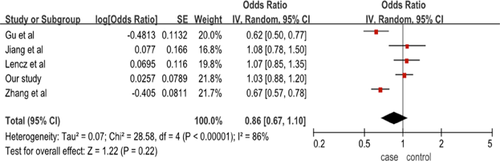
Recent association studies evaluating the polymorphisms of NDST3 and schizophrenia were searched. We recruited four studies focusing on schizophrenia in which SNPs examined overlapped with ours in the meta-analysis. After further scrutinize for studies performed in the Asian population and the Han Chinese population, rs11098403 was fit into the meta-analysis. The characteristics of the studies included in our meta-analysis were listed in supplemental Table S1. After pooling all data from previous and the present data, we found that there was no evidence of significant association between rs11098403 and schizophrenia yielded in the meta-analysis (p = 0.22, OR = 0.86[95%CI = 0.67–1.10]), which further supported our own results in the Han Chinese population (Figure 2).
4 DISCUSSION
In this study, we investigated the association between the SNPs in NDST3 gene and SCZ, BPD, and MDD in the Han Chinese population. As of now, large scale association studies have already found many complex diseases susceptible risk alleles (Ripke et al., 2014). However, risk polymorphisms identified in one population are not always reproducible in another population. That may be an inevitable rising problem in genetic studies. There have been some variants obtained in European samples, while they could not be largely validated in Asian populations as another ancestry. Many of the risk associations are population specific. In 2013, a genome-wide association study first demonstrated that NDST3 gene located in chromosome 4q26 might be implicated in major mental disorders. A novel susceptibility locus rs11098403 located in the vicinity of NDST3 was indicated to be associated with SCZ in an Ashkenazi Jewish population by the large scale genome wide association study (Lencz et al., 2013). However, the significant association was not replicated in the Japanese ancestry samples. Recently, two independent small replication studies in Chinese samples have confirmed a significant association between rs11098403 and schizophrenia risk in Han Chinese (Gu et al., 2014; Zhang et al., 2016). Meanwhile, the OR of the G allele of rs11098403 for schizophrenia in the Caucasian population and Han Chinese were 1.41 (p = 6.55 × 10−9) and 0.70 (p < 0.001) (Lencz et al., 2013; Zhang et al., 2016). It signified that rs11098403 G allele is associated with increased risk of schizophrenia in the Caucasian population and decreased risk in the Han Chinese population. In Jiang et al's study, there was negative association found between rs11098403 and SCZ in an independent Chinese samples from Kunming (Xiao et al., 2016). In our results, we observed no significant difference in either allelic or genotypic frequency of the eight SNPs between cases and normal controls in SCZ (p > 0.05). Our meta-analysis further confirmed our results by pooling our sample with these previous studies.
Schizophrenia, bipolar disorder, and major depressive disorder share many core manifestations including cognitive dysfunction, affective disturbance, psychosis, and suicidality. Comorbidity between schizophrenia and bipolar disorder, as well as bipolar disorder and major depressive disorder was found in family studies (Stefansson et al., 2003; Valles et al., 2000). It is therefore of great interest to investigate the shared risk effect by NDST3 for the three disorders. In 2013, PGC (Psychiatric Genomics Consortium) identified cross-disorder effects of a broader set of genome-wide significant loci for five psychiatric disorders. In the large scale genome wide association study in the Caucasian population, it was reported that the significance of NDST3 across BD and SCZ (Lencz et al., 2013). However, Zhang et al. (2016) failed to observe the significant frequency differences of rs11098403 between the BD and controls in the Han Chinese population, which was in accordance with the previous GWAS results gained in a Taiwan Han Chinese population (Lee et al., 2011). Similarly, we observed no significant difference in individual SNP marker genotypes or allele distributions between our BD and control samples. Therefore in the current study, it showed that NDST3 was not positively associated with BD in the Han Chinese population. The difference in study ethnic lines was an important course, but the high prevalence of psychotic symptoms existing in BD patients could not be ignored, which could yield greater power in genetic studies when investigating BD by subtype category (Zhang et al., 2016). As a promising susceptibility gene for major mental disorders, there has been no study to research the association between NDST3 and major depressive disorder so far. It is the first time to investigate NDST3 with MDD in our study, which nine SNPs included rs11098403 were selected. Our results did not found significant association between NDST3 and MDD in the Han Chinese population.
It is crucial to recognize shared genetic risk components across different races while it could be in favor of exploring the pathogenesis mechanism and discovery of new therapeutic and drug targets. In Xiao's meta-analysis, 19 SNPs were showed genome-wide significant associations with SCZ in populations of European ancestry, among them nine SNPs were positive in Asian populations (Xiao et al., 2016). As for complex diseases genetic association analysis, the age, gender, or geography might probably give rise to the non-significant result. And this observed heterogeneity was also due to other exogenous factors such as culture, lifestyle, or environmental exposure specific to different populations (Xiao et al., 2016). The effect of single variant could be restricted by the polygenic pathogenesis and heritability of psychiatric disorders and the limited testing sample size could account for the discrepancy between different studies of the same population. Further investigations with a larger sample size and strict study design are required for a better understanding of the relationship between NDST3 and MDD, BD, as well as SCZ.
Taken together, we conducted an analysis to validate the association between NDST3 polymorphisms and SCZ, BD, and MDD in the Han Chinese population. In the present study, no positive association between NDST3 gene and the three major mental disorders was detected in the current sample set. Our results for BD were in line with all current association results published in Han Chinese population (Lee et al., 2011; Zhang et al., 2016); our findings for SCZ was consistent with the previous Japanese reports and Jiang et al's study (Lencz et al., 2013; Xiao et al., 2016; Xiao et al., 2016). More studies with participants of larger sample size and different ethnic groups may be worthwhile to further verification.
ACKNOWLEDGMENTS
We appreciate Li Ming's group from Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology to provide us the unpublished data in Xiao's study. We thank for the contribution of the members participating in this study, as well as the psychiatrists who helped us with diagnosis. This work is supported by the 973 Program (2010CB529600), the National 863 project (2012AA02A515, 2012AA021802), the national Key R&D Program-Special Project on Precision Medicine(2016YFC0903402),the Natural Science Foundation of China (81121001, 81171271, 81421061, 81501154), the Program of Shanghai Academic Research Leader (15XD1502200), the Foundation for the Author of National Excellent Doctoral Dissertation of China (201026), Shanghai Key Laboratory of Psychotic Disorders (13dz2260500), the Research Project of Shanghai Health and Family Planning Commission (201440552), Interdisciplinary Program of Shanghai Jiao Tong University (YG2014QN13), the National Science Foundation of China(21375139,31571012).