Brain calcification process and phenotypes according to age and sex: Lessons from SLC20A2, PDGFB, and PDGFRB mutation carriers
Abstract
Primary Familial Brain Calcification (PFBC) is a dominantly inherited cerebral microvascular calcifying disorder with diverse neuropsychiatric expression. Three causative genes have been identified: SLC20A2, PDGFRB and, recently, PDGFB, whose associated phenotype has not yet been extensively studied. We included in the largest published case series of genetically confirmed PFBC, 19 PDGFB (including three new mutations), 24 SLC20A2 (including 4 new mutations), and 14 PDGFRB mutation carriers, from two countries (France and Brazil). We studied clinical features and applied our visual rating scale on all 49 available CT scans. Among the symptomatic mutation carriers (33/57, 58%), the three most frequently observed categories of clinical features were psychiatric signs (72.7%, 76.5%, and 80% for PDGFB, SLC20A2, and PDGFRB, respectively), movement disorders (45.5%, 76.5%, and 40%), and cognitive impairment (54.6%, 64.7%, and 40%). The median age of clinical onset was 31 years, 25% had an early onset (before 18) and 25% a later onset (after 53). Patients with an early clinical onset exhibited mostly isolated psychiatric or cognitive signs, while patients with a later onset exhibited mostly movement disorders, especially in association with other clinical features. CT scans rating allowed identifying four patterns of calcification. The total calcification score was best predicted by the combined effects of gene (SLC20A2 > PDGFB > PDGFRB mutations), sex (male), and (increasing) age, defining three risk classes, which correlated with the four patterns of calcification. These calcification patterns could reflect the natural history of the calcifying process, with distinct risk classes characterized by different age at onset or rate of progression. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Primary familial brain calcification (PFBC, also known as idiopathic basal ganglia calcification (IBGC) or Fahr's disease) is a primary cerebral microvascular calcifying disorder. PFBC is thought to be rare but its prevalence has not been assessed. This disease is inherited as an autosomal dominant trait, and three causative genes have been identified recently. First, heterozygous loss-of-function mutations of SLC20A2, encoding PiT2, a sodium-inorganic phosphate cotransporter, cause PFBC most likely through an impairment of the cerebral metabolism of inorganic phosphate [Wang et al., 2012; Jensen et al., 2013]. Second, heterozygous loss-of-function mutations of PDGFRB [Nicolas et al., 2013b], the gene encoding the platelet-derived growth factor receptor β (PDGFRβ), or of PDGFB [Keller et al., 2013], the gene encoding its main ligand, PDGFB, are responsible for an impairment of the blood–brain barrier, a mechanism which could be involved in the development of the calcifications seen in both patients with PFBC and mouse models [Keller et al., 2013] (see also https://coppolalab.ucla.edu/lovd/genes where mutations in the three genes are reported). Putative links between these pathways are still under debate and deserve more investigation [Arts et al., 2015; Betsholtz and Keller, 2014]. A causative mutation within one of these genes was found in 35% of families with autosomal dominant PFBC [20%, 10%, and 5% in the SLC20A2, PDGFB, and PDGFRB genes, respectively) in the French case series [Nicolas et al., 2014b] while an SLC20A2 mutation was found in up to 50% of PFBC families in other studies [Hsu et al., 2013; Yamada et al., 2014] (where PDGFB and PDGFRB screening were not reported), highlighting possibly different inclusion criteria, the diagnosis of probable PFBC (with no identified genetic cause) needing the exclusion of numerous differential diagnoses [Manyam, 2005]. Besides inheritance, PFBC can have a sporadic presentation, which is frequently difficult to ascertain because it requires (1) a normal CT scan in both parents; and (2) an even more extensive etiological assessment than in autosomal dominant presentations [de Oliveira et al., 2013]. We previously reported the cases of a woman with a de novo PDGFB mutation [Nicolas et al., 2014] and another one with a de novo SLC20A2 mutation [Ferreira et al., 2014], but most sporadic cases might in fact be due to mutations inherited from an asymptomatic, undiagnosed parent [Nicolas et al., 2014b]. In the French case series, we identified a causative or probably causative mutation within one of the three genes in 34.4% of patients with sporadic presentation of PFBC [Nicolas et al., 2014b] (17.9%, 13.8%, and 3.4% in the SLC20A2, PDGFB, and PDGFRB genes, respectively; after reevaluation of PDGFRB mutations by functional studies [Arts et al., 2015], the p.Glu1071Gly mutation was considered as probably not causative).
Clinical features associated with PFBC are diverse, as are ages of onset (ranging from early childhood to ∼80 years, clinical expression being inconsistent). We previously reported that psychiatric signs, cognitive impairment and movement disorders were each found in ∼55 to ∼59% of symptomatic patients, while gait, speech, cerebellar, and pyramidal signs were found in 23–43% of them. Other signs, like seizures, were found in less than 8% of symptomatic patients [Nicolas et al., 2013]. Several patients were also described with chronic headache, including migraine [Hsu et al., 2013], but this is still unclear whether these observations were coincidental or truly related to PFBC. Evaluation of brain calcifications on computerized-tomography (CT) scans using an original visual rating scale revealed that the total calcification score (TCS) strongly correlated with age and was more severe in SLC20A2 compared to PDGFRB mutation carrier. The PDGFB gene was identified as PFBC-causative more recently [Keller et al., 2013] and the clinical and radiological phenotype of patients with a PDGFB mutation has not yet been precisely assessed especially in comparison with others.
With the aim to describe the phenotype associated with the lastly identified PFBC-causing gene, PDGFB, we conducted a binational study (France and Brazil) including 57 patients with a PDGFB, PDGFRB, or SLC20A2 mutation. Within this largest series of genetically confirmed PFBC patients, we report three new PDGFB mutations, compare the clinical and radiological phenotypes associated with each gene, and identify the main factors associated with calcification severity and the symptomatic status. We also describe four patterns of calcification which could be related to the natural history of brain calcification in mutation carriers.
MATERIALS AND METHODS
Patients
Patient records, CT images, and blood samples from multiple French and Brazilian centers were referred to two centers of expertise: Inserm U1079 (Rouen, France) and University of Pernambuco (Recife, Brazil), respectively. We diagnosed with probable PFBC, probands presenting calcification of at least both lenticular nuclei on brain imaging if (1) the TCS was above the age-specific threshold and (2) no cause was found following an extensive clinical–radiological–biological assessment [Nicolas et al., 2013]. Sanger sequencing of all protein-coding exons and exon-intron boundaries of the SLC20A2, PDGFB, and PDGFRB genes was performed, as previously described [Wang et al., 2012; Keller et al., 2013; Nicolas et al., 2013b], and copy number variantions involving these genes were also assessed according to ref. Nicolas et al. [2014c]. We defined single nucleotide and indel variants as probably causative following the methods previously described [Nicolas et al., 2013]. From them, we excluded variants with no effect when functional studies where available. The nomenclature of the mutations refers to transcripts NM_006749 (SLC20A2), NM_002608.2 (PDGFB), and NM_002609.3 (PDGFRB). Patients with a probably causative mutation were then diagnosed with genetically confirmed PFBC. We also diagnosed relatives of these probands with genetically confirmed PFBC if they carried the familial mutation as well as calcification affecting at least both lenticular nuclei (note that no relative with a mutation had a normal CT scan).
Only patients (probands and affected relatives) with genetically confirmed PFBC were eventually included in this study. From the medical records, we noted the age at clinical onset, the age at last clinical evaluation, the age at CT scan, the sex, and the type of symptom among the list provided by Manyam et al. [2001] (Table I). Patients were considered symptomatic if they presented at least one sign among this list. In addition, we noted when patients presented migraine with or without aura, following International Headache Classification criteria [Headache Classification Subcommittee of the International Headache, 2004].
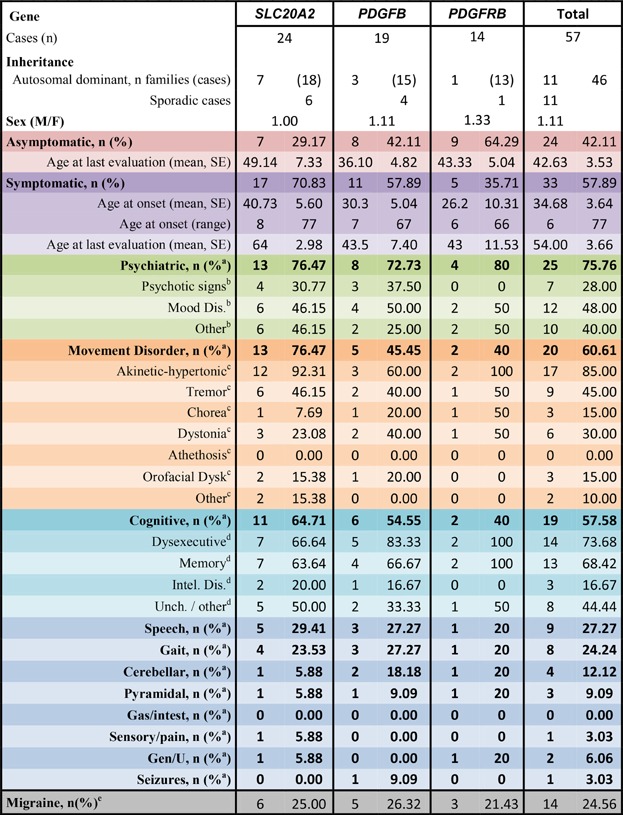
- Note that several patients exhibited combinations of clinical features.
- aAmong symptomatic patients.
- bAmong patients with psychiatric signs.
- camong patients with movement disorder.
- damong patients with cognitive impairment.
- eamong all patients.
Calcification Rating Score
When available, CT scans were rated according to our previously published visual rating scale [Nicolas et al., 2013]. Briefly, two of us (G.N., D.H.) applied separately and then jointly the visual scale to each cerebral location (from 0, no calcification, to 5, severe and confluent; in the following locations: lenticular, caudate, thalamus nuclei, subcortical white matter, cortex, cerebellar hemispheres, vermis, midbrain, pons, and medulla). The total calcification score (TCS, ranging from 0 to 80) was obtained by adding all location-specific scores for each patient. Both investigators were blind from the genotypes during CT-scan rating.
Statistics
Analyses were performed on the R statistical software version 3.0.2. Fisher exact tests and Mann–Whitney tests were used to compare clinical characteristics between genetic groups. Results are expressed as mean ± standard error of the mean (SEM) unless otherwise specified. We compared clinical data of patients with a PDGFB mutation only to those with a SLC20A2 mutation because 13/14 patients with a PDGFRB mutation belonged to the same family, which could have induced misinterpretation of proportion of symptomatic and asymptomatic patients, and only 5 of them were symptomatic. We analyzed calcification rating scores as follows. First, a negative binomial distribution was adopted to model the TCS, allowing for over-dispersion of count data, when accounting for the combined effects of age, sex, and causative genes. Second, logistic regression was used to perform multivariate analysis of the symptomatic status including the TCS among the analyzed criteria. Third, a heatmap (Fig. 3) was built, representing the grid of quantification scores for each location and patient on a colored scale. On each axis, locations and patients displaying similar score profiles were ordered and clustered together by ascending hierarchical clustering based on the Euclidean distance and Ward's aggregation criterion. The resulting clustering trees are shown on the x and y axes. To compare scores of locations rated twice (left and right, rated /10) versus locations rated once (median structures (vermis, pons, and medulla) and cortex, each rated /5), we doubled the score of the last-mentioned locations.
RESULTS
Inclusions
We included a total of 57 patients with genetically confirmed PFBC: 24 with a SLC20A2 mutation (18 from 7 families; 6 with a sporadic presentation), 19 with a PDGFB mutation (15 from 3 families; 4 with a sporadic presentation) and 14 with a PDGFRB mutation (13 from one family and one with a sporadic presentation) (Table 1, 10 patients from Brazil, and 47 from France). Mutation details are listed in Supplementary Table S1. Among the PDGFB mutations included, three were previously unreported: one disruptive and two missense (Supplementary Table S1). The c.64.-3C>G mutation is predicted to avoid splicing of exon 2, introducing a frameshift and a premature stop codon. We confirmed these predictions by studying the proband's RNA (data not shown). Among the SLC20A2 mutations, four were recently listed by Lemos et al. [2015]: three disruptive (frameshift short deletions) and one missense (Supplementary Table S1).
Clinical Features
Thirty-three patients (57.9%) were considered symptomatic (Table I). The median age of onset was 31 years, ranging from 6 to 77. Their repartition is shown in Supplementary Figure S1 (among the 31/33 symptomatic patients with available age at onset). Interestingly, 25% of the symptomatic patients had a disease onset before 18 and 25% after 53 years. In total, 57.9% of the PDGFB mutation carriers and 70.8% of the SLC20A2 mutations carriers were symptomatic (Non Significant, NS) with no significant differences in mean ages at last evaluation of asymptomatic carriers between the two groups (36.1 years (95% confidence interval, [26.7–45.6]) and 49.1 (95% confidence interval, [34.8–63.5]), respectively). No significant differences were noted when looking at categories of clinical features either.
Overall, psychiatric signs were the most frequent clinical features (75.8% of symptomatic patients), followed by movement disorder (60.6%) and cognitive impairment (57.8%) (Table I). In particular, akinetic-hypertonic syndrome was found in 85% of patients with movement disorder, associated with tremor in 9/17. Akinetic-hypertonic syndrome was declared by physicians and patients to be Dopa-sensitive in four of them, not in two, and Dopa therapy was not tried in the others because akinetic-hypertonic syndrome was either thought to be iatrogenic or it was not severe enough.
Patients with impaired episodic memory presented pathological retrieval with inefficient cueing in 7/13, while cueing was efficient in five patients (free and cued recall test; not done in one patient).
The sex ratio was 1.11, with 60% of men and 55.6% of women considered as symptomatic (NS). The mean age at onset was 33.9 ± 4.3 in men and 35.7 ± 7.0 years in women (NS).
If we focus on the eight patients with early clinical onset (in the first quartile), two had a PDGFB mutation (p.*242Tyrext*89 stop loss mutation), four an SLC20A2 mutation (2 p.Pro184Leu, 1 splice c.431-1G>T, 1 p.Tyr187Cys), and two a PDGFRB mutation (p.Leu658Pro). The first reported sign or syndrome was isolated psychiatric syndrome in four patients (bipolar disorder in one, anxiety disorder with obsessive compulsive signs in one, attention deficit hyperactivity disorder in one, personality disorder in one), isolated intellectual disability in two, psychosis with intellectual disability in one and seizures in one.
If we focus on the eight patients with later onset (fourth quartile), six had an SLC20A2 mutation (p.Asn587SerFs*7, p.Asn509LysFs*7, c.431-1G>T, p.Leu380CysFs*75, and two patients with p.Ala251LeuFs*67), one had a PDGFB mutation (p.Ala95Thr), and one had a PDGFRB mutation (p.Arg987Trp). At presentation, 6/8 were affected by movement disorders (akinetic-hypertonic syndrome in 4, with tremor in one and with tremor plus dystonia and chorea in one; chorea in one; orofacial dyskinesia in one), and akinetic-hypertonic syndrome was noted several years later in the other two patients. Cognitive impairment was noted at presentation in six of them, depression in two, pyramidal signs in two (with no paresis), cerebellar syndrome marked by gait disorder and dysarthria in two, anxiety in one, and urinary incontinence in one. Three had only one sign at presentation (akinetic-hypertonic syndrome, cognitive impairment, and major depressive episode).
In addition, 14/57 patients (24.6%) presented migraine, respectively noted in 9/24 of the otherwise asymptomatic carriers (33.3%) and 5/33 of the symptomatic carriers (15.15%, OR = 3.17 95%CI = [0.78 −14.42], P = 0.117). Migraine was found in 16.7% of males and 33.3% of females (NS).
Calcification Rating Analyses
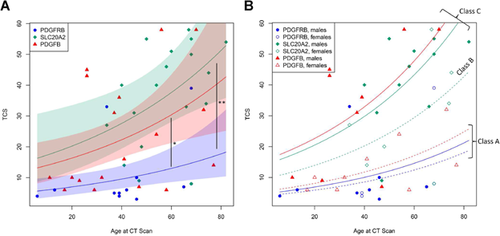
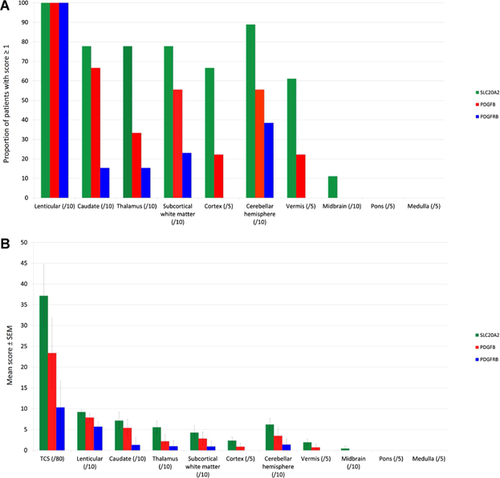
Brain CT scans were available for 49/57 patients (for 7, quality of the images was not sufficient to allow accurate rating and one patient had only MRI). Analyses of CT scans confirmed that the TCS strongly correlated with the age at CT scan (P = 0.00023). Assuming a binomial negative regression model adjusted for age, PDGFB mutation carriers had a greater expected TCS compared to PDGFRB (P = 0.0005), and we confirmed that SLC20A2 mutation carriers had an even greater expected TCS compared to PDGFRB mutation carriers (P = 3.10−5). While SLC20A2 mutation carriers had an apparently more severe TCS compared to PDGFB (suggesting a hierarchy SLC20A2 > PDGFB > PDGFRB), this difference was not significant (P = 0.36, Fig. 1A). The same hierarchy was nonetheless observed concerning each location of calcification: SLC20A2 mutation carriers had a more severe mean calcification score and were more frequently affected by extra lenticular calcification than PDGFB mutation carriers, followed by PDGFRB mutation carriers (Fig. 2). Cortical and vermian calcifications were exclusive to SLC20A2 mutation carriers, and, less frequently and with a lesser mean severity, to PDGFB mutation carriers (Fig. 2).
We then aimed to determine the best associated criterion of the symptomatic status among the following: sex, age at CT scan, gene, and TCS. After logistic regression, we identified the TCS as the most significantly correlated criterion with the symptomatic status (P = 0.005; P value for age: 0.036). After adjustment on TCS, adding one or several of the other criteria did not bring any significant complementary information (Supplementary Table S2).
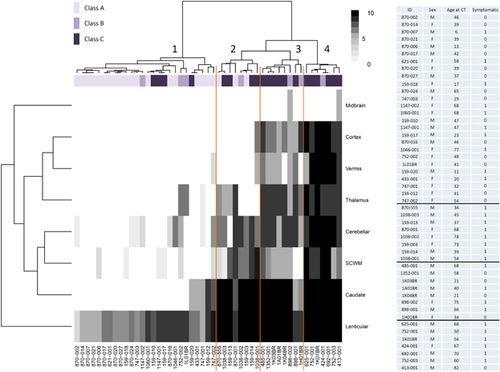
With the aim to determine the factors associated with the TCS, we then performed forward backward model selection based on Akaike Information Criterion with gene, sex, age at CT scan, and symptomatic status as criteria. The best model including only one criterion identified the gene as the most significant predictor of TCS (P = 6.66.10−5; P = 0.001 for age, P = 0.007 for symptomatic status; P = 0.044 for sex Supplementary Table S3). Combination of gene, age at CT scan, and sex was the best model combining three criteria (P = 0.002, Supplementary Table S3). We then tested the interaction between each other. Among them, the interaction between sex and gene was most significant for PDGFB (P = 0.048). It allowed us to determine three classes in the prediction of the TCS (Fig. 1B): class A (PDGFRB, both sexes; and PDGFB, women), class B (SLC20A2, women), and class C (SLC20A2, men; and PDGFB, men). We interpreted classes A, B, and C respectively as classes with increasing risk for presenting a greater TCS in our dataset. We then represented the score in each location by mutation carrier after performing ascending hierarchical clustering and representing it on a heatmap (Fig. 3). The algorithm permitted the identification of four categories of calcification hereafter named patterns 1, 2, 3, and 4, which were significantly associated with risk classes (Fisher exact test P-value=5.6.10−5, Supplementary Table S4). Pattern 1 regroups 26 patients with the lowest TCS, most of them presenting isolated lenticular calcifications or associated with caudate calcifications. Patients from class A account for 19 of them and have a mean age of 40.42 at CT scan, while four patients are from class C and have a mean age of 32 years at CT scan in this pattern. Pattern 2 regroup 8 patients with severe calcifications of both lenticular and caudate nuclei, associated with calcification of the subcortical white matter and the cerebellar hemispheres with an intermediate severity, as well as thalamic calcifications for four of them. Patients from the three risk classes are represented in this pattern, the mean age of patients from class A being 58.33 years, and 43.75 years for patients from class C. All patients from class A are in patterns 1 and 2. Patterns 3 and 4 regroup the patients with the highest TCS, marked by severe and confluent lenticular and caudate calcification in nearly all, calcification of all locations except the midbrain in all but two, with a mean severity higher in pattern 4 than pattern 3. Only patients from class B and C are found among these calcification patterns, with a mean TCS of 58 and 35.5 (respectively for patterns 4 and 3, patients from class B) and of 55.17 and 42.83 (respectively for patterns 4 and 3, patients from class C). In pattern 4, patients are older than in pattern 3 (67 years vs. 54.5, class B; 64.33 vs. 47.33, class C). Interestingly, only 38% of patients with the calcification pattern 1 were symptomatic, compared to the 100% with pattern 2, 50% with pattern 3 and 85% with pattern 4 (Fig. 3).
To further examine the effect of sex, we re-analyzed our results obtained from CT scans of 600 controls (unselected patients consecutively hospitalized in our Neurology department) [Nicolas et al., 2013]. We found that 43/284 men (15.1%) had a TCS > 0, compared to 47/316 women (14.9%, NS). The mean TCS was 2.02 among the 43 men with calcification and 1.87 among the 47 women with calcification (NS), and the medians were 2 and 1, respectively, with a non-different mean age (69.0 ± 1.8 in men and 68.5 ± 2.5 in women). As hormone differences could explain a non-observed difference in this group of controls from all age groups, we then considered only controls younger than 50, but we did not observe a significant difference (13/119 (10.9%) men had a TCS > 0 compared to 17/175 (9.7%) women).
DISCUSSION
This is the largest phenotypical study of genetically confirmed PFBC, and the first one comparing PDGFB-associated phenotype with other genes. We report the phenotype associated with new and previously reported mutations in a binational study and bring new clues to understanding the progressive development of calcification in patients' brains.
Clinical Phenotype
Strikingly, no significant clinical difference was noted between PDGFB and SLC20A2 mutation carriers. Considering all PFBC genetically confirmed patients, the most frequent category of clinical features was psychiatric signs (75.8% of symptomatic patients). This confirms, with a larger sample and a binational recruitment, what we had previously observed [Nicolas et al., 2013]. We reported that only 50% (19/38) of the symptomatic patients with probable PFBC (no identified mutation) presented psychiatric signs, and that, among them, four out of the seven thereafter found to have a PDGFB mutation had psychiatric signs. Psychiatric expression of PFBC seems therefore to be more frequent in genetically confirmed PFBC than probable PFBC, where patients may have other genetic causes of PFBC, or multifactorial origin.
Importantly, the median age of onset was at 31 years old, which seems consistent with the most commonly thought that disease onset is mainly between ages 30 and 60 [Sobrido et al., 2004] or after 40 years [Westenberger and Klein, 2014]. However, ages of onset ranged from 6 to 77 and the repartition of ages of onset showed that no category of age of onset was overrepresented. This may have significant consequences in genetic counseling and patient information and we think that the above-mentioned statements should not yet be used.
Among the patients with the earliest clinical onset, psychiatric signs and cognitive impairment were the most frequent presenting clinical features, especially isolated, while motor signs were not observed at this age in our series. On the contrary, movement disorder (especially akinetic-hypertonic syndrome) was prominently noted when patients had a later disease onset, where association of several clinical features was frequent at presentation.
Patients with an early onset and a late onset sometimes carried the same mutation, while other mutation carriers from the same families remained asymptomatic. This was not specific to a gene or a given mutation.
Migraine was found in 24.6% of mutations carriers, with no significant difference in gene and tended to be more frequent in patients with no other clinical features. Prevalence of migraine was previously estimated at 14.7% in the general population [Vos et al., 2012]. However, migraine did not segregate with PFBC in several families (e.g., family 870 with a PDGFRB mutation [Nicolas et al., 2013b] and family 1H01BR with a de novo SLC20A2 mutation in the proband, who suffered from migraine, as well as her unaffected mother [Ferreira et al., 2014]). This suggests that patients with PFBC might present migraine more frequently than in the general population and confirms the difficulty to interpret such a frequent sign within cosegregation studies or case series.
Brain Calcifications
We confirmed the strong association between age and TCS and identified patients with a PDGFB mutation as exhibiting calcification of intermediate severity, between PDGFRB and SLC20A2 mutation carriers, the latter being confirmed as exhibiting the highest TCS in this two-nation study. After regression, we identified the combination of gene, age, and sex as the best TCS prediction model with combined effect of three criteria. The symptomatic status was significantly associated with a higher TCS when considering the effect of one or combining two criteria (with the gene), but there was no gain to add to the model the information on the symptomatic status to the other two criteria when considering the best three-criteria combination. Conversely, after logistic regression on symptomatic status, the TCS showed the most significant correlation. This suggests that age, sex, and gene are factors strongly associated with TCS, which is itself correlated with the symptomatic status (Supplementary Figure S2).
We identified four patterns of calcification, whose hierarchy from 1 to 4 correlated positively with age. We suggest that these patterns could reflect the natural progression history of calcification, a time-related process putatively arising in the brain of all PFBC patients. This calcifying process would follow a similar pathway in each patient (pattern 1, then 2, 3, and 4, regardless of gene and sex) but with different ages at onset and speed of progression. The affected gene (and most likely several mutations, for which power was insufficient for performing an accurate analysis) and the sex would influence, amongst other (unidentified) factors, the beginning of the calcifying process as well as its speed. In this hypothesis, patients from class A (all PDGFRB mutation carriers and PDGFB female carriers included here) would not grow old enough to pass into the 3rd and 4th patterns of calcification, particularly in areas involving vermis and cortex. We hypothesize that they had a delayed calcification onset (as suggested by the lowest TCS in the youngest patients), and/or a slower progression of the calcifying process than in PFBC due to other mutations. As calcification onset is quite impossible to measure, comparative longitudinal data would be necessary to test this hypothesis. Caution is needed however in the generalization of our results regarding other mutations of these genes, as the TCS comparisons between genes and the identification of risk classes deeply relied on the mutations studied in this work (e.g., a putative disruptive mutation of PDGFRB could have been responsible for more severe, SLC20A2-like, calcifications), and can also be subject to family biases. A future analysis focusing on particular mutations or groups of mutations should be undertaken, based on an increased number of mutations.
Effect of Sex
The male:female sex ratio was 1.36 in the study with review of the literature by Manyam et al. [2001] and 1.40 in our previous study [Nicolas et al., 2013]. It was interpreted as a higher incidence of calcification in men [Manyam et al., 2001; Westenberger and Klein, 2014]. Although in the present study, where we recruited mutation carriers only, the sex ratio may seem more even (1.11), we show that the male sex is associated with an increased TCS—which reflects calcification extension and severity, this effect appearing stronger in PDGFB mutation carriers. In our series of controls however, no sex bias was noted. Beyond the calcification process frequently and fortuitously observed in controls, men could present abnormal calcification earlier in life, which could be detected more easily, and they could be included in series of “Fahr's” disease earlier than women. The putative mechanisms underlying the effect of sex are unknown, a modifying gene on X chromosome or the effect of hormones might be assessed. We did not identify differences in mean age at onset or proportion of symptomatic patients between males and females, contrary to a previous study [Manyam et al., 2001]. This indirectly reinforces the fact that the correlation between TCS and clinical status, although true, is not self-sufficient.
CONCLUSIONS
We report in this two-nation study the phenotype associated with the three first PFBC causal genes. After the inclusion of new patients and the identification of the PDGFB mutation carriers, we confirm the three main categories of clinical features associated with PFBC, and highlight the particularly high prevalence of psychiatric disturbances in these patients. Importantly, information to relatives should be carefully given when dealing with ages of onset, as clinical onset was found to be much more dispersed over lifespan than commonly thought. In addition, we show that the calcification severity is determined by the combination of the affected gene, the age, and the (male) sex, and is itself associated with the symptomatic status. Other factors influencing the calcification process as well as the symptomatic status, the types of clinical features, and their severity remain research challenges for the future. Finally, further research is warranted to identify environmental, genetic and epigenetic factors influencing the clinical expression and course, as well as new biomarkers.
ACKNOWLEDGMENTS
We are grateful to Tracey Avequin for her help in editing the manuscript. The French IBGC study group are: Gaël Nicolas, Mathieu Anheim, Xavier Ayrignac, Jean-Philippe Azulay, Camille Charbonnier, Nathalie Derache, Franck Durif, Olivier Guillin, Lucie Guyant-Maréchal, Didier Hannequin, Mathilde Hodille-Renaud, Pierre Labauge, Isabelle Le Ber, Romain Lefaucheur, David Maltête, Jérémie Pariente, Anne-Claire Richard, Olivier Rouault, Christel Thauvin-Robinet, Pauline Rudolf, Christine Tranchant, Christophe Verny, David Wallon. The French group (GN, CC, DW, ACR, OG, TF, DC, DH) is funded by Inserm and the CNR-MAJ. JRMO acknowledges funding from FACEPE (APQ 1831-4.01/12) and CNPq (457556/2013-7;480255/2013-0; 307909/2012-3). DHG and GC acknowledge NIH/NINDS support (R01 NS040752). The funding source had no specific role. We have no conflict of interest.




