Genetic overlap of schizophrenia and bipolar disorder in a high-density linkage survey in the Portuguese Island population†
How to Cite this Article: Fanous AH, Middleton FA, Gentile K, Amdur RL, Maher BS, Zhao Z, Sun J, Medeiros H, Carvalho C, Ferreira SR, Macedo A, Knowles JA, Azevedo MH, Pato MT, Pato CN. 2012. Genetic Overlap of Schizophrenia and Bipolar Disorder in a High-Density Linkage Survey in the Portuguese Island Population. Am J Med Genet Part B 159B:383–391.
Abstract
Recent family and genome-wide association studies strongly suggest shared genetic risk factors for schizophrenia (SZ) and bipolar disorder (BP). However, linkage studies have not been used to test for statistically significant genome-wide overlap between them. Forty-seven Portuguese families with sibpairs concordant for SZ, BP, or psychosis (PSY, which includes either SZ or psychotic BP) were genotyped for over 57,000 markers using the Affymetrix 50K Xba SNP array. NPL and Kong and Cox LOD scores were calculated in Merlin for all three phenotypes. Empirical significance was determined using 1,000 gene-dropping simulations. Significance of genome-wide genetic overlap between SZ and BP was determined by the number of simulated BP scans having the same number of loci jointly linked with the real SZ scan, and vice versa. For all three phenotypes, a number of regions previously linked in this sample remained so. For BP, chromosome 1p36 achieved significance (11.54–15.71 MB, LOD = 3.51), whereas it was not even suggestively linked at lower marker densities, as did chromosome 11q14.1 (89.32–90.15 MB, NPL = 4.15). Four chromosomes had loci at which both SZ and BP had NPL ≥ 1.98, which was more than would be expected by chance (empirical P = 0.01 using simulated SZ scans; 0.07 using simulated BP scans), although they did not necessarily meet criteria for suggestive linkage individually. These results suggest that high-density marker maps may provide greater power and precision in linkage studies than lower density maps. They also further support the hypothesis that SZ and BP share at least some risk alleles. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
In the past several years, considerable advances have been made in uncovering the genetic basis of both schizophrenia (SZ) and bipolar disorder (BP). Genome-wide linkage scans have been performed in several ethnic groups for about two decades [Segurado et al., 2003; Ng et al., 2008], resulting in numerous positive findings. However, inconsistent results have been the rule, with no genomic region significantly linked in multiple samples. Nevertheless, some of these regions have yielded replicated susceptibility genes [Serretti and Mandelli, 2008; Schwab and Wildenauer, 2009]. More recently, whole genome association studies have identified a number of novel genes for both SZ [Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009] and BP [Ferreira et al., 2008; Sklar et al., 2008; Scott et al., 2009; Smith et al., 2009]. A major limitation of GWAS is that they have so far used genotyping platforms consisting of only common SNPs. Furthermore, DNA chip platforms are currently limited to about 1 million SNPs, and may therefore miss risk alleles, which are not in LD with SNPs on the platform.
Linkage therefore remains a potentially important method. It has been suggested that linkage signals may be produced by multiple common as well as rare variants that are not so deleterious as to significantly impair reproductive fitness, and therefore, can segregate in families [Holmans et al., 2009]. Because of their much greater marker density, marker maps consisting of large numbers of single nucleotide polymorphisms (SNPs) may have greater polymorphism information content (PIC) than standard microsatellite maps. We previously reported significant linkage of BP to 6q using a 10,000 SNP marker map, but not a microsatellite map [Middleton et al., 2004]. This region went on to be validated in a meta-analysis of multiple samples [McQueen et al., 2005].
In the current study, we sought to follow up our previous linkage scan of SZ, which utilized several hundred microsatellite markers [Sklar et al., 2004], as well as that of BP [Middleton et al., 2004], in the Portuguese Island Collection. Families were genotyped for approximately 57,000 SNP markers. We hypothesized that we would confirm linkage of SZ to 5q, and BP to 6q and 11q, as well as to detect regions of linkage to both disorders, which may have gone undetected in our previous studies due to their lower PIC.
One of the more far-reaching but contentious issues in recent psychiatric genetic studies has been the genetic overlap of a number of psychiatric disorders, most prominently, SZ and BP. A very large recent family study demonstrated co-aggregation of the two disorders [Lichtenstein et al., 2009]. GWA studies [Purcell et al., 2009; Wang et al., 2010; Ripke et al., 2011; Sklar et al., 2011] have demonstrated jointly associated loci, as well as overall sharing of polygenic risk between them [Purcell et al., 2009]. We sought to extend these results to the linkage framework using Genome Scan Correlation, which we have described previously [Fanous et al., 2007].
METHODS
Subject Ascertainment and Assessment
Full details of subject ascertainment and assessment have been presented in detail elsewhere [Sklar et al., 2004]. Families were ascertained from the systematic screening of clinicians, treatment facilities, social services, and extensive family interviews. In the Azores and Madeira, all psychiatric and general hospitals participated. On the mainland, families were identified by collaborators at the University of Coimbra. Informed consent was obtained from participants in the genetic and family studies. Collection of blood and family history information was approved by all of the appropriate institutional review boards. On the islands, families were ascertained if both of the proband's parents and all four of the grandparents were native to the islands. Diagnoses were made from data obtained with the Diagnostic Interview for Genetic Studies (DIGS) [Nurnberger et al., 1994]. Best-estimate diagnoses according to the Diagnostic and Statistical Manual, fourth edition (DSM-IV), were made by two independent blinded researchers after review of clinical information, the DIGS, the Operational Criteria Checklist for Psychotic Illness [McGuffin et al., 1991], and written narratives. Diagnostic and rating procedures were identical for the Azores and Madeira.
Laboratory Methods
DNA was isolated from 10 ml of venous blood collected from subjects in the clinic or in their homes. DNA extraction and preparation for the Human Mapping Assay was performed in the laboratory of FM, and have been described elsewhere in detail. Samples were processed according to the GeneChip Mapping Assay Manual (Affymetrix, Santa Clara, CA). In the current study, we used the GeneChip Human Mapping Xba array 240, which contains 58,624 probe sets (SNPs). Each DNA sample was added to a Xba array and hybridized at 48°C for 16 hr in an Affymetrix GeneChip Hybridization Oven at 60 rpm. After 16 hr, the probe arrays were washed and stained according to the GeneChip Mapping Assay Manual by use of the DNAARRAY_WS2 protocol on the Affymetrix Fluidics Station 400. Arrays were scanned once with the Agilent GeneArray 2500 scanner and analyzed with Affymetrix GeneChip DNA Analysis Software (GDAS) to generate genotype calls for each of the SNP probes on the array.
Linkage Analysis
Prior to linkage analysis, all genotypes were checked for non-Mendelian inheritance and excessive recombination events using the “–error” function in Merlin [Abecasis et al., 2002]. Erroneous genotypes were removed using the Pedwipe routine. We employed autosomal genome-wide multipoint nonparametric linkage analysis in Merlin. The Sall statistic was calculated, which uses all relative pairs. Both NPL and Kong and Cox LOD scores [Kong and Cox, 1997] were calculated. Allele frequencies were calculated from pedigree founders only. Markers not in Hardy–Weinberg equilibrium (P ≤ 0.001) were excluded. We used three definitions of affection: SZ (including schizophrenia and schizoaffective disorder, depressed type), BP, and PSY (adding bipolar disorder with psychotic features to the SZ definition). These three definitions included 52, 44, and 90 total genotyped sibling pairs, respectively, from 47 families. Distributions of genotypes siblings were as follows. There were 27 families with BP sibpairs only; of these, 22 had 2 affected siblings, 4 had 3 affected siblings, and one had 4 affected siblings. There were 19 families with SZ sibpairs only; of these, 12 had 2 affected siblings, 4 had 3 affected siblings, 2 had 4 affected siblings, and one had 6 affected siblings. Six BP families had siblings with psychotic BP. These were added to the SZ families for the PSY phenotype, which included 25 families with 2 affected siblings, 11 with 3, 3 with 4, and one with 6. One family had 3 BP and 2 SZ siblings. Fewer SZ families were included in this study compared to our previous scan, due to budgetary constraints, which could have reduced the power to detect linkage.
We adjusted for the effects of marker-to-marker LD using the –rsq option in Merlin with a r2 cutoff of 0.05, as previously suggested by simulation studies [Levinson and Holmans, 2005]. Mean inter-marker distance was 48,228.44 bp. Mean PIC (SD) was 0.946 (0.018). There were a total of 57,418 SNP markers in Hardy–Weinberg equilibrium. Unlikely genotypes were removed using the –error and –pedwipe routines, and occurred at a rate of 0.54%. Based on these, 18,877 clusters were retained by Merlin for linkage analysis. Clusters with non-Mendelian inheritance and obligate recombinants were dropped by Merlin in the families in which they occurred.
Genome-wide significance levels were calculated from 1,000 simulated genome scans constructed using the method of gene-dropping, in Merlin [Abecasis et al., 2000]. Each simulated data set used the original phenotypes, and generated simulated genotypes with the same allele frequencies, marker spacing, and missing data pattern as the original genotypes. These simulated data sets were used to define NPL and Kong and Cox LOD criteria for suggestive (less than once per scan given the null hypothesis of no linkage) and significant linkage (less than once per 20 scans) [Lander and Kruglyak, 1995]. Simulated scans also used the –rsq option with r2 cutoff of 0.05.
Genome-Scan Correlation
- (1)
At each locus, we ranked the two NPLs, SZ, and BP, from highest to lowest.
- (2)
We sorted all loci from highest to lowest, by the least of their two NPL's: either SZ or BP. This resulted in a series of “peaks” comprising loci with high NPLs for both SZ and BP.
- (3)
To eliminate the effects of spatial autocorrelation, we retained only the highest such locus per chromosome.
- (4)
For each of the highest joint linkage peaks we observed in (3), we counted the chromosomes in the real scan with an NPL at least as high for both SZ and BP.
- (5)
We repeated steps 1–4, but using the real SZ scan and each of the 1,000 simulated BP scans.
- (6)
An empirical P-value (real SZ-sim BP P) was calculated as (d + 1)/(n + 1), where d is the number of simulated BP scans exceeding the number observed in (4), and n is the total number of simulated BP scans (n = 1,000).
We then repeated these steps, but this time using the real BP and simulated SZ scans to calculate empirical significance (real BP-sim SZ P). We opted not to examine the distribution of joint linkage in only the simulated scans of both phenotypes, as this is a relationship between two null distributions, which could have inflated our observed empirical significance. Following analogous rank-based methods, such as ordered subsets analysis [Hauser et al., 2004], we started this procedure with the highest observed jointly linked peak. If that was significant, we stopped. If not, we proceeded to progressively smaller jointly linked peaks until we encountered a peak NPL that was significant, and then stopped.
RESULTS
Criteria for Suggestive and Significant Linkage
We calculated the NPLs corresponding to significant linkage from our gene-dropping simulations as 4.07 for SZ, 3.99 for BP and 4.04 for PSY. NPLs corresponding to suggestive linkage were, respectively, 3.10, 3.02, and 3.09. Significant Kong and Cox LOD scores were calculated as 3.52 for SZ, 3.48 for BP, and 3.52 for PSY, and suggestive Kong and Cox LODs were, respectively, 2.11, 2.04, and 2.02.
Linked Regions of Note
A number of chromosomal regions were suggestively linked using empirical criteria. All are listed in Table I. We have presented NPL and/or Kong and Cox LOD traces, as appropriate, for notable linkage regions in Figure 1. We report linkage peaks as 95% confidence intervals, corresponding to the 1-LOD drop-down region, based on the NCBI36 (hg18) assembly. However, we opted to report such peaks even if they were closer than 30 cM from the nearest peak. We did this because the high PIC of the marker map may increase the spatial resolution of disease gene location estimates.
| SNP | Chr | cM | Maximum NPL (nearest maximum LOD) | 95% CI's of NPL (MB) | Bands 95% CI's of NPL | ||
|---|---|---|---|---|---|---|---|
| Bipolar | Schizophrenia | Psychosis | |||||
| rs1361912 | 1 | 21.881 | 3.96 (3.51*) | −0.37 | 0.55 | 11.54–15.71 | 1p13.2–1q21.3 |
| rs2033015 | 2 | 80.041 | 3.31 (2.08) | −0.64 | 1.3 | 55.00–57.53 | 2p16.1–2p16.1 |
| rs10496148 | 2 | 91.502 | 3.28 (2.49) | −0.57 | 0.65 | 64.41–72.60 | 2p14–2p13.2 |
| rs16760 | 6 | 90.41 | 3.17 (2.01) | 2.24 | 2.88 | 77.69–82.32 | 6q14.1–6q14.1 |
| rs9285394 | 6 | 111.88 | 3.1 (1.74) | 0.71 | 2.39 | 106.08–111.28 | 6q21–6q21 |
| rs2022476 | 6 | 127.27 | 3.01 (1.51) | −0.47 | 1.88 | 117.33–129.53 | 6q22.1–6q22.33 |
| rs988322 | 11 | 89.72 | 4.15* (3.44) | 0.58 | 1.86 | 83.16–84.42 | 11q14.1–11q14.1 |
| rs1573567 | 11 | 95.39 | 3.08 (2.24) | 1.26 | 1.46 | 91.00–94.29 | 11q14.3–11q21 |
| rs1387381 | 16 | 111.733 | 3.81 (2.64) | −0.33 | 0.09 | 81.52–82.68 | 16q23.3–16q23.3 |
| rs175304 | 20 | 38.814 | 3.75 (2.24 | 0.73 | 1.41 | 13.21–16.27 | 20p12.1–20p12.1 |
| rs874590 | 3 | 171.58 | 0.92 (0.21) | 3.17 (1.52) | 3.07 (1.54) | 166.89–171.43 | 3q26.1–2q26.2 |
| rs4705049 | 5 | 150.111 | −1.04 | 3.18 (3.08) | 3.04 | 146.44–147.95 | 5q32–5q32 |
| rs10516059 | 5 | 177.699 | 1.64 | 3.18 (2.29) | 3.67 | 149.06–158.84 | 5q32–5q33.3 |
| rs9324684 | 5 | 156.566 | −0.3 | 2.94 | 3.15 (2.28) | 149.98–158.85 | 5q34–5q35.2 |
| rs10516069 | 5 | 179.175 | 1.72 | 3 | 3.93 (3.35) | 166.38–170.03 | 5q34–5q35.1 |
| rs10508608 | 10 | 45.014 | −0.74 | 1.61 | 3.22 (2.46) | 16.73–24.74 | 10p13–10p12.1 |
| rs1414686 | 10 | 73.635 | −0.09 | 2.59 | 3.89 (2.97) | 52.51–60.37 | 10q11.23–10q21.1 |
| rs3848643 | 19 | 41.544 | 2.07 | 2.53 | 3.49 (2.71) | 15.86–35.02 | 19p13.12–19q12 |
| rs6077578 | 20 | 29.168 | 2.63 | 1.92 | 3.49 (2.38) | 5.62–10.61 | 20p12.3–20p12.2 |
| rs3850528 | 20 | 59.239 | 2.26 | 0.54 | 3.38 (2.57) | 31.23–40.51 | 20q11.21–20q12 |
| rs237459 | 20 | 75.831 | 3.15 | 1.2 | 3.44 (2.15 | 42.91–48.27 | 20q13.12–20q13.13 |
| rs2154924 | 22 | 2.719 | 2.36 | 1.06 | 3.26 (2.23) | 14.92–18.01 | 22q11.1–22q11.21 |
- In this table, maximum NPL's and their respective markers and 95% confidence intervals are listed. Bold type indicates suggestive significance based on 1,000 gene-dropping simulations. For suggestive maximum NPL's, the closest maximum LOD is given in parentheses. Maximum LODs were physically very close to maximum NPL's as seen in Figure 1. In the case of the significant linkage of rs988322 to BP, they were at the same marker. The juxtaposition of NPLs for SZ, BP, and PSY at the same markers highlights several loci, which attain NPL's close to ≥2.0 for SZ and BP, which are mutually exclusive phenotypes. MB positions are derived from the Genome Reference Consortium human genome build 37. We opted to report linkage peaks even if they were closer than 30 cM from the nearest peak. We did this because the high polymorphism information content of the marker map may increase the spatial resolution of disease gene location estimates.
- * Eempirical genome-wide significant linkage.
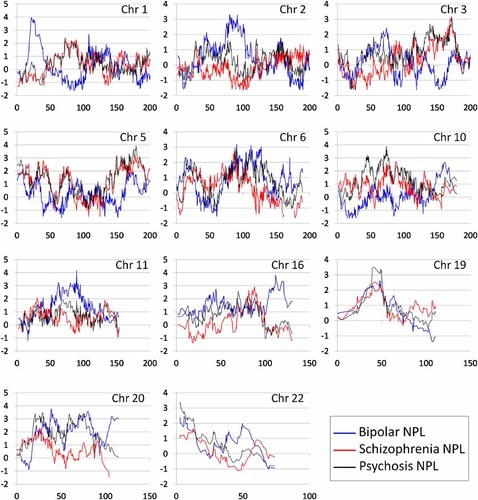
NPL traces at selected chromosomes in an autosomal genome-wide linkage study of schizophrenia, bipolar disorder, and psychosis. NPL statistics (Y-axis) are plotted against centimorgan position (X-axis) at selected chromosomes for schizophrenia, bipolar disorder, and psychosis. Schizophrenia NPLs are in red, bipolar NPLs are in blue, psychosis NPLs are in black. Chromosomes 5p, 6q, 19q, and 22q all contain loci at which both SZ and BP had NPLs of ≥1.98.
Schizophrenia and Psychosis
Our previously linked region on chromosome 5q [Sklar et al., 2004] attained a maximum NPL of 3.18 for SZ at 149.1–158.4 MB. The NPL trace for PSY was very similar in shape and location, but had a maximum NPL of 3.93. Although not achieving empirical criteria, BP had NPLs in the 1.8–2.0 range around 159–169.7 MB. In addition, we observed another distinct and very narrow peak for SZ and PSY at 146.44–148 MB. The maximum LOD for this latter region (3.08, at the same marker) was considerably higher than that in the larger 149.1–158.4 MB region (2.29).
The only other region suggestively linked to SZ was 3q26.1–q26.3 (166.89–171.43 MB). However, a number of others met suggestive criteria for the PSY phenotype. This included chromosome 10 at 17–25 MB, as well as at 53–60 MB; chromosome 20 at three peaks: 6–11, 31–41, and 43–48 MB; and chromosome 22 at 14.92–18.01 cM.
Bipolar Disorder
Two regions attained significant linkage using either NPL or Kong and Cox LOD criteria. Marker rs988322 had an NPL of 4.15, on chromosome 11q14.1. The maximum LOD in this region was 3.44, at the same marker, and just below the LOD significance criterion of 3.48. Marker rs1339365, on chromosome 1p36.21, had a significant LOD of 3.51. The 95% LOD CI was 11.54–15.60 Mb; while the 95% NPL CI corresponded closely, at 11.54–15.71 Mb.
Chromosome 2 (55–58 and 64–73 MB), chromosome 16 (82–83 MB), and chromosome 20 (13–16 MB) were suggestively linked as in our previous study [Middleton et al., 2004]. Chromosome 6q, while previously significantly linked, was now only suggestively linked (NPLs of 3.17, 3.1, and 3.01 at 107–111, 117–130, and 70–90 MB, respectively).
Overlap Between Linkage Signals in Scans of Bipolar Disorder and Schizophrenia
We observed a number of loci at which both BP and SZ attained high NPLs. A locus at chromosome 19 (39.54 cM) attained a NPL of at least 2.32 for both phenotypes (SZ NPL = 2.36, BP NPL = 2.32) and was the top jointly linked locus in the genome. However, one chromosome with both phenotypes achieving NPL ≥ 2.32 was not empirically significant based on the simulated scans. However, we observed four loci with NPL ≥ 1.98 for both phenotypes, on chromosomes 5, 6, 19, and 20. The occurrence of four loci with NPL ≥ 1.98 in both BP and SZ was more than would be expected by chance based on the distribution of such jointly linked chromosomes in our simulated scans (empirical P = 0.01 using simulated SZ scans; 0.07 using simulated BP scans). These four loci are presented in Table II.
| Chr | cM | Maximum NPL | 95% CI's of NPL in cM | 95% CI's of NPL in MB | Chromosomal Bands of 95% CI's of NPL | |
|---|---|---|---|---|---|---|
| Bipolar | Schizophrenia | |||||
| 5 | 8.439 | 1.98 | 2.13 | 0–10.658 | 0–4.031 | 5p15.33–5p15.33 |
| 6 | 90.41 | 3.17 | 2.24 | 84.224–90.683 | 70.987–80.555 | 6q13–6q14.1 |
| 19 | 39.542 | 2.32 | 2.36 | 38.594–52.282 | 16.629–31.138 | 19p13.11–19q12 |
| 20 | 25.789 | 2.08 | 2.27 | 17.399–34.834 | 5.542–11.696 | 20p12.3–20p12.2 |
DISCUSSION
We report a family-based genome-wide linkage survey of schizophrenia and bipolar disorder with the densest marker map that we know of to date, using over 50,000 SNP markers. At several chromosomes, we observed considerable narrowing or breaking up of linked regions previously observed in our lower density scans [Middleton et al., 2004; Sklar et al., 2004]. Simulation studies have indicated that disease gene location estimates of microsatellite-based scans are imprecise [Roberts et al., 1999]. Furthermore, localization of complex trait genes with high resolution requires many crossovers, and hence, sample sizes much larger than ours [Kruglyak and Lander, 1995]. However, the precision of very high-density linkage maps has not been assessed. We have therefore opted to discuss below potential candidate genes in selected narrow linked regions, as it has not been ruled out that such high-density maps improve precision. Furthermore, the narrowness of the peaks we observed is consistent with that seen in other recent studies, suggesting that they may in fact represent an increase in precision [Fallin et al., 2010; Fradin et al., 2010].
Schizophrenia and Psychosis
3q26.1–q26.31
Few studies have previously implicated this region in SZ [Ng et al., 2008]. However, this region has been linked to autism [Allen-Brady et al., 2009], which may etiologically overlap with SZ. Further searches for SZ susceptibility genes in this region are warranted.
5q32–q33.3
As we have previously demonstrated in a sparser, independent microsatellite map in a portion of this sample, chromosome 5q provides considerable, although not genome-wide significant, evidence of linkage in this Portuguese sample. In addition to the main linkage peak we observed on 5q at 149.06–158.84, there was an additional and much narrower one at 146.4–148 MB. Furthermore, the latter peak had considerably higher LODs than the former, more telomeric peak, suggesting a lower probability of false-positive linkage. While these two peaks are too close together to be resolved on the basis of intervening crossovers, the 146.1–148 MB peak contains a number of potentially relevant genes. These include Dihydropyrimidinase-like 3 (DPYSL3), which is involved in neuronal differentiation, axonal outgrowth and neuronal regeneration [Kowara et al., 2008]; protein phosphatase 2, regulatory subunit B, beta (PPP2R2B), which causes autosomal dominant spinocerebellar ataxia 12 (SCA12), a neurodegenerative disorder [Dagda et al., 2008]; SH3 domain and tetratricopeptide repeats 2 (SH3TC2), which can cause autosomal recessive Charcot–Marie–Tooth disease type 4C, also a neurodegenerative disease [Roberts et al., 2010]. The involvement of these three genes would be consistent with neurodevelopmental [Weinberger, 1996] and neurodegenerative etiologies of SZ [Woods, 1998]. The serotonin 4 receptor (HTR4) is present at the telomeric end of this narrow region, the involvement of which would be consistent with other evidence suggesting serotonergic dysfunction in SZ, such as the blockade of 5HT receptors by atypical antipsychotics [Kapur and Remington, 2001].
Chromosome 10p-q
Chromosome 10p12 has been linked to SZ in a number of independent samples [Ng et al., 2008; Holmans et al., 2009], including significant linkage in a large, multicenter study [Holmans et al., 2009]. It has also been linked to psychotic BP [Cheng et al., 2006], which is consistent with the increase in significance observed in the current study when psychotic BP was added to SZ in the PSY phenotype. The linked region in our microsatellite-based study [Sklar et al., 2004] appeared to divide into two distinct regions attaining suggestive linkage in the current study: 16.73–24.74 MB (10p13–p12.1) and 52.51–60.37 MB (10q11.23–q21.1). These are likely to be far enough apart to be resolved by intervening crossovers.
19p13.2–q12
This chromosome 19 region had not previously been linked in our sample using a traditional microsatellite map, but now met empirical criteria for suggestive linkage to PSY. The linkage traces of SZ and BP, although not meeting suggestive criteria individually, are remarkably similar in shape and location (Fig. 1). It has not been linked in many previous scans. A population based linkage method was used to find linkage to chromosome 19q13 (46.5–48.8 MB) in SZ and BP samples from Scotland and Germany [Francks et al., 2008], while linkage to BP has been reported to 19q12–q13 [Badenhop et al., 2002]. Other studies have reported linkage at more telomeric regions of 19q to phenotypes including neurocognitive performance [Almasy et al., 2008], Deficit Syndrome in SZ [Fanous et al., 2008], and BP [Venken et al., 2008].
22q11.1
We observe suggestive linkage to PSY to this region, whereas our previous studies found none, in any phenotype. This region has been linked in a number of previous studies of both BP [Badner and Gershon, 2002] and SZ [Pulver et al., 1994], as well as psychotic BP [Potash et al., 2003]. A recent large, multicenter study reported heterogeneity at this locus [Holmans et al., 2009]. One of the most prominent cytogenetic findings in psychiatric illness has been a deletion on chromosome 22q11 known to cause velocardiofacial syndrome (VCFS) [Ivanov et al., 2003] in which about a third of patients have psychiatric conditions, including SZ and autism. However, the critical genes and regions responsible for susceptibility to psychosis remain unknown.
Bipolar Disorder
Our current study confirmed regions we previously reported to be suggestively linked, such as chromosomes 2 and 16q [Middleton et al., 2004], while chromosome 4 no longer met criteria. Chromosome 6q no longer met criteria for significance, although it remained suggestively linked, and has been amply discussed elsewhere [Middleton et al., 2004; McQueen et al., 2005].
1p36
This region was not even suggestively linked in our previous scan of BP, but now met criteria for significant linkage. Previous studies have reported suggestive [Cichon et al., 2001] and significant linkage [Schumacher et al., 2005] of BP to 1p36, as well as to recurrent major depressive disorder [McGuffin et al., 2005]. This region should now be considered to be definitively linked and merits thorough follow-up with sequencing and association studies.
11q13.3–q21
We previously reported significant linkage to chromosome 11 at 45–68 MB [Middleton et al., 2004]. At our current density, we observe that the peak is now at 83.16–84.42 MB, which has not been as frequently linked to BP as has 11p [Serretti and Mandelli, 2008]. Furthermore, we observed an extremely narrow 95% CI, which meets criteria for a peak using the strict criterion of having no other peak within 30 cM (unlike the 5q and 6q peaks). As with the 1p31.1 peak, this one is too narrow to be resolved by linkage, but merits further scrutiny, as its 95% CI's match very closely to the coordinates of the gene DLG2, which encodes postsynaptic density protein PSD-93.
DLG2 is a membrane-associated guanylate kinase (MAGUK), which is thought to couple synaptic NMDA receptors to binding complexes, which mediate long-term potentiation, and binds to the neuregulin receptor ErbB-4 [Garcia et al., 2000]. This is of interest given the widely held glutamatergic hypothesis of schizophrenia [Goff and Coyle, 2001]. DLG2 has recently been found to be disrupted by structural variants [Walsh et al., 2008], and its expression altered in the anterior cingulated cortex, in SZ [Kristiansen et al., 2006]. In our recent integrative analysis of a putative schizophrenia gene network with enriched signal in GAIN and CATIE GWAS datasets, DLG2 was found to be top ranked in a subnetwork that also included ErbB-4, NOS1, GRID1, GRIN1, and GRIN2B. It was additionally highlighted in a glutamate receptor signaling pathway based subnetwork analysis [Sun et al., 2010]. These subnetworks are likely to be involved in SZ. Considering the possibility that the narrowness of this peak could be due to precipitous drops in PIC on either side of it, rather than a true linkage signal, we calculated PIC of all the SNP clusters employed by Merlin on the chromosome. We did not observe any drastic changes in PIC in the clusters surrounding this peak. Nevertheless, it is unlikely that our sample size could contain enough crossovers to resolve a linkage signal to such a fine scale. Our results do, however, strengthen the evidence for a BP locus on chromosome 11, given these results and our previous report [Middleton et al., 2004].
Overlap of Schizophrenia and Bipolar Disorder
While a number of linkage studies have demonstrated regions linked to both BP and SZ [Maziade et al., 2001, 2009; Francks et al., 2008], none have previously tested whether such overlap is statistically significant on a genome-wide basis. Furthermore, the most complete linkage meta-analyzes for each disorder have not provided evidence of regions commonly linked [McQueen et al., 2005; Ng et al., 2009]. More recently, large-scale GWAS studies have demonstrated shared polygenic factors for both [Purcell et al., 2009] as well as joint association at top GWAS hits for either, including CACNA1C [Ripke et al., 2011] and ZNF804A [Steinberg et al., 2010]. Using our method of Genome Scan Correlation, we observed a greater number of loci jointly linked to SZ and BP than would be expected by chance, as modeled in our gene-dropping simulations. This method required NPLs for BP and SZ to occur at the same marker in order for that marker to be counted as jointly linked. This was conservative, as linkage, even at this density, should not be considered to be precise in its estimates of location [Roberts et al., 1999]. It was also conservative in only counting one such marker per chromosome, as multiple regions in the same chromosome could harbor susceptibility genes for either or both disorders. Our finding that the number of jointly linked loci is greater than what would be expected by chance is broadly consistent with other studies suggesting that SZ and BP do in fact have shared genetic risk factors [Lichtenstein et al., 2009; Purcell et al., 2009]. The four jointly linked loci we observed, which were more than would be expected by chance, did not themselves attain criteria for suggestive or significant linkage. Therefore, although this finding was empirically significant, it is not likely to account for a large portion of the putative genetic overlap between the two disorders, with many of their genetic risk factors being unique to only one of them. Furthermore, this is a relatively small sample. Therefore, our power to detect loci linked to either disorder may have been limited by type II error. An additional limitation is the possibility of greater than 50% sharing of alleles between siblings, which could violate the assumptions of the simulation study and inflate the observed overlap. However, this excess sharing is likely to be very small (50.43% in a previous study in the Hutterite population) [Zollner et al., 2004].
PSY was not independent of either SZ or BP, and furthermore, most PSY subjects had SZ rather than BP. Therefore, we did not implement empirical tests of overlap of PSY with either BP or SZ. However, we did note that PSY peaks at 5q and 10pq were considerably larger than those seen for SZ, suggesting that some cases of psychotic BP are have predisposition loci at these chromosomes, which have been linked to SZ in a number of studies. This therefore supports the use of broad definitions of affection in genetic studies of SZ.
Acknowledgements
We thank the families for their participation. Financial support for this study was provided by awards from the National Alliance for Research on Schizophrenia and Depression (to C.N.P., M.T.P., and J.L.K.), as well as the NIMH (MH52618 and MH058693 [to C.N.P. and M.T.P.]) and the Department of Veterans Affairs Merit Review Program (to A.H.F, M.T.P., and C.N.P.) We would also like to acknowledge Dr. Silviu-Alin Bacanu for helpful discussions and analyses of an earlier, smaller subset of this dataset, and to thank Ms. Laura Chopko for editorial assistance.




