Intra-individual variability in genetic and environmental models of attention-deficit/hyperactivity disorder†
How to cite this article: Perry GML, Sagvolden T, Faraone SV. 2010. Intra-Individual Variability in Genetic and Environmental Models of Attention-Deficit/Hyperactivity Disorder. Am J Med Genet Part B 153B:1094–1101.
Abstract
The frequent observation of intra-individual variability (IIV) in the expression of ADHD symptoms suggest that IIV is an integral component of the disorder. We tested IIV in ADHD-like phenotype from five different studies of rodent models of ADHD, including studies with Spontaneous Hypertensive Rats (SHR/NCrl and SHR/N), Wistar-Kyoto Hyperactive Rats (WKHA/N), Wistar-Kyoto Hypertensive rat (WKHT), PCB-126 and -153-treated Lewis rats and behaviorally normal Wistar/Mol, Wistar-Kyoto (WKY/N and WKY/NMol), and untreated Lewis rats. Averages of the absolute residual deviation of ADHD-like behavior from individual means (“individual phenotypic dispersion,” PDi) were used to represent IIV in the fixed-interval (FI) and extinction (EXT) phases of operant behavioral activity. Across all studies, SHR rats had higher PDi than WKY rats (P < 0.0001) for all ADHD-like traits, and higher PDi for hyperactivity than WKHT and WKHA/N rats. Male SHR rats in particular had higher PDi for hyperactivity than male or female WKYs, SHR females for EXT hyperactivity, and higher dispersion for inattention than WKY females. These findings strongly suggest the genetic control of IIV, and suggest that the SHR may be a useful model for the identification of genes for IIV in human ADHD. These findings also obliquely support the SHR as a useful model for ADHD overall. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a serious behavioral disorder affecting 8–12% of adolescents [Faraone et al., 2005]. Besides hyperactivity, inattention and impulsiveness, ADHD-affected individuals frequently have high variability in reaction time during stop-signal, Go/No-Go and continuous performance tasks [Castellanos et al., 2005; Nigg et al., 2005; Klein et al., 2006; Di Martino et al., 2008], termed intra-individual variability (IIV). IIV is common and may itself be an endophenotype of ADHD [Castellanos et al., 2005; Sagvolden et al., 2005a; Johnson et al., 2007; Geurts et al., 2008; Johansen et al., 2009]. Little is known of the physiological/genetic basis for IIV although various works suggest deficiencies in energy supply to active neurons or the poor re-establishment of ionic homeostasis across neuronal membranes [Russell et al., 2006], poor noradrenergic/dopaminergic co-function [Cho et al., 2008], deficits in neurocognitive timing [Castellanos et al., 2005], or poor behavioral mechanisms due to dopamine hypofunction [Aase and Sagvolden, 2005; Sagvolden et al., 2005a; Aase et al., 2006; Johansen et al., 2009].
Various rodent models of ADHD exist. The Spontaneous Hypertensive Rat (SHR), in particular, expresses all major features of ADHD, including hyperactivity, impulsiveness and inattention [Sagvolden et al., 2005b; Kantak et al., 2008], smaller brain volume [Bendel and Eilam, 1992], sex differences in ADHD-like phenotype [Sagvolden and Berger, 1996; Berger and Sagvolden, 1998; Bucci et al., 2008] and differences in the expression of genes for catecholaminergic functioning, neuronal metabolism, and ion channels compared to controls [Mill et al., 2005; Russell et al., 2005; Das-Banerjee et al., 2008]. The Wistar-Kyoto Hyperactive Rat (WKHA) expresses hyperactivity specifically [Moisan et al., 2003]. Environmental contaminants such as polychlorinated biphenols (PCBs) also induce hyperactivity and impulsiveness, the prenatal period and weaning periods being the most sensitive for neurological damage by PCBs [Pantaleoni et al., 1988; Lilienthal and Winneke, 1991; Morse and Brouwer, 1995]. Ortho-substituted congeners are common [McFarland and Clarke, 1989] and have marked effects on neurological behavior [Holene et al., 1995; Schantz et al., 1995]. Prenatal exposure to PCBs leads to deficits in executive function and ADHD in humans [Boucher et al., 2009]. Rats exposed prenatally to PCBs also show ADHD-like symptoms [Daly et al., 1989; Holene et al., 1998; Berger et al., 2001] and dysregulated expression of catecholaminergic genes [Das-Banerjee et al., 2008].
There appears to be a genetic component to IIV: Andreou et al. 2007 found that familial elements of reaction time variability (as much as 70% of phenotypic variance) were shared with familial variance components in ADHD. Wood et al. 2009 found that genetic variance associated with ADHD and with mean reaction time was also highly correlated with variability in reaction time. SHR have higher IIV in correctly locating target holes during nose-poking investigations: SHR located the correct hole a third of the time with initial investigations, while WKY rats nose-poked the correct hole approximately half the time [Johansen et al., 2007]. However, little is known of IIV in other genetic or environmental models of ADHD, or of IIV in traits other than reaction time. Our objective was to test IIV in ADHD-like traits in rodent models of ADHD and their controls. The identification of such differences might permit the mapping and eventual identification of candidate genes or physiological systems responsible for IIV.
MATERIALS AND METHODS
Experimental Samples
We collected data on rat operant training and response to operant challenge from five studies: Berger et al. [Sagvolden et al., 1992, 1993; Berger and Sagvolden, 1998; Boix et al., 1998; Holene et al., 1998]. Operant behavioral testing in each of these groups was similar to those described and used by Sagvolden [Sagvolden, 2006; Sagvolden and Xu, 2008; Sagvolden et al., 2008].
The comparisons included 6–10 individuals from several strain/environmental classes in each study, including male and female SHR/N and SHR/NMol, WKY/N, WKY/NMol, Lewis, Wistar-Kyoto Hyperactive (WKHA/N), and Wistar-Kyoto Hypertensive (WKHT) rats (see Table I). Conditions for testing were identical in all studies with the exception of Holene et al. 1995, which tested the effects of exposure to PCB-126 3,3′,4,4′,5-penta-CB; IUPAC # 126) and PCB-153 (2,2′,4,4′,5,5′-hexa-CB; IUPAC #153) on operant behavior; PCB exposure is known to cause ADHD-like symptoms in operant behavioral challenges [Holene et al., 1995] and to cause dysregulation of ADHD candidate genes in the brains of Sprague-Dawley rats (Dasbanerjee, 2008 #22475). Dams of experimental rats in this study were exposed to either (i) 5 mg/kg BW PCB153 or (ii) 2 µg/kg BW PCB-126 via gavage of contaminated corn oil and (Cambridge Isotope Laboratories, Andover, MA) see [Holene et al., 1995].
| Strain/treatment | n | Source | Source article |
|---|---|---|---|
| SHR/NMol | 8 | MBC |
Boix et al. 1998 |
| WKY/NMol | 8 | MBC | |
| SHR/NMol, male | 8 | MBC |
Berger and Sagvolden 1998 |
| SHR/NMol, female | 8 | MBC | |
| WKY/NMol, male | 6 | MBC | |
| WKY/NMol, female | 10 | MBC | |
| Untreated DA/OLA/HSD females × Lewis males | 8 | OLA; Harlan, UK |
Holene et al. 1998 |
| PCB126-treated DA/OLA/HSD females × Lewis males | 8 | OLA; Harlan, UK | |
| PCB153-treated DA/OLA/HSD females × Lewis males | 8 | OLA; Harlan, UK | |
| SHR/N | 8 | NIH |
Sagvolden et al. 1992 |
| WKHA/N | 8 | NIH | |
| WKHT/N | 8 | NIH | |
| WKY/N/NMol | 8 | NIH; MBC | |
| PVG/NMol | 9 | MBC |
Sagvolden et al. 1993 |
| SHR/NMol | 10 | MBC | |
| SPRD NMol | 10 | MBC | |
| Wistar/NMol | 9 | MBC | |
| WKY/NMol | 10 | MBC |
- Male and female Spontaneous Hypertensive Rats (SHR) and Wistar-Kyoto rats (WKY) rats, and untreated, PCB-126 and PCB-153 treated rats, were used as separate factors in analysis of the datasets of Boix et al. 1998 and Holene et al. 1995. All other comparisons involved strains only (Wistar-Kyoto Hyperactive Rats (WKHA/N), Wistar-Kyoto Hypertensive rat (WKHT)). Sample size (n) and institutional source for the rats in each test (MBC, øllenberg Breeding Center, Denmark. OLA, Oak Ridge National Laboratory. NIH, National Institutes of Health) are given.
All rats were trained and tested in Campden Instruments operant chambers. The working space for the rats consisted of a ventilated, sound-insulated 25 cm × 25 cm × 20 cm operant chamber equipped with two levers and a 2.8-W house light, separated from the liquid reinforcement delivery chamber by a 7 cm × 5 cm plastic lid, operable by light paw or nose push and monitored by microswitch. Operant training was begun at 5 weeks of age, running 5 days per week. Experimentation consisted of a 2-min fixed interval (FI) of reinforcement, followed by a 5-min extinction (EXT) period with no reinforcer [Berger and Sagvolden, 1998; Holene et al., 1998]. The FI period was divided into twelve 10-sec segments and the EXT period into 1-min bins. The house light was on during the FI and off during the EXT period to signal reinforcement state to the rats. Water was available for 3 sec after the reinforcement cubicle door was opened. The daily test time for each rat was randomized within a block of 10 AM–2 PM. Rats were given ad libitum access to water for one hour, followed by 22 hr of water deprivation. Environment was maintained at 23°C (±1°C), 50% humidity and a 12:12 photoperiod.
Behavioral Measurement
Behavior was recorded by a SPIDER array (Paul Fray, Ltd., Waterbeach, UK) and microcomputer. Within each segment, the proportion of correct and incorrect openings of the water reward cubicle ([(openings during FI)]/[(openings during FI + openings during EXT)]) were recorded to reflect attention, the number of responses with short (<0.67 sec) inter-response times (IRT) as impulsiveness and the total number of lid openings as a measure of activity [Sagvolden, 2006; Sagvolden et al., 2008; Sagvolden and Xu, 2008]. Hyperactivity and impulsiveness were recorded in the FI and EXT phases.
Intra-Individual Variability
We obtained individual measures of IIV from the average of absolute phenotypic dispersion for each individual (PDi) among all experimental segments 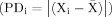 in the FI and EXT periods, where Xi is ADHD-like phenotype for individual rat i and
in the FI and EXT periods, where Xi is ADHD-like phenotype for individual rat i and  the average phenotype for a given rat across all FI or EXT segments within each strain type or contrast (see Table I) within each of the five studies. Differences in mean PDi among strains or contrast groups (Table I) were calculated using Wilcoxon ranks in Kruskal–Wallis nonparametric tests [SAS, 2000]. Differences in PDi between groups in each test were then identified using Kruskal–Wallis tests, and the significance of these individual differences adjusted using Bonferroni correction. Means and standard errors were calculated for each group. Cross-study hypothesis tests were not possible except for the SHR and WKY, which occurred in all five studies. For these strains, we performed a parametric linear contrast analysis using a general linear model (GLM) [SAS, 2000] for PDi in each trait.
the average phenotype for a given rat across all FI or EXT segments within each strain type or contrast (see Table I) within each of the five studies. Differences in mean PDi among strains or contrast groups (Table I) were calculated using Wilcoxon ranks in Kruskal–Wallis nonparametric tests [SAS, 2000]. Differences in PDi between groups in each test were then identified using Kruskal–Wallis tests, and the significance of these individual differences adjusted using Bonferroni correction. Means and standard errors were calculated for each group. Cross-study hypothesis tests were not possible except for the SHR and WKY, which occurred in all five studies. For these strains, we performed a parametric linear contrast analysis using a general linear model (GLM) [SAS, 2000] for PDi in each trait.
RESULTS
Intra-Individual Variability
In each of the original studies, the SHR had higher hyperactivity, impulsiveness and inattention than the WKY rats [Sagvolden et al., 1992, 1993; Berger and Sagvolden, 1998; Boix et al., 1998; Holene et al., 1998]. PCB-treated rats had higher lever-pressing activity and shorter IRTs [Holene et al., 1998].
SHR rats had significantly higher PDi for FI hyperactivity than WKY rats in three of the four studies in which they were tested against the latter (Table II, Fig. 1; P < 0.05). PDi in SHR males was significantly higher than in WKYs of either sex (P < 0.05) after Bonferroni correction. Dispersion for hyperactivity during the EXT phase resembled that during FI; SHRs had higher PDi for hyperactivity than the WKY in all tests involving both strains, and higher PDi for hyperactivity than SPRD rats (Table II, Fig. 2; P < 0.05). As for the FI phase, SHR males had higher PDi than male (P < 0.01) and female WKYs (P < 0.05) after Bonferroni correction, and female SHR higher PDi than male WKYs (Table II, Fig. 2; P < 0.05). PDi for hyperactivity was significantly higher in SHR than in WKY across the five studies using contrast analysis (P < 0.0001; Table III).
| Behavior/period | Study | n | χ2 | P |
|---|---|---|---|---|
| Hyperactivity—FI |
Boix et al. 1998 |
17 | 7.25 | 0.0071 |
|
Berger and Sagvolden 1998 |
32 | 17.6 | 0.0005 | |
|
Sagvolden et al. 1992 |
32 | 11.3 | 0.0103 | |
| Hyperactivity—EXT |
Boix et al. 1998 |
17 | 10.1 | 0.0015 |
|
Berger and Sagvolden 1998 |
32 | 18.6 | 0.0003 | |
|
Sagvolden et al. 1992 |
32 | 8.49 | 0.0369 | |
|
Sagvolden et al. 1993 |
48 | 10.7 | 0.0302 | |
| Impulsiveness—FI |
Boix et al. 1998 |
17 | 4.48 | 0.0343 |
|
Berger and Sagvolden 1998 |
32 | 14.5 | 0.0023 | |
|
Sagvolden et al. 1992 |
32 | 7.88 | 0.0486 | |
| Impulsiveness—EXT |
Boix et al. 1998 |
17 | 8.34 | 0.0039 |
|
Berger and Sagvolden 1998 |
32 | 13.7 | 0.0033 | |
|
Sagvolden et al. 1992 |
32 | 12.0 | 0.0072 | |
|
Sagvolden et al. 1993 |
48 | 13.1 | 0.0110 | |
| Attention |
Boix et al. 1998 |
17 | 5.79 | 0.0161 |
|
Berger and Sagvolden 1998 |
32 | 10.1 | 0.0177 | |
|
Sagvolden et al. 1993 |
48 | 9.50 | 0.0498 |
- χ2 and P values are derived from Kruskal–Wallis tests of differences for the entire data set.
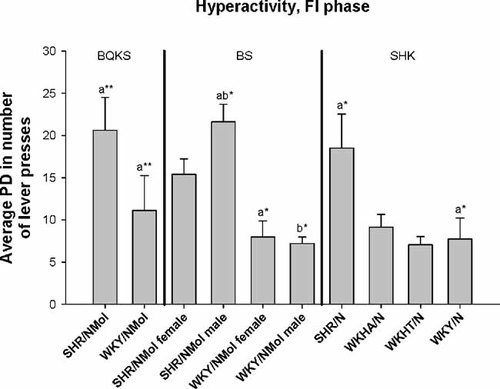
Means of absolute phenotypic dispersion (PDi) for hyperactivity during the fixed-interval (FI) phase of operant training in male and female Spontaneous Hypertensive Rats (SHR/NMol), male and female Wistar-Kyoto (WKY/NMol) rats, Wistar-Kyoto Hyperactive (WKHA/N), and Wistar-Kyoto Hypertensive (WKHT) rats (from Boix et al. 1998, Berger and Sagvolden 1998, and Sagvolden et al. 1992). Significance of differences among means for PDi in FI hyperactivity after Bonferroni correction for multiple testing is indicated as *P < 0.05, **P < 0.01, ***P < 0.001.
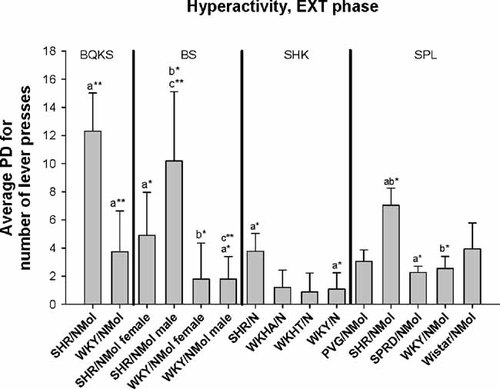
Means for absolute phenotypic dispersion (PDi) in hyperactivity during the extinction phase (EXT) of operant activity in male and female Spontaneous Hypertensive Rats (SHR/NMol), male and female Wistar-Kyoto (WKY/NMol) rats, Wistar-Kyoto Hyperactive (WKHA/N) rats, Wistar-Kyoto Hypertensive (WKHT) rats, hooded rats (PVG/NMol), Sprague–Dawley rats (SPRD/NMol), and Wistar rats (from Boix et al. 1998, Berger and Sagvolden 1998, Sagvolden et al. 1992, 1993). The significance of differences among groups for PDi after Bonferroni correction is indicated as *P < 0.05, **P < 0.01, ***P < 0.001. Data originally from Berger and Sagvolden 1998 and Sagvolden et al. 1993.
| Trait | F | P | 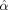 (95% CI) (95% CI) |
|---|---|---|---|
| Impulsiveness, fixed-interval | 17.6 | <0.0001 | −3.56 (1.83) |
| Impulsiveness, extinction interval | 3.21 | 0.0756 | −0.821 (0.893) |
| Hyperactivity, fixed-interval | 36.3 | <0.0001 | −17.1 (5.57) |
| Hyperactivity, extinction interval | ns | — | — |
| Inattention | ns | — | — |
SHR rats also had higher PDi for impulsiveness in the FI phase than WKY rats in two of the four studies in which they were tested. SHR males and females both had significantly (P < 0.05) higher PDi for FI impulsiveness than female WKY rats in Boix et al. 1998 (Table II; Fig. 3). The SHR had higher a priori PDi for FI impulsiveness than the WKY in the data of Sagvolden et al. 1992, but these differences were not significant post-Bonferroni correction (Table II; Fig. 3). As in the FI phase, SHR had higher PDi for EXT impulsiveness than the WKY in two of the four studies comparing their dispersion. SHR males had higher PDi for impulsiveness than WKY females (P < 0.05) in the study of Berger and Sagvolden 1998; unlike the FI phase, SHR also had higher PDi than WKHT for EXT impulsiveness (P < 0.05) in the data of Sagvolden et al. 1992, and higher PDi than PVG (P < 0.05) and SPRD rats (P < 0.01) from Sagvolden et al. 1993( Fig. 4). As for hyperactivity, contrast analysis indicated that PDi for FI and EXT impulsiveness was significantly higher in the SHR across all four studies overall than the WKY (P < 0.0001; Table III).

Means for absolute phenotypic dispersion (PDi) in hyperactivity in the fixed interval (FI) phase among male and female Spontaneous Hypertensive Rats (SHR/NMol), male and female Wistar-Kyoto (WKY/NMol) rats, Wistar-Kyoto Hyperactive (WKHA/N), and Wistar-Kyoto Hypertensive (WKHT) rats (from Boix et al. 1998, Berger and Sagvolden 1998, and Sagvolden et al. 1992). The significance of differences in mean PDi among rat strains after Bonferroni correction for multiple testing is given as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
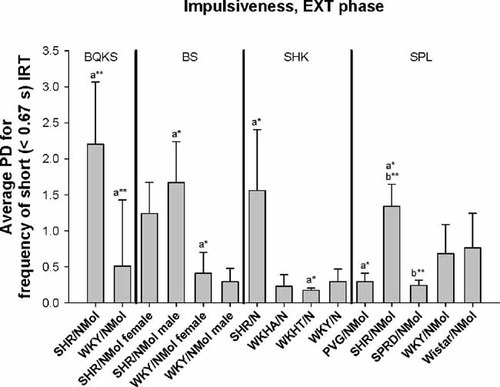
Means of absolute phenotypic dispersion (PDi) for impulsiveness during the extinction (EXT) phase in male and female Spontaneous Hypertensive Rats (SHR/NMol), male and female Wistar-Kyoto (WKY/NMol) rats, Wistar-Kyoto Hyperactive (WKHA/N) rats, Wistar-Kyoto Hypertensive (WKHT) rats, hooded rats (PVG/NMol), Sprague–Dawley rats (SPRD/NMol), and Wistar rats (from Boix et al. 1998, Berger and Sagvolden 1993, 1998, Sagvolden et al. 1992). Significance of differences among means after correction for multiple testing given as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
SHR had significantly higher PDi for inattention than WKY rats (P < 0.05) from the data of Boix et al. 1998 and male SHR significantly higher PDi than WKY females from Berger and Sagvolden 1998 (Table II; Fig. 5). SHR also had higher PDi than SPRD rats (P < 0.05) (from Sagvolden et al. 1993). As hyperactivity and impulsiveness, contrast analysis indicated that PDi for inattention was significantly higher in the SHR than the WKY across all four studies in which they were represented (P < 0.0001) (Table III).
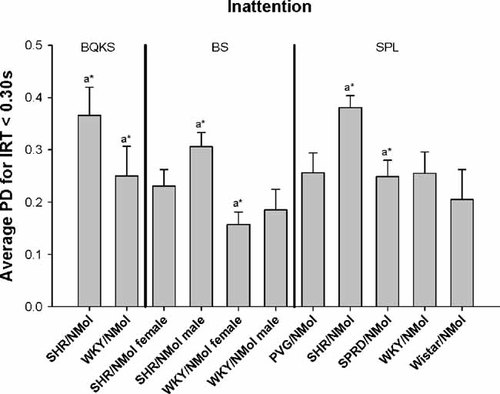
Phenotype dispersion (PDi) means for inattention in male and female Spontaneous Hypertensive Rats (SHR/NMol), male and female Wistar-Kyoto (WKY/NMol) rats, Wistar-Kyoto Hyperactive (WKHA/N), and Wistar-Kyoto Hypertensive (WKHT) rats (from Boix et al. 1998, Berger and Sagvolden 1998, and Sagvolden et al. 1992). The significance of differences among PDi means after Bonferroni correction for multiple testing is indicated as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
Our results indicate genetic effects on IIV (measured here as PDi) and illustrate that the SHR has higher IIV for ADHD-related characters than other strains, including WKY. As in children with ADHD [Castellanos et al., 2005; Klein et al., 2006; Di Martino et al., 2008; Geurts et al., 2008], the SHR strain, and male SHR in particular, were more variable in their expression of ADHD-like phenotype than WKY controls. This recommends both that SHR as a model of IIV and the WKY strain as a control line in mapping genes for IIV. Our interpretation is partially cautioned by the use of dispersion values uncorrected for the mean, raising the possibility of heterscedasticity. However, the extremely high values of operant phenotype expressed by the SHR in the four source studies that include that strain [Sagvolden et al., 1992, 1993; Berger and Sagvolden, 1998; Boix et al., 1998] make such a transform highly susceptible to overcorrection for SHR phenotype. Mean-transformed values for dispersion (not shown) resulted in a range of results, with mean PDi in the SHR ranging everywhere from significantly higher to significantly lower PDi than our central control strain, the WKY. This suggests that such an approach may be overly conservative. We expect to be able to separate means and variances for operant behavioral phenotype from that of the SHR and WKY strains using F2 SHR × WKY intercrosses, permitting us to simultaneously search for classically acting genes and those responsible for IIV.
There are a number of mechanisms by which IIV in ADHD symptoms might occur. Russell et al. [Russell et al., 2006] proposed that IIV resulted from deficiencies in astrocyte function. Under this hypothesis, insufficient production of ATP to active neurons and inadequate supply of lactate for energy by neighboring astrocytes [Sanchez et al., 2001; Kasischke et al., 2004], resulting short-term IIV [Russell et al., 2006]. A deficiency in ATP production might also limit the activity of Na+/K+ATPase and Ca+ATPase pumps, disrupting transmembrane ionic gradients and affecting the ability of neuronal cells to release neurotransmitters [Russell et al., 2006]. Astrocytes do appear to affect neurotransmission [Grimaldi et al., 1997; Moldrich et al., 2002; Miyazaki et al., 2004]. The state regulation hypothesis of Johnson et al. 2009 strongly resembles this case; in this hypothesis, task efficiency (and thus variability) is a function of short-term energetic status and the arousal/activation state of a stream of task response elements including stimulus recognition, the search of memory, cognitive decision and finally motor activity. IIV occurs generally in several other human behavioral disorders such as schizophrenia [Schwartz et al., 1989], cognition after head injury [Stuss et al., 1989; Walker et al., 2000; Murtha, 2002] and Tourette's syndrome [Peterson and Leckman, 1998; Geurts et al., 2008], which jointly suggests dysfunction in basal physiology such as energetic metabolism. This is also suggested by IIV in Tourette's Syndrome, which has been linked to individual variability in motor neuron activity [Walters et al., 2001].
IIV, as an essentially random process, might result from poor regulation of normal periodicity in neural physiology, such as that associated with autonomous functions like heart rate and respiration [Castellanos et al., 2005]. This could result from poor co-ordination of catecholaminergic physiology, which appears to modulate executive function and attention [Faraone, 2004, 2006; Faraone et al., 2005; Staller and Faraone, 2007; Cho et al., 2008; Mick and Faraone, 2008]. This presumably occurs via variability in noradrenergic output by the locus coeruleus, causing variability in prefrontal cortex regulation and increased noise in neuron function and attention [Arnsten et al., 1996; Cho et al., 2008]. Notably, the expression of genes involved in dopaminergic/catecholaminergic physiology in the SHR differs to that of control rats [Mill et al., 2005; Russell et al., 2006; Das-Banerjee et al., 2008].
The dynamic developmental theory of ADHD [Sagvolden et al., 2005a] suggests that reduced dopamine activity changes fundamental behavioral selection mechanisms by causing deficient reinforcement of successful behavior combined with deficient EXT of unsuccessful behavior. Such deficient selection mechanisms will slow the association (“chunking”) of simple response units into longer, more elaborate chains of adaptive behavioral elements that function as higher-order response units [Aase and Sagvolden, 2005; Aase et al., 2006]. When response units are chunked together into a chain, one response unit reliably precedes the next with high predictability within the chain. Deficient or slowed chunking of behavior means that the reliable and predictable pattern of responses is absent, resulting in the increased IIV observed in ADHD [Johansen et al., 2009].
It has been argued that the phenomenon of IIV in ADHD represents either a nuisance element, or an underlying genetic liability intrinsically associated with ADHD [Castellanos et al., 2005; Russell et al., 2006]. The latter seems more likely; the 10-repeat allele at the dopamine transporter (DAT), previously associated with ADHD [Faraone et al., 2005], has also been associated with IIV in specific behavioral [Bellgrove et al., 2005] and neurological [Loo et al., 2003] phenotypes. The patterns of differences in IIV among strains (especially for SHR-WKY contrasts) in this study obliquely support our hypothesis that IIV is under genetic control rather than representing random error. Notably, the total and the posterior area of the corpus callosum has been negatively associated with IIV in reaction time [Anstey et al., 2007]; as ADHD-affected humans [Paule et al., 2000; Castellanos et al., 2002] SHR have smaller brain size compared to controls [Bendel and Eilam, 1992].
Our findings suggest a genetic component to IIV in ADHD. However, clinical implications of IIV on ADHD and its genetics are not entirely clear. IIV could create difficulties in the diagnosis of borderline ADHD-affected individuals with high variance in their symptoms, or require the restructuring of cognitive tasks used for the diagnosis of ADHD, such as the division of longer tasks into shorter ones with more rapid reinforcement [Sagvolden et al., 2005a; Russell et al., 2006; Cho et al., 2008]. In the broader sense, a genetic basis for IIV in ADHD suggests the existence of genes causing IIV in other traits and other model organisms, for which there is already some evidence [SanCristobal-Gaudy et al., 1998; Perry et al., 2003; Sorensen and Waagepetersen, 2003; Mackay and Lyman, 2005]. The genetic control of IIV would require a major reevaluation of the fundamental Fisherian theorem [Fisher, 1918] to include estimates of the genetic control of residual variability, radically altering the entire continuum of all quantitative genetic research.