Evidence for three loci modifying age-at-onset of Alzheimer's disease in early-onset PSEN2 families†
How to Cite this Article: Marchani EE, Bird TD, Steinbart EJ, Rosenthal E, Yu CE, Schellenberg GD, Wijsman EM. 2010. Evidence for Three Loci Modifying Age-at-Onset of Alzheimer's Disease in Early-Onset PSEN2 Families. Am J Med Genet Part B 153B:1031–1041.
Abstract
Families with early-onset Alzheimer's disease (AD) sharing a single PSEN2 mutation exhibit a wide range of age-at-onset, suggesting that modifier loci segregate within these families. While APOE is known to be an age-at-onset modifier, it does not explain all of this variation. We performed a genome scan within nine such families for loci influencing age-at-onset, while simultaneously controlling for variation in the primary PSEN2 mutation (N141I) and APOE. We found significant evidence of linkage between age-at-onset and chromosome 1q23.3 (P < 0.001) when analysis included all families, and to chromosomes 1q23.3 (P < 0.001), 17p13.2 (P = 0.0002), 7q33 (P = 0.017), and 11p14.2 (P = 0.017) in a single large pedigree. Simultaneous analysis of these four chromosomes maintained strong evidence of linkage to chromosomes 1q23.3 and 17p13.2 when all families were analyzed, and to chromosomes 1q23.3, 7q33, and 17p13.2 within the same single pedigree. Inclusion of major gene covariates proved essential to detect these linkage signals, as all linkage signals dissipated when PSEN2 and APOE were excluded from the model. The four chromosomal regions with evidence of linkage all coincide with previous linkage signals, associated SNPs, and/or candidate genes identified in independent AD study populations. This study establishes several candidate regions for further analysis and is consistent with an oligogenic model of AD risk and age-at-onset. More generally, this study also demonstrates the value of searching for modifier loci in existing datasets previously used to identify primary causal variants for complex disease traits. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Alzheimer's disease (AD; MIM104300) is the most prevalent form of dementia, affecting 5–10% of individuals ≥65 years of age and as many as 45% of individuals over the age of 85 [Evans et al., 1989; Canadian Study of Health and Aging Working Group, 1994]. AD is characterized by progressive memory loss and decline of cognitive abilities associated with neurofibrillary tangles, amyloid-β plaques, and deposition of amyloid in cerebral blood vessels [Wisniewski et al., 1993; Price et al., 1998; Dickson, 2001]. This devastating neurodegenerative disease is responsible for high financial and social costs to both patients and caregivers [Ernst and Hay, 1994; Bloom et al., 2003; Lopez-Bastida et al., 2006], which could be reduced dramatically if onset of the disease could be delayed [Brookmeyer et al., 1998; Zhu et al., 2006].
AD has a complex genetic basis, resulting in variation in age-at-onset. Highly penetrant mutations in three genes (APP, PSEN1, PSEN2) are associated with rare Mendelian-dominant early-onset (EOAD) forms of the disease [Goate et al., 1991; Schellenberg et al., 1992; Levy-Lahad et al., 1995a,b; Rogaev et al., 1995; Sherrington et al., 1995]. However, the majority of AD cases are late-onset (LOAD), and APOE is the only risk locus for LOAD to consistently replicate in both association [Coon et al., 2007; Grupe et al., 2007; Abraham et al., 2008; Beecham et al., 2009] and linkage studies [Pericak-Vance et al., 2000; Myers et al., 2002; Sillen et al., 2008]. The APOE ε4 allele reduces age-at-onset in a dose-dependent manner in both LOAD [Saunders et al., 1993; Strittmatter et al., 1993; Farrer et al., 1997] and some EOAD pedigrees [Pastor et al., 2003; Wijsman et al., 2005]. This suggests shared biological pathways between the early- and late-onset forms of AD.
It is likely that additional loci may be identified that explain variation in AD onset. The EOAD mutations in APP, PSEN1, and PSEN2 are highly penetrant, but they are rare at the population level [Campion et al., 1999]. The more-common APOE ε4 allele is neither necessary nor sufficient to cause LOAD [Corder et al., 1993; Strittmatter et al., 1993; Evans et al., 1997; Slooter et al., 1998], and variation within APOE is estimated to explain only 4–15% of the variance in age-at-onset [Bennett et al., 1995; Slooter et al., 1998; Daw et al., 2000; Tunstall et al., 2000]. As heritability measures estimate that up to 80% of disease risk may be due to genetic factors [Pedersen et al., 2004; Gatz et al., 2006], it appears that additional risk loci remain to be found. There is no shortage of candidate loci, as evidence for linkage or association with AD has been reported for every autosome in the human genome [Bertram et al., 2007]. Validated identification of modifier and risk variants would likely illuminate the mechanisms involved with the etiology and progression of AD, and could provide additional tools for screening, treatment, delay of onset, and prevention.
Special strategies are necessary to identify risk or modifier loci. Several studies have combined samples from multiple centers in an attempt to increase power to detect these loci using association- [Coon et al., 2007; Reiman et al., 2007; Harold et al., 2009; Lambert et al., 2009] or linkage-based approaches [Pericak-Vance et al., 1998, 2000; Li et al., 2002; Scott et al., 2003; Hamshere et al., 2007]. This strategy, not to be confused with a meta-analysis of published studies, has a limit: as more datasets are merged, fewer remain available for replication. If the majority of datasets must be merged to detect a linkage or association signal, it is not likely the remaining independent datasets will have adequate power to replicate that signal. Alternatively, some investigators have successfully identified modifier loci for other complex diseases by focusing on phenotypic variation within families segregating monogenic forms of the disease [Riazuddin et al., 2000, 2006; Hasstedt et al., 2004; Daw et al., 2008]. Although all possible modifier loci are unlikely to be identified within a single population or pedigree, this focus on monogenic forms of a disease improves the power to detect those loci segregating within the dataset. The power to detect modifier loci or risk loci can be further increased by (i) the analysis of large pedigrees [Heath et al., 1997; Williams et al., 1997]; (ii) the analysis of quantitative phenotypes [Wijsman and Amos, 1997]; and (iii) the reduction of missing data, including censored data, and population stratification [Balding, 2006].
We have performed a genome scan for age-at-onset (AGEON) modifiers in the Volga German EOAD families [Bird et al., 1988]. Our method of analysis combines the power of an oligogenic model for mapping disease loci with the ability to simultaneously account for the effects of APOE genotype and a shared causal PSEN2 variant (N141I) [Levy-Lahad et al., 1995a]. Analysis of all families detected strong evidence of linkage of AGEON to a single chromosome, while single-family analyses identified another two chromosomes with strong evidence of linkage within a single large pedigree.
MATERIALS AND METHODS
Samples and Markers
Subjects from nine families of Volga German descent were ascertained and evaluated by the University of Washington Alzheimer's Disease Research Center, as previously described [Bird et al., 1988]. The families ranged in size from 3 to 73 individuals, totaling 265 individuals. This study obtained informed consent from all participants and was approved by the University of Washington Institutional Review Board. AGEON is modeled with two components: age-at-onset of AD (AGEAD) for affected individuals, and the age at which an unaffected individual was last known to be free of AD (AGECEN). AGECEN was available for 133 individuals, while AGEAD [Bird et al., 1989] was available for 82 individuals. Individuals without affectation status and AGEON were considered to be missing phenotype data. Table I provides a summary of affectation status for the three largest families in our sample and for all families combined. The remaining families ranged in size from 3 to 21 members.
| All families | HB family | HD family | R family | |
|---|---|---|---|---|
| N | 265 | 73 | 46 | 65 |
| Number affected | 92 | 21 | 15 | 26 |
| Mean (SD) AGEAD | 55.56 (9.55) | 60.89 (8.62) | 55.53 (8.81) | 51.79 (9.39) |
| Range in AGEAD | 39–85 | 49–75 | 46–75 | 40–85 |
| Number censored | 133 | 37 | 20 | 36 |
| Mean (SD) AGECEN | 63.48 (18.39) | 67.19 (15.93) | 70.65 (17.16) | 60.44 (17.73) |
| Range in AGECEN | 16–95 | 27–95 | 30–94 | 28–89 |
The Volga German EOAD families share a highly penetrant Asn141Ile PSEN2 mutation and exhibit a wide range in AGEON that is only partially explained by APOE genotype [Bird et al., 1988, 1996; Wijsman et al., 2005]. APOE and PSEN2 genotypes were obtained as previously described [Hixson and Vernier, 1990; Levy-Lahad et al., 1995a] for 119 and 120 individuals, respectively. Multiallelic STR genome-scan markers (ABI Prism Linkage Mapping set, Version 2.5, MD10, http://www.appliedbiosystems.com) with an average spacing of ∼10 cM apart were genotyped on 114 individuals using either an ABI377 or ABI3100 analyzer. Sex-averaged marker map positions from the Marshfield Center for Medical Research Web Site [Broman et al., 1998] were converted to Haldane positions for statistical analysis.
Statistical Methods
Trait Model
We used a Bayesian MCMC approach to perform oligogenic segregation and linkage analyses using AGEON as a quantitative censored trait [Heath, 1997; Daw et al., 1999]. The oligogenic trait model allows the analysis of continuous censored traits and adjustment for major gene covariates within a family framework, while also capturing a complex mode of inheritance. The reversible-jump MCMC approach provides a computationally tractable way to: (i) perform simultaneous multipoint linkage analyses using large pedigrees; (ii) cope with the large amount of missing data typical of late-onset disease pedigrees; (iii) estimate missing APOE and PSEN2 genotypes for phenotyped individuals, thereby increasing the available information compared to the use of residuals from a complete data analysis [Dickson et al., 2008]; and (iv) estimate trait model parameters without pre-specifying the number of trait loci. The power and reliability of these methods have been well described using simulated [Heath et al., 1997; Wijsman and Amos, 1997; Shmulewitz and Heath, 2001] and real [Daw et al., 1999, 2008; Gagnon et al., 2003] datasets. A detailed review of this methodological approach, as well as the interpretation of results, is available [Wijsman and Yu, 2004].
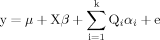 (1)
(1)As described in Equation (1), AGEON (y) is modeled as a linear combination of the baseline age-at-onset (µ), covariate effects, QTL effects, and a normally distributed residual effect (e). AGECEN is treated as a special case of missing data for AGEON: rejection sampling is used to generate pseudo AGEAD values that are greater than the observed AGECEN for each MCMC iteration, as previously described [Daw et al., 1999]. Covariate adjustment takes the form of Xβ, where X represents the covariate incidence matrix and β is the vector of covariate effects. The variable k indicates the number of inferred QTLs present in a given iteration of the MCMC process, Qi is the incidence matrix for the ith QTL, and αi is the vector of genotype effects for Qi. These QTLs may remain unlinked, or may visit different chromosomal locations across MCMC iterations of the linkage analysis, where the concept of “visiting” reflects the fact that the value of the location parameter varies across iterations. All QTL models are diallelic and parameterized by the frequency of the A allele (pA) and genotype means (µAA, µAB, µBB) defined as the difference between the mean phenotypic value among individuals of a given genotype relative to a baseline effect. From these values, the percent of total (VT) and genetic (VG) variance explained can be obtained. PSEN2 and APOE are included as major gene covariates in the oligogenic trait model to reduce the residual variance, as variants within both genes are known to influence affectation status and AGEON within these Volga German families [Bird et al., 1988, 1996; Wijsman et al., 2005]. The performance of the MCMC process was evaluated by checking for proper mixing [Daw et al., 1999; Wijsman and Yu, 2004], and parameter summaries were estimated from the expectation of the parameter of interest across a full run of the MCMC analysis. Since the stochastic nature of the MCMC process produces variability across runs, the results presented here are representative of several such runs and should be interpreted as approximations [Daw et al., 1999].
Segregation analyses
Bayesian MCMC oligogenic segregation and linkage analyses were performed using Loki2.4.7 (http://www.stat.washington.edu/thompson/Genepi/pangaea.shtml). We chose the following values for the required prior distributions: (i) marker allele frequencies were estimated from the observed and inferred data; (ii) the number of QTLs was Poisson with mean 1 and maximum of 17; (iii) QTL allele frequencies were drawn from a uniform 0–1 distribution; and (iv) QTL genotype effects were drawn from a N (0,τ) distribution.
We performed marker-free oligogenic segregation analyses to: (i) identify QTL models consistent with the distribution of phenotypic data within the pedigrees; (ii) estimate total broad-sense heritability within the sample; and (iii) to identify the τ that maximized the genetic variance and number of QTLs in the model [Wijsman and Yu, 2004]. Except where noted, analyses were performed with τ = 640 and a standard-length run of 2,000,000 iterations, saving every 20th iteration for estimation of posterior distributions after an initial 1,000 iteration burn-in period. Segregation and linkage analyses performed with and without pedigree loops did not differ, and so pedigree loops were cut where present to increase the speed of analysis.
Linkage analyses
Genome scans were performed jointly among all pedigrees (all-families) and individually within each of the three largest pedigrees (HB, HD, and R). To minimize computational time, each genome scan was performed using 500,000 iterations, with an initial burn-in period of 1,000 iterations. Evidence of linkage was measured using the Bayes' factor (BF) [Kass and Raftery, 1995]. The BF over a given interval (here, 2 cM) is simply the posterior odds of linkage given the data divided by the prior odds of linkage. Exact BF values are presented in the text for precision, while figures are drawn using log10 (BF) to better illustrate trends. Chromosomes showing evidence of linkage in the initial scan, as defined by a maximum BF (maxBF) > 10, were subject to standard-length runs and included in further analyses if the linkage signal was replicated in the majority of these longer runs.
Single-marker analyses were performed to identify those markers contributing to multipoint linkage signals. Signals supported by multiple markers are less likely to be false signals triggered by map misspecification [Daw et al., 2000]. Single-marker support of linkage was defined by evidence of a signal with a clear peak near the location of the multipoint signal.
“Interesting regions” on each chromosome were defined as the interval including all markers supporting linkage, as well as two flanking markers on either side. We performed marker resimulation over these intervals under the null hypothesis of no linkage to evaluate the empirical significance of the observed linkage signals, as previously described [Igo and Wijsman, 2008]. All P-values presented were obtained from such simulations. We simulated 5,000 marker sets for the chromosome 17p region in the R pedigree, and 1,000 markers sets for each of chromosomes 1, 7, and 11 due to computational time constraints (combined cpu time = 4,564 hr). P-values are estimated by the proportion of iterations with maxBFs at least as large as the observed value within the same 2 cM bin as the original signal (bin-specific) or within the entire interesting region (region-wide). These P-values are approximate, as estimating the tail of a distribution is difficult with limited observations, and should be interpreted as such. Simulated maxBFs >10 were rare, present in as few as 1.1% (R-family, chromosome 7) to as many as 7.7% (all-families, chromosome 1) of simulated datasets. This suggests that using maxBF ≥10 as our threshold to identify moderate linkage signals in the genome scan was reasonable.
Marker data for all chromosomes showing evidence of linkage were analyzed simultaneously within each of the three large pedigrees and among all-families. These multi-chromosomal analyses were used to investigate whether QTL models visiting different chromosomes could coexist, and to identify what pedigrees were contributing to each linkage signal. Multi-chromosomal linkage analyses were performed using long runs of 8,000,000 iterations after an initial burn-in of 1,000 iterations. We sampled 25,000 evenly spaced iterations from these 8,000,000 to generate the posterior distributions.
We evaluated the sensitivity of multi-chromosomal linkage analyses to trait model specifications. Linkage signals were robust to the assigned prior values of τ and mean number of QTLs. Sensitivity analyses excluding either APOE or PSEN2 as major gene covariates were performed, as were analyses of chromosome 19 excluding APOE as both a covariate and a marker.
Additional genotyping was performed to increase marker density surrounding observed linkage signals, including D1S2768, D1S2844, D7S500, D7S509, D7S2473, D11S1308, D11S1755, D11S915, D17S1584, and D17S952. Single-marker and multi-chromosomal analyses were subsequently performed in an effort to both eliminate any spurious signals caused by a lack of marker information and to improve true linkage signals. Because the linkage signal on chromosome 11 is supported by a single marker located ∼1 cM away from a single candidate gene (BDNF), we also genotyped the single marker (rs6265) within BDNF that demonstrated significant evidence of association to AD in the AlzGene meta-analysis [Bertram et al., 2007]. Standard-length linkage analysis runs were performed on chromosome 11 including this SNP as a major gene covariate to determine whether this SNP could explain the observed linkage signal.
Cross-chromosomal correlation caused by a small sample size, such as a single family, may lead to spurious evidence for linkage on multiple chromosomes. When a single family provided evidence of linkage to four chromosomes, we used the mlink program in the FASTLINK/LINKAGE linkage analysis package [Lathrop et al., 1984; Cottingham et al., 1993; Schaffer et al., 1994] to compute lod scores between markers in interesting regions and all other markers in the genome for members of this family. P-values were estimated as the fraction of lod scores genome-wide at least as high as that observed between a given pair of markers.
RESULTS
Segregation Analyses
Segregation analyses estimate that AGEON is highly heritable within the Volga German families (h2 = 0.93), and identified two major QTL models associated with AGEON. Results from the all-families segregation analysis are presented in Figure 1, which shows the distribution of AB and BB genotype means relative to the AA genotype mean. In model 1, the A allele (pA ∼ 0.17) is recessive, reducing AGEON by nearly 40 years among A-allele homozygotes. This model appears slightly overdominant for B-allele carriers, though this is likely an artifact of the ascertainment process [Ma et al., 2007]. In model 2, the A allele (pA ∼ 0.66) is dominant, reducing AGEON by ∼20 years among heterozygotes and A-allele homozygotes. Each model explains ∼10% of the VG in AGEON. This is much less than that explained by PSEN2 (∼74% VG), but nearly triple that explained by APOE (∼3% VG) within this sample.
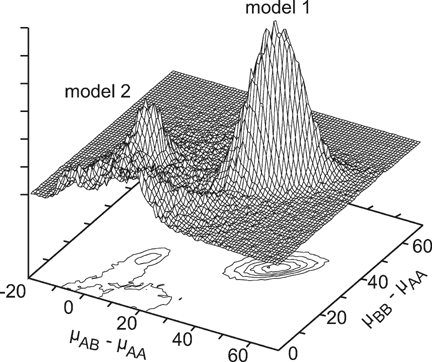
QTL models identified in the segregation analysis of all-families. The heterozygote genotype mean (µAB) relative to the AA genotype mean (µAA) is plotted on the X-axis, while the BB genotype mean (µBB) relative to µAA is plotted on the Y-axis. Genotype means are measured in years, and reflect changes to age-at-onset of AD. The Z-axis is a measure of frequency, the contour and surface plots illustrating the frequency of each QTL model in the segregation analysis run.
Linkage Analyses
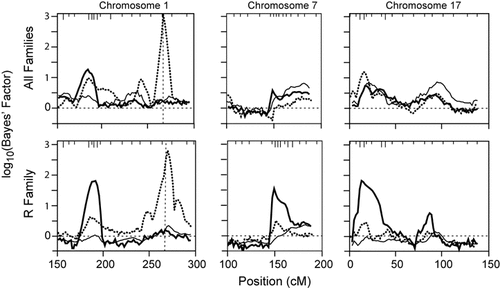
The genome scan for AGEON identified two strong and four moderate linkage signals, shown in Figure 2. A strong signal on chromosome 1 is present both in the all-families analysis and within the R pedigree (∼187 cM, maxBF = 61.51 and 54.25, respectively). Several linkage signals are evident only in the R pedigree: a strong signal on chromosome 7 (∼151 cM, maxBF = 69.75), and modest signals on chromosomes 11 (∼31 cM, maxBF = 11.45), 17p (∼15 cM, maxBF = 13.23), 17q (∼85 cM, maxBF = 12.60), and 18 (∼133 cM, maxBF = 11.51). The HD pedigree has a single modest linkage signal on chromosome 4 (∼201 cM, maxBF = 12.31). Replicate linkage analyses with longer MCMC runs confirmed the signals on chromosomes 1, 17p, 7, and 11, while the initial signals on chromosomes 4, 17q, and 18 failed to consistently replicate and were not pursued further. Similarly, subsequent analyses of the HB and HD families showed no strong evidence of linkage (maxBF < 10), and are not presented.
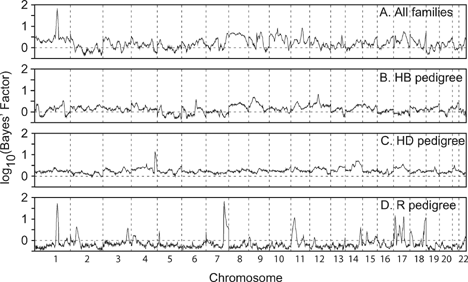
Genome scan for age-at-onset of AD. Chromosomal position is plotted against evidence of linkage, as measured by the log10(Bayes' Factor). Panel A displays the results from analyzing all families, while panels B–D illustrate the results based upon each of the three largest pedigrees. The horizontal line at log10(Bayes' Factor) = 0 indicates the prior and posterior odds of linkage are equivalent. The vertical dotted lines denote chromosomal boundaries.
Chromosome 1 (chr01) exhibited the strongest evidence of linkage in both all-families and R-family analyses. Exclusive analysis of chr01 provided significant evidence of linkage (P < 0.0010) near 1q23.3 in both all-families and R-family analyses (Table II), and identified six markers individually supporting this linkage signal near D1S484 (Supplemental Table A). Multi-chromosomal analyses (Fig. 3) strongly supported linkage to chr01 among all-families (maxBF = 38.70) and within the R-family (maxBF = 16.13). Although all-families analyses did not show strong support for chr01 when evidence of linkage to chromosome 17 was strong, no pair of linkage signals within the multi-chromosomal analyses was mutually exclusive.
| Family | Linkage region | Chromosome-specific maxBF | Empirical P-value analyses | ||||
|---|---|---|---|---|---|---|---|
| Observed maxBF | Region-wide P-value | Bin-specific P-value | Critical value (α = 0.01) | Critical value (α = 0.05) | |||
| All | 1q23.3 | 13.74 | 33.29 | 0.0060 | <0.0010 | 26.74 | 12.52 |
| R | 1q23.3 | 25.95 | 40.41 | 0.0020 | <0.0010 | 18.83 | 7.05 |
| R | 7q33 | 67.33 | 4.57 | 0.0240 | 0.0170 | 11.54 | 2.88 |
| R | 11p14.2 | 12.43 | 4.57 | 0.0320 | 0.0170 | 21.66 | 3.37 |
| R | 17p13.2 | 12.52 | 58.26 | 0.0032 | 0.0002 | 27.24 | 6.08 |
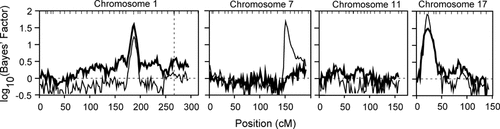
Simultaneous multipoint linkage analysis of chromosomes 1, 7, 11, and 17. All-families results are shown in bold, while R-family results are shown with a thin line. Linkage signals on chromosomes 1 and 17 in the all-families analysis are taken from separate runs, as they did not coincide within a single analysis run. The horizontal line at log10(Bayes' Factor) = 0 indicates the prior and posterior odds of linkage are equivalent. The vertical dotted line marks the location of PSEN2 on chromosome 1. Tic-marks represent marker locations.
Evidence for linkage to chromosome 17 (chr17) was strong in the R-family, and also emerged in multi-chromosomal all-families analyses. Evidence for linkage near D17S938 was highly significant (P = 0.0002; Table II) within the R-family, and supported by individual analyses of four markers (Supplemental Table A). Multi-chromosomal analyses within the R-family (Fig. 3; maxBF = 81.72) and all-families (maxBF = 30.85) both overwhelmingly supported linkage to 17p13.2.
Strong evidence for linkage was detected on chromosome 7 (chr07), but only within the R-family. Analysis of chr07 generated a strong linkage signal near D7S640 (maxBF = 67.33) with nominal significance (Table II), supported by individual analysis of five markers within the R-family (Supplemental Table A). Multi-chromosomal analyses (Fig. 3) of the R-family maintained strong support for linkage, although the chr07 signal was absent from all-families analyses.
Chromosome 11 (chr11) showed promising signs of linkage within the R-family, but the signal dissipated when multiple chromosomes were analyzed at once. Analysis of chr11 alone produced a moderate (maxBF = 12.43) and nominally significant linkage signal in the R-family (Table II) supported by a single marker, D11S904 (Supplementary Table A). Multi-chromosomal analyses of both all-families and the R-family failed to support linkage to 11p14.2 (Fig. 3).
The two QTL models observed in Figure 1 reappeared in the linkage analysis, and a third model, model 3, emerged with the inclusion of marker data. Under model 3, the A allele (pA ∼0.90) is recessive, decreasing AGEON by ∼20 years. Homozygote genetic effects were more variable under model 3, although the model explained the same amount of VG as models 1 and 2. Model 2 was closely associated with chr07 in the R-family analyses. Although both models 1 and 3 were associated with the linkage signals on chr01 and chr17, the relationships were complicated: (i) the linkage signals on chr01 and chr17 tended to share a single model within an analytical run; (ii) either chr01 or chr17 showed strong signs of linkage within a run, not both; and (iii) model 1 was not present in R-family analyses.
Linkage signals on all chromosomes were highly dependent on the major gene covariates, but robust to increased marker density near linkage signals (Fig. 4). Multi-chromosomal linkage analyses excluding APOE as a covariate showed no signs of linkage to chromosomes 1, 7, 11, or 17, while exclusion of APOE both as a marker and a covariate failed to produce a linkage signal near APOE on chromosome 19. Not surprisingly, the exclusion of PSEN2 as a covariate reduced linkage signals to loci other than PSEN2. Although slight evidence of linkage to our interesting regions on chromosomes 1 and 17 was present, these signals were not strong enough to merit further analysis had they been observed in the initial genome scan. Increasing marker density yielded only subtle changes to the observed linkage signals (Fig. 4), although it did identify additional markers supporting linkage to chromosomes 1, 17, and 7 (Supplementary Table A). We found no significant evidence of marker–marker linkage between interesting regions in this family (maximum lod score = 0.39; P > 0.05), suggesting the observed linkage signals on multiple chromosomes are not the result of chance cross-chromosomal linkage.
Sensitivity of linkage signals to major gene covariates and additional marker data. Multi-chromosomal linkage analyses with greater marker density within interesting regions are drawn in bold. Using the original marker panel, APOE sensitivity analyses (thin line) excluded APOE as a marker and covariate, while the PSEN2 sensitivity analyses (dashed line) excluded PSEN2 as a covariate. The horizontal dotted line at log10(Bayes' Factor) = 0 indicates the prior and posterior odds of linkage are equivalent, while the vertical dotted line marks the location of PSEN2 on chromosome 1. Tic-marks represent marker locations, with longer tic-marks indicating markers that support linkage in single-marker analyses. Chromosome 11 did not provided a linkage signal in any of these analyses, and is not shown.
DISCUSSION
Our analysis of AGEON within the Volga German EOAD families has identified linkage signals on 1q23.3, 17p13.2, and 7q33 that merit further analysis. The Volga German families offered a dataset well suited for identifying AGEON modifier loci, as (i) the early AGEAD observed in these families results in less missing phenotype data; and (ii) the families share a single PSEN2 mutation, eliminating allelic heterogeneity within PSEN2 as a source of variation in AGEON. Joint oligogenic segregation and linkage analysis with MCMC sampling allowed us to tackle a censored quantitative trait adjusted for major gene covariates within these large pedigrees. Our linkage signals on chromosomes 1, 17, 7, and 11 are consistent with previous linkage and association results, and most signals lie within 3 Mb of functional candidate genes. Our results suggest that the study of early-onset forms of late-onset disease may bear fruit for the disease in general: if multiple risk loci can be identified in severely affected pedigrees, the power of future analyses may be increased by either limiting testing to a subset of the genome or by incorporating this information into the analysis model.
Our linkage signal on chromosome 1 shares markers with the linkage signal observed in Dutch [Liu et al., 2007] and Finnish [Hiltunen et al., 2001] LOAD samples, and appears to be near that observed in NIMH [Kehoe et al., 1999; Blacker et al., 2003; Dickson et al., 2008], and other [Grupe et al., 2007] LOAD samples. Furthermore, two bins with suggestive evidence of linkage (P < 0.005) in a recent meta-analysis [Butler et al., 2009] flank our linkage signal at 1q23.3. This region also encompasses variants in CHRNB2 and other surrounding genes previously associated with AD [Cook et al., 2004; Bertram et al., 2007, 2008]. Interestingly, one of the single markers most strongly associated with this linkage peak, D1S484, is ∼1.2 Mb away (3′) from the serum amyloid P component precursor gene SAP, and only ∼0.5 Mb from the gene encoding nicastrin (NCSTN). The relative positions of SAP and NCSTN to our markers are shown in Supplemental Figure A. SAP is bound to the amyloid plaques and neurofibrillary tangles diagnostic of AD and other dementias [Duong et al., 1989; Kalaria et al., 1991; Rostagno et al., 2007]. SAP stimulates amyloid-β (Aβ) and pro-inflammatory cytokine production [Veerhuis et al., 2003] and, similar to APOE, is also associated with atherosclerotic lesions [Ezzahiri et al., 2006]. As a new drug compound has been demonstrated to remove SAP from serum and cerebrospinal fluid in AD patients [Kolstoe et al., 2009], confirmation of the pathogenic role played by SAP variants in AD could have a direct impact on the clinical treatment of AD. Nicastrin, like presenilin, is a component of the γ-secretase complex necessary for production of Aβ from the amyloid precursor protein (APP) [Francis et al., 2002; Edbauer et al., 2003; Tanzi and Bertram, 2005]. Linkage studies in Dutch [Dermaut et al., 2002] samples and possibly a Finnish sample [Helisalmi et al., 2004] have found evidence of linkage to NCSTN. With linkage and association signals detected in several independent samples, this region clearly merits further study.
Similarly, the linkage signal on 17p13.2 also coincides with previous linkage [Lee et al., 2008] and association [Grupe et al., 2007] signals for LOAD. The single marker with the strongest evidence of linkage on chromosome 17, D17S938, is within 3 Mb of two candidate genes: Netrin-1 (NTN1) and tyrosine kinase, non-receptor, 1 (TNK1) (Supplemental Figure A). NTN1 is a ligand for APP and controls Aβ production in a transgenic AD mouse model [Lourenco et al., 2009], while TNK-1 is involved with intracellular signaling and has shown significant evidence of association with AD by AlzGene's meta-association analysis [Bertram et al., 2007]. This region harbors several biological candidates and also deserves further attention.
Although the linkage signal on chromosome 7 is observed only in the R pedigree in this study, this signal overlaps a previously described linkage signal identified in a Dutch EOAD sample [Rademakers et al., 2005]. The interesting region surrounding the linkage signal on 7q33 also encompasses linkage and association signals identified in Caribbean Hispanic [Lee et al., 2008], Amish [Hahs et al., 2006], and Israeli-Arab [Farrer et al., 2003] AD samples. The persistence of this linkage signal across ethnic and geographic boundaries suggests further research into its possible role in AD is merited.
The linkage peak on chromosome 11 is supported by a single marker, D11S904, which is located ∼1 cM away from BDNF. Reports of association between variants in BDNF and LOAD have been published in several populations [Kunugi et al., 2001; Riemenschneider et al., 2002; Ventriglia et al., 2002], including the Caucasian population in the AlzGene meta-analysis (rs6265) [Bertram et al., 2007]. Although we do not have strong multipoint support of linkage to this region, the position of the linked marker relative to BDNF was intriguing. Further analyses found no evidence that variation in rs6265 contributed to this linkage signal on 11p14.2, with the credible interval of BDNF genotypic effects including zero. It is still possible that other variants within BDNF may influence AGEON in this sample.
The appearance of a third QTL model in the linkage analyses raises a number of issues. There appears to be a relationship between models 1 and 3, as both were associated to chromosomes 1 and 17, they share extreme allele frequencies, and model 3 is only present in multi-chromosomal analyses of all-families, while it is always present in the R-family. It is possible that the assumptions within our analytical model are violated by the presence of a non-additive relationship between the QTL models, or a multiallelic QTL. Our small dataset cannot resolve this variability, though the persistence of these linkage signals suggests that chromosomes 1q23.3 and 17p13.2 merit further investigation in other datasets.
Our analytic approach dramatically improved our ability to detect linkage to AGEON through the careful choice of covariates. Inclusion of APOE and PSEN2 as major gene covariates in our analytical model proved essential: without them, all other linkage signals dissipated, and would likely not have been detected. APOE and PSEN2 were able to explain much of the previously identified genetic variance in AGEON, which allowed patterns in the remaining genetic variance to become detectable. Despite its important role as a covariate, the region surrounding APOE did not produce a linkage signal when APOE was excluded as both a marker and a covariate, probably due to a lack of power given its modest effect (∼3% VG) within the Volga German families. This highlights the importance of including as much solid prior information as possible into the model when searching for modifier loci.
Single large pedigrees with very complete genotype and phenotype data may have an advantage for identifying modifier loci. Previous genome scans have successfully identified modifier loci in such pedigrees when the major gene variants appeared to have incomplete penetrance [Riazuddin et al., 2000; Hasstedt et al., 2004]. In our study, the R family had a similar advantage (Table I): it had the least censored phenotype data and the most complete genotype data of the large Volga German pedigrees, while exhibiting excess genetic variance in AGEON not explained by the major gene covariates. This may explain the detection of additional linkage regions in the R family not evident in the all-families analysis. This also demonstrates that carefully chosen pedigrees may have the power to detect modifier loci, even though they have relatively small sample sizes. This also suggests that existing datasets previously used to identify major gene variants in such pedigrees may provide excellent sources of data for the identification of modifier loci.
Acknowledgements
We thank the generosity of the participants for their contribution to this study. Financial support was provided by Veterans Affairs research funds and National Institute of Health (NIH) grants AG05136, AG00258, and GM46255.




