Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk
Abstract
Schizophrenia is one of the most severe psychiatric disorders, with a high heritability of up to 80%. Several studies have reported telomere dysfunction in schizophrenia, and common variants in the telomerase reverse transcriptase (TERT) gene. TERT is a key component of the telomerase complex that maintains telomere length by addition of telomere repeats to telomere ends, and has repeatedly shown association with mean lymphocyte telomere length (LTL). Thus, we hypothesized that TERT may be a novel susceptibility gene for schizophrenia. Using a Taqman protocol, we genotyped eight tag SNPs from the TERT locus in 1,072 patients with paranoid schizophrenia and 1,284 control subjects from a Chinese Han population. We also measured mean LTL in 98 cases and 109 controls using a quantitative PCR-based technique. Chi-square tests showed that two SNPs, rs2075786 (P = 0.0009, OR = 0.76, 95%CI = 0.65–0.90) and rs4975605 (P = 0.0026, OR = 0.73, 95%CI = 0.60–0.90), were associated with a protective effect, while rs10069690 was associated with risk of paranoid schizophrenia (P = 0.0044, OR = 1.23, 95%CI = 1.07–1.42). Additionally, the rs2736118-rs2075786 haplotype showed significant association with paranoid schizophrenia (P = 0.0013). Moreover, mean LTL correlated with rs2075786 genotypes was significantly shorter in the patient group than the control group. The present results suggest that the TERT gene may be a novel candidate involved in the development of paranoid schizophrenia. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Schizophrenia affects approximately 1% of the general population worldwide and is associated with substantial mortality as well as personal and societal costs [Knapp et al., 2004]. Family, twin, and adoption studies consistently demonstrate a contribution of genetic factors (>80%) and environmental influences to the etiology of schizophrenia [Sullivan et al., 2003]. Paranoid schizophrenia is the most common subtype of the disease and has a stable clinical feature.
Traditional linkage analysis and candidate gene studies, as well as genome-wide association studies (GWAS), have identified a number of genetic risk variants [Shi et al., 2011; Rietschel et al., 2012]. Recently, the Schizophrenia Working Group of the Psychiatric Genomics Consortium performed a single, systematic analysis of the largest number of samples to date, and identified 108 Loci that exceeded genome-wide significance [Schizophrenia Working Group of the Psychiatric Genomics, 2014]. Nevertheless, this is not enough to unequivocally ascertain the genetic architecture of schizophrenia, and many more susceptibility genes of relatively small effect remain to be identified.
In humans, chromosomal telomeres consisting of simple tandem hexameric nucleotide repeats (TTAGGG) and associated proteins [Moyzis et al., 1988] form the ends of linear chromosomes, protecting chromosomal termini from the loss of genetic material, and end-to-end fusion, which is crucial for maintaining chromosomal integrity. Several lines of evidence have implicated abnormal telomere length (TL) in various somatic diseases such as cancer [Walsh et al., 2014], cardiovascular diseases [Mourkioti et al., 2013], and hypertension [Morgan et al., 2014]. Telomere dysfunction has also been reported in schizophrenia [Kao et al., 2008] and other psychiatric disorders including phobic anxiety [Kananen et al., 2010; Okereke et al., 2012], mood disorders [Simon et al., 2006], and major depression [Needham et al., 2014; Shalev et al., 2014].
To date, many susceptibility genes (e.g., TERT, TERC, KRT80, OBFC1) have been associated with mean TL [Codd et al., 2010; Levy et al., 2010; Codd et al., 2013]. Among them, common variants of telomerase reverse transcriptase (TERT) show repeated association with mean lymphocyte TL (LTL) in GWAS [Codd et al., 2013; Liu et al., 2014]. In terms of function, TERT is crucial for maintaining reproductive activity in cells by adding telomeres to chromosomes ends, and has various physiological functions. TERT is inducible in postmitotic neurons after ischemic brain injury, and prevents N-methyl-D-aspartate receptor (NMDA) receptor-mediated neurotoxicity via shuttling of cytosolic free Ca2+ into mitochondria [Kang et al., 2004]. Additionally, Francesca et al. reported that TERT plays a pro-survival role in fully differentiated neurons by associating with RNA granules [Iannilli et al., 2013]. These findings suggest that TERT dysfunction may play a role in schizophrenia pathogenesis. However, to our knowledge, genetic association analysis between TERT variants and paranoid schizophrenia has not been documented before.
Here, we hypothesize that the genetic variants of the TERT gene may influence susceptibility in patients with paranoid schizophrenia. We genotyped eight tag single nucleotide polymorphisms (SNPs) of TERT and analyzed association of the TERT locus with paranoid schizophrenia in a Chinese Han population.
METHODS
Subjects
In this study, we recruited a total of 1,072 unrelated patients with paranoid schizophrenia (563 males and 509 females, aged 37.8 ± 12.8 years) from the Institute of Mental Health, Peking University, Beijing, China in the period between January 2006 and August 2009. These patients were clinically assessed by at least two consultant psychiatrists according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria based on the Structured Clinical Interview for DSM-IV (SCID). None of these patients received psychotropic medication within 4 weeks. Subjects with the following conditions were excluded from this study, i.e., pregnancy, serious medical conditions, abnormal laboratory baseline values, unstable psychiatric features (e.g., suicidal), epileptic seizures, organic brain disorders, alcoholism, drug abuse, major neurological diseases as well as concomitant Axis I psychiatric disorder. Meanwhile, 1,284 unrelated healthy subjects (669 males and 615 females, aged 33.6 ± 8.9 years) were recruited as controls from local communities. These control subjects were interviewed to obtain detailed information about their medical and family histories. Those who had a history of major psychiatric or neurological disorders, psychiatric treatment or drug abuse, or family history of severe forms of psychiatric disorders were excluded. All the subjects gave written consent to participate in this study. The study was reviewed and approved by the local Ethical Committee of Human Genetics Resources. DNA samples were isolated from peripheral blood leukocytes by the standard phenol/chloroform procedure, which had also been used in our previous association study [Chen et al., 2012].
Selection of SNPs Genotyped
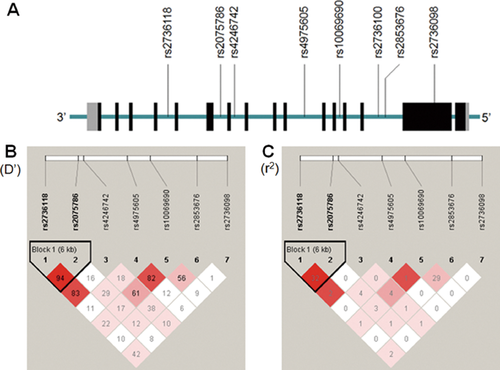
A total of eight SNPs (rs2736118, rs2075786, rs4246742, rs4975605, rs10069690, rs2736100, rs2853676, and rs2736098) within the TERT gene were selected using the Haploview 4.2 program (http://www.broad.mit.edu/mpg/haploview) based on SNP tagging of HapMap database (Han Chinese in Beijing population, http://hapmap.ncbi.nlm.nih.gov/). The distribution of these SNPs was shown in Figure 1A and Table I. All the SNPs selected have a minor allele frequency (MAF) of greater than 5% based on the NCBI SNP database.
| SNP | Positiona | Sample | N | Allele frequency (%) | OR (95%CI) | χ2 | P-valueb | Genotype frequency (%) | χ2 | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs2736118 | 1260080 | A | G | AA | AG | GG | |||||||
| SCZ | 1056 | 1978 (93.7) | 134 (6.3) | 0.87 (0.69–1.09) | 1.53 | 0.22 | 930 (88.1) | 118 (11.2) | 8 (0.7) | 0.15 | 0.70 | ||
| CTR | 1281 | 2376 (92.7) | 186 (7.3) | 1103 (86.1) | 170 (13.3) | 8 (0.6) | |||||||
| rs2075786 | 1266195 | T | C | TT | TC | CC | |||||||
| SCZ | 1007 | 1734 (86.1) | 280 (13.9) | 0.76 (0.65–0.90) | 11.00 | 0.0009 | 746 (74.1) | 242 (24.0) | 19 (1.9) | 3.54 | 0.06 | ||
| CTR | 1280 | 2112 (82.5) | 448 (17.5) | 872 (68.1) | 368 (28.8) | 40 (3.1) | |||||||
| rs4246742 | 1267241 | A | T | AA | AT | TT | |||||||
| SCZ | 1029 | 1388 (67.4) | 670 (32.6) | 1.00 (0.88–1.13) | 0.01 | 0.94 | 480 (46.7) | 428 (41.6) | 121 (11.8) | 1.09 | 0.30 | ||
| CTR | 1280 | 1724 (67.3) | 836 (32.7) | 577 (45.1) | 570 (44.5) | 133 (10.4) | |||||||
| rs4975605 | 1275413 | G | T | GG | GT | TT | |||||||
| SCZ | 1072 | 1978 (92.3) | 166 (7.7) | 0.73 (0.60–0.90) | 9.06 | 0.0026 | 915 (85.4) | 148 (13.8) | 9 (0.8) | 0.03 | 0.96 | ||
| CTR | 1281 | 2299 (89.7) | 263 (10.3) | 1029 (80.3) | 241 (18.8) | 11 (0.9) | |||||||
| rs10069690 | 1279675 | C | T | CC | CT | TT | |||||||
| SCZ | 1049 | 1642 (78.3) | 456 (21.7) | 1.23 (1.07–1.42) | 8.10 | 0.0044 | 658 (62.7) | 326 (31.1) | 65 (6.2) | 13.40 | 0.0003 | ||
| CTR | 1281 | 2091 (81.6) | 471 (18.4) | 849 (66.3) | 393 (30.7) | 39 (3.0) | |||||||
| rs2853676 | 1288432 | G | A | GG | GA | AA | |||||||
| SCZ | 1032 | 1698 (82.3) | 366 (17.7) | 1.04 (0.89–1.21 | 0.22 | 0.64 | 701 (68.0) | 296 (28.7) | 35 (3.4) | 1.89 | 0.17 | ||
| CTR | 1276 | 2113 (82.8) | 439 (17.2) | 868 (68.0) | 377 (29.6) | 31 (2.4) | |||||||
| rs2736098 | 1293971 | G | A | GG | GA | AA | |||||||
| SCZ | 1002 | 1239 (61.8) | 765 (38.2) | 1.12 (0.99–1.27) | 3.45 | 0.06 | 377 (37.6) | 485 (48.4) | 140 (14.0) | 1.96 | 0.16 | ||
| CTR | 1276 | 1646 (64.5) | 906 (35.5) | 523 (41.0) | 600 (47.0) | 153 (12.0) | |||||||
- SCZ, paranoid schizophrenia; CTR, control.
- a Chromosomal positions based on GRCh38.
- b Global P-value was 0.006 after 10,000 permutations.
Genotyping of SNPs
Before genotyping of SNPs, DNA samples were diluted to a concentration of 30 ng/μl and the qualities of genomic DNAs were checked using the agarose gel electrophoresis.
All SNPs were genotyped by a Taqman protocol. Briefly, a 10-μl PCR reaction was performed to genotype each SNP on an CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with the following PCR constituents: 5 μl 2x GoldStar TaqMan Mixture (CWBIO, China), 450 nM of primer F/R, 250 nM of probes and 30 ng genomic DNA.
Thermal cycling conditions were as follows: initial denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec and annealing/extension at 60°C∼69°C for 1 min. The primers and probes of SNPs were designed and synthesized by GeneCore BioTechnologies (Shanghai, China). Sequences of each primer and probe used were given in Table SI.
To ensure optimal analysis, internal controls with known genotypes selected through DNA sequencing and negative controls with water were used. Cases and controls were randomized during genotyping and 10% duplicates of randomly chosen samples were performed to assess the genotyping error rate (genotype concordance was 100%). The genotype call rate for these SNPs was 98.1% on average.
Measurement of Telomere Length
The mean LTL was measured using a previously reported quantitative PCR–based technique [Kao et al., 2008], with minor modifications. This method applied the ratio of telomere repeat length (T) to copy number for a single-copy gene (S) to indicae LTL in each sample. The qPCR reactions for both the telomere and single-copy gene were performed separately in triplicate using LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) and 25 ng template DNA on an CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA), ensuring that the T and S reactions for each sample were located in precisely the same well position.
To amplify telomeres, 270 nM of tel one primer (GGTTTTTGAGGGTGAGGGTGAGGGT GAGGGTGAGGGT) and 900 nM tel two primer (TCCCGACTATCCCTATCCCTATCCCTAT CCCTATCCCTA) were used in each reaction. After an initial heating step to activate the polymerase, the cycling conditions for telomere PCR included denaturation at 95°C for 10 sec and 40 cycles of annealing/extension at 54°C for 2 min with plate read.
The hemoglobin gene (Hgb) was used as single copy control. The cycling conditions for single copy gene included initial incubation step at 95°C for 5 min, followed by 40 cycles of amplification with 10 sec at 95°C, 30 sec at 58°C, and 20 sec at 72°C with data collection, using 300 nM Hgb1 primer (GCTTCTGACACAACTGTGTT CACTAGC) and 700 nM Hgb2 primer (CACCAACTTCATCCACGTTCACC).
Variations between plates were controlled using the standard curve method prepared by two fold serial dilutions of a reference genomic DNA sample ranging from 12.6–100.4 ng (12.6, 21.2, 35.6, 59.7, 100.4 ng, referred as STD1 to STD5). The mean of the correlation coefficient of the standard curves was more than 0.99 for both the T and S reaction. The inter-plate coefficients of variance (CV) of Ct value for the calibrator sample were 2.04% for T and 0.82% for S. DNA samples with a CV > 5% between triplicated tests or a Ct value of >36 were excluded from further analysis (n = 8). The T/S ratio was calculated according to the comparative 2−△△Ct method, where △△Ct = ΔCtSample−ΔCtCalibrator sample, ΔCt = CtT−CtS and CtCalibrator sample = (CtSTD2 + CtSTD3)/2 [Martinsson et al., 2013].
Statistic Analysis
Student t-test (two-tailed) was applied to compare age-distribution between the patient group and the control group. Pearson chi-square (χ2) was performed to test sex-distribution between the two groups, and the χ2 goodness-of-fit test to estimate the Hardy–Weinberg equilibrium (HWE) for the genotypic distributions of each SNP. The Haploview program (version 4.2) was applied to define the haplotype blocks and to estimate linkage disequilibrium (LD) using r2 algorithm between paired SNPs. The UNPHASED program (version 3.1.4, http://homepages.lshtm.ac.uk/frankdudbridge/software/unphased/) was applied for analysis of allelic, genotypic, and haplotypic associations [Dudbridge, 2008]. Rare frequency of haplotype was set at 0.01 in either controls or cases. The odds ratio (OR) with 95% confidence interval (CI) was calculated to represent the effect size of the alleles and haplotypes associated with paranoid schizophrenia. To circumvent inflation of false positive rate due to multiple testing [van den Oord, 2005], 10,000 permutations were performed using the UNPHASED program for the global null hypotheses in which all the odds ratios were equal. Sex and age of the participants were included as covariates in order to rule out confounding effects. Analysis of covariance was applied to compare LTL between cases and controls using SPSS 17.0.
RESULTS
Genetic Association of the TERT Gene With Paranoid Schizophrenia
Of the eight SNPs selected in this study, the genotypic distribution of rs2736100 (χ2 = 83.84, P = 5.4 E-20) deviated from HWE in the control samples, therefore this SNP was removed from further analysis (Table SII). When odds ratio (OR) is set at >1.5, our sample has a minimum power of >70% to detect association for an allele with a minor allele frequency (MAF) ≥0.05.
As shown in Table I, three SNPs in the TERT gene were found to be significantly associated with paranoid schizophrenia, including rs2075786 (χ2 = 11.00, P = 0.00091, OR = 0.76, 95%CI = 0.65-0.90), rs4975605 (χ2 = 9.06, P = 0.0026, OR = 0.73, 95%CI = 0.60-0.90), and rs10069690 (χ2 = 8.10, P = 0.0044, OR = 1.23, 95%CI = 1.07–.42). The global P-value was 0.006 after 10,000 permutations. Genotypic association was also detected for rs10069690 (χ2 = 13.40, df = 2, P = 0.00025).
Haploview analysis showed that of the seven SNPs, rs2736118, and rs2075786 were present in one linkage disequilibrium (LD) block in the control samples, whereas the remaining five SNPs were not in LD with the other SNPs (Fig. 1B and C). The rs2736118-rs2075786 haplotype showed strong association with paranoid schizophrenia (χ2 = 13.28, df = 2, P = 0.0013). As shown in Table II, the A-T haplotype frequency was significantly higher in cases than controls (χ2 = 12.21, adjusted P = 0.0015) while the A-C haplotype frequency was significantly lower in cases than controls (χ2 = 10.42, adjusted P = 0.0012).
| Combination | Haplotype | SCZ, N (%) | CTR, N (%) | OR (95%CI) | χ2 | Individual P-value | Global P-value (df = 2) |
|---|---|---|---|---|---|---|---|
| rs2736118-rs2075786 | A-T | 1723 (86.2) | 2095 (82.4) | 1 | 12.21 | 0.0005a | 0.0013 |
| A-C | 156 (7.8) | 270 (10.6) | 0.70 (0.57–0.86) | 10.42 | 0.0012b | ||
| G-C | 119 (6.0) | 177 (7.0) | 0.82 (0.64–1.04) | 1.86 | 0.1724 |
- a,bThe adjusted P-values after Bonferoni correction were 0.0015 and 0.0036, respectively.
Association of Genetic Variants With Mean LTL
Age- (ranging from 41 to 45 years) and sex-matched samples were chosen for mean LTL measurement as age and sex are reported to have an effect on mean LTL [Blasco, 2005; von Zglinicki and Martin-Ruiz, 2005]. As shown in Table SIII, there were no significant differences between paranoid schizophrenia and controls in age (t = 1.012, P = 0.31), or sex (χ2 = 0.01, P = 0.92). As previous studies have shown that both age and sex have an effect on LTL, correlation analysis was used to investigate the effect size of age and sex in our samples. We found that mean LTL was negatively associated with age in controls (rs = −0.259, P = 0.007). Moreover, although mean LTL was not significantly different between male and female subjects (rs = 0.158, P = 0.103), females had longer LTL than males (1.651 vs. 1.454) (Table SIV). To observe significant differences in mean LTL between cases and controls, we selected only paranoid schizophrenia cases with extreme characteristics (full positive symptoms scores).
In accordance with our expectations, mean LTL was significantly shorter in paranoid schizophrenia cases (N = 98) than healthy individuals (N = 109) (F = 225.02, P < 0.0001, adjusted for age and sex) (Fig. 2A).
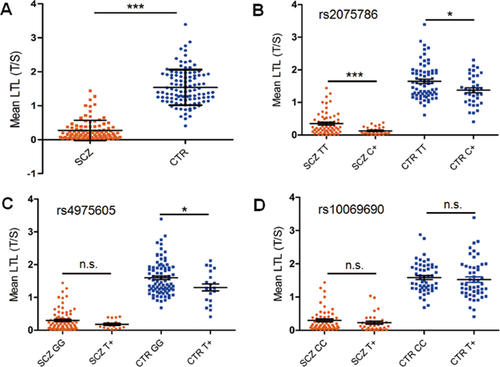
Based on our genetic association results, three SNPs (rs2075786, rs4975605, and rs10069690) were selected for association analysis with mean LTL. Because of the big difference in mean LTL between cases and controls, patients, and controls were analyzed separately. We found longer LTL in rs2075786 TT carriers compared with C+ carriers (TC + CC genotypes) in both patients (P = 0.0006) and controls (P = 0.025) (Fig. 2B). Additionally, rs4975605 was genotypically associated with mean LTL in only the control group, although this SNP showed the strongest association with paranoid schizophrenia. Healthy individuals with GG genotypes had longer mean LTL than T+ carriers (GT and TT genotypes) (P = 0.044) (Fig. 2C). Genotypes at rs10069690 did not show any association with the mean LTL in either cases or in controls (Fig. 2D).
DISCUSSION
In the present study, we identified three SNPs at the TERT locus, SNP rs2075786, rs4975605, and rs10069690, which are associated with paranoid schizophrenia in our Chinese Han population. Our data further enriches the genetic architecture of paranoid schizophrenia.
The Schizophrenia Working Group of the Psychiatric Genomics Consortium (PGC) performed a GWAS using the largest number of samples to date, and identified 108 independent genomic loci that exceed genome-wide significance [Schizophrenia Working Group of the Psychiatric Genomics, 2014]. Although TERT identified in our study was not on this list of schizophrenia risk loci, this is reasonable for the following two reasons. First, population stratification may be involved, which can largely be dealt with statistically due to allele frequency differences among subpopulations [Burmeister et al., 2008; Campbell et al., 2008]. Second, we narrowly focused on paranoid schizophrenia, which is clinically characterized by hallucinations and delusions as well as absence of negative symptoms, and such a specific subtype of schizophrenia may share unique genetic variations.
Our data also showed correlation between different rs2075786 genotypes and LTL in both paranoid schizophrenia patients and controls (Fig. 2B). Bellido et al. previously reported association of rs2075786 with LTL, and in silico studies predicted that this SNP may cause early telomerase activation [Bellido et al., 2013]. However, this study also showed that rs2075786 AA genotype carriers had shorter telomeres, which is inconsistent with our findings. For SNP rs4975605, we found that different genotypes are associated with LTL in controls only (Fig. 2C), while rs10069690 shows no association with mean LTL in either cases or controls (Fig. 2D). In contrast, rs10069690 has shown association with TL in a population from Utah [Pellatt et al., 2012]. We believe that population stratification should be the main cause for these discrepancies. Although additionally, they may be partially explained by different sex or age distributions since previous studies have shown that younger, female subjects have longer telomeres [Blasco, 2005; von Zglinicki and Martin-Ruiz, 2005].
Our results revealed that those schizophrenia risk-associated SNPs, including rs4975605 and rs10069690, might have no effect on telomere length. Similarly, although certain TERT SNPs are significantly associated with TL, they are not yet risk loci for schizophrenia, e.g., rs2735940 [Burke et al., 2013]. In short, these findings suggest that TERT may increase the risk of paranoid schizophrenia through roles other than telomere length. Although the precise mechanism of TERT participating in the pathophysiological process of schizophrenia remains unknown, several studies have reported the functions of TERT in the nervous system. For example, transgenic mice overexpressing murine TERT exhibit significant resistance to ischemic brain injury and NMDAR-mediated excitotoxicity [Kang et al., 2004]. In addition, Wang et al. reported significant mprovements in learning and memory in rats with vascular dementia that received bone marrow mesenchymal stem cells (BMSCs) co-expressing nerve growth factor (NGF)-TERT, compared with those receiving BMSCs without NFG-TERT transfection [Wang et al., 2014]. Furthermore, high TERT expression and wnt/β-catenin pathway activation are closely linked. TERT is a direct target of the wnt/β-catenin pathway and activation of this pathway can upregulate TERT expression [Zhang et al., 2012], while high TERT expression may activate wnt/β-catenin signaling [Ueda et al., 2011]. Considering that T-cell factor 4 (TCF4) is strongly associated with schizophrenia, TERT may be involved in schizophrenia pathogenesis by participating in the wnt/β-catenin pathway. All of these studies provide clues for investigating TERT function in paranoid schizophrenia in the future.
Another interesting finding of our study was that the mean LTL is significantly shorter in paranoid schizophrenia cases than controls, which is consistent with several previously reported results. Kao et al. found significantly shorter telomeres in schizophrenia patients (N = 51) compared with healthy family members (N = 24) and controls (N = 53) [Kao et al., 2008]. Similarly, in a study of 41 individuals with non-affective psychosis (including 27 with schizophrenia and nine with schizophreniform disorder), and 41 controls [Fernandez-Egea et al., 2009], significantly decreased telomere DNA content was found in individuals with non-affective psychosis compared with controls. In contrast, Mansour et al. investigated LTL in a cohort of 60 schizophrenia cases and 60 controls, but failed to find association between schizophrenia and TL [Mansour et al., 2011]. More surprisingly, Nieratschker et al. observed that LTL was slightly longer in schizophrenia patients (N = 539) than in controls (N = 519) [Nieratschker et al., 2013]. We believe these conflicting results are mainly due to the extensive genetic and phenotypic heterogeneity of schizophrenia, and varying LTL in schizophrenia patients may reflect association at the endophenotype level. Psychotropic medication may also explain the controversy. Savolainen et al. reported that psychotropic medications have antioxidative effects, and thereby prevent telomere attrition [Savolainen et al., 2012]. Unfortunately, our sample failed to include information on lifetime amount of psychotropic medication, and we were unable to determine if telomere dysfunction in our paranoid schizophrenia samples is due to psychotropic medication. Finally, our findings might be biased by the relatively small sample size.
ACKNOWLEDGMENTS
We thank all of the subjects, including both paranoid schizophrenia patients and healthy controls, for their active participation in this work. This work was supported by the 973 Project (2013CB531301, 2010CB529603, 2012CB517902), PCSIRT (IRT1006), NSFC (31222031, 81000583, 81278412), the Fundamental Research Funds for the Central Universities (2012S05), PUMC Youth Fund (2012J09, NCET-12-0071).