Association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and age of onset in schizophrenia†‡
All authors reported no biomedical financial interests or potential conflicts of interest relevant to the subject matter of this manuscript.
How to Cite this Article: Vares M, Saetre P, Deng H, Cai G, Liu X, Hansen T, Rasmussen HB, Werge T, Melle I, Djurovic S, Andreassen OA, Agartz I, Hall H, Terenius L, Jönsson EG. 2009. Association Between Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Age of Onset in Schizophrenia. Am J Med Genet Part B 153B:610–618.
Abstract
Different lines of evidence indicate that methylenetetrahydrofolate reductase (MTHFR) functional gene polymorphisms, causative in aberrant folate–homocysteine metabolism, are associated with increased vulnerability to several heritable developmental disorders. Opposing views are expressed considering the possible association between MTHFR and susceptibility for schizophrenia. In order to evaluate if age of onset could explain some of this discrepancy we investigated the relationship between two functional MTHFR gene polymorphisms and age at onset in this disorder. Scandinavian patients (n = 820) diagnosed with schizophrenia, schizoaffective disorder, and schizophreniform disorder were investigated. Two functional MTHFR single nucleotide polymorphisms (SNPs; rs1801131 and rs1801133) were genotyped and the effect of MTHFR polymorphisms on the age of onset was examined with survival analysis. In an attempt to replicate the findings from the Scandinavian sample, the association between rs1801133 and age at onset was also analyzed in Chinese high-risk families, with two or more affected siblings (n = 243). Among the Scandinavian patients the functional MTHFR SNP rs1801133 (C677T) significantly affected age at onset of schizophrenia in a dose-dependent manner (P = 0.0015), with lower age of onset with increasing numbers of the mutant T-allele. There was no evidence of rs1801131 (A1298C) affecting age of onset in schizophrenia. Within the Chinese high-risk families carriers of the MTHFR 677T allele showed earlier age at onset than siblings being homozygous for the wild-type allele (P = 0.008). The MTHFR C677T polymorphism may play a role as a modifying factor for age of onset in schizophrenia. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Methylenetetrahydrofolate reductase (MTHFR) plays a key roll in the one-carbon cycle that generates precursors of deoxyribonucleic acid (DNA) bases, and is involved in methylation of membrane lipids and DNA. Normal activity of this enzyme maintains the pool of circulating folate and methionine and reduces levels of homocystine. Dysfunctional reactions involving the methionine–homocysteine metabolism are anticipated to be significant in schizophrenia etiology [Muntjewerff et al., 2006]. Folate deficiency has also been linked to disturbed metabolism of serotonin, dopamine, and noradrenaline, that is, neurotransmitter abnormalities possibly contributing to schizophrenia development [Bottiglieri et al., 2000].
The MTHFR gene is located on chromosome 1p36.3 [Goyette et al., 1994], a region for which linkage to schizophrenia has been reported in one study [Kohn et al., 2004]. Two single nucleotide polymorphisms (SNPs) of this gene, C677T and A1298C, each results in a thermolabile enzyme with decreased activity [Frosst et al., 1995; van der Put et al., 1998; Lievers et al., 2001]. These MTHFR polymorphisms have been the focus for numerous studies analyzing cardiovascular anomalies, neurological conditions, and psychiatric disorders during a recent few years. The associations of these SNPs with schizophrenia have been tested in different studies, but results have been inconsistent. Some authors report association between one or both of these MTHFR polymorphisms and the disorder [Arinami et al., 1997; Joober et al., 2000; Sazci et al., 2003; Kempisty et al., 2006, 2007], whereas others failed to verify this association [Kunugi et al., 1998; Virgos et al., 1999; Tan et al., 2004; Yu et al., 2004; Vilella et al., 2005; Philibert et al., 2006; Jönsson et al., 2008]. A few studies report that the association between the MTHFR C677T polymorphism and schizophrenia is gender-specific with significant results only in men [Sazci et al., 2005] or women [Muntjewerff et al., 2005]. Other studies were not able to replicate these gender-specific results [Philibert et al., 2006; Jönsson et al., 2008]. However outcome of several meta-analyses still indicate that MTHFR gene variations, especially C677T, are associated with higher risk of schizophrenia [Lewis et al., 2005; Muntjewerff et al., 2006; Zintzaras, 2006; Gilbody et al., 2007; Allen et al., 2008; Jönsson et al., 2008; Shi et al., 2008]. MTHFR C677T polymorphism has also been examined in relation to some schizophrenia phenotypes suggesting possible association with negative symptoms and executive function deficits [Roffman et al., 2007, 2008] as well as metabolic complications during atypical antipsychotic medication [Ellingrod et al., 2008].
Schizophrenia is a complex disorder with significant heritability shown by numerous epidemiological and molecular genetic studies [Gottesman et al., 1982]. Still schizophrenia is a heterogenous disorder, and clinical characteristics have been used to delineate more homogenous genetic subgroups. Among single phenotype characteristics of schizophrenia age of onset has been regarded as important with evidence for underlying genetic components [Cardno et al., 2001]. Consequently, different candidate genes for schizophrenia have been investigated concerning possible relationships with age of onset. Significant earlier age of onset has been associated with a brain-derived neurotrophic factor (BDNF) gene polymorphism [Numata et al., 2006] and as a result of interaction between functional BDNF and dopamine receptor D3 (DRD3) polymorphisms [Gourion et al., 2005]. An epidermal growth factor (EGF) gene polymorphism was linked with an earlier onset in male patients [Hänninen et al., 2007], whereas onset after 40 years has been associated with an allele in the chemokine receptor 5 (CCR5) gene [Rasmussen et al., 2006]. Also in a Chinese study of families with two or more affected siblings, the MTHFR C677T polymorphism was linked to schizophrenia in patients with an age of onset before 25 years [Deng et al., 2002]. In the present report the possible relationship between the MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms and age at onset of schizophrenia was studied in a Scandinavian sample. In addition we re-evaluated the Chinese affected sib-pair study [Deng et al., 2002] to examine to what extent the MTHFR C677T polymorphism is associated with age of onset within high-risk families.
MATERIALS AND METHODS
Scandinavian Samples
The clinical samples originate from the Scandinavian Collaboration on Psychiatric Etiology (SCOPE) and were collected in Denmark (DK), Norway (NO), and Sweden (SE). Affected individuals were diagnosed with schizophrenia (n = 717), schizoaffective disorder (SCA, n = 87), or schizophreniform disorder (SCP, n = 16), according to ICD-10 (DK) or DSM-III-R/DSM-IV (NO and SE) (Table I). Age at first admission to a Psychiatric Hospital Department served as the measure of disease onset. For 20 of the 840 patients previously reported in a separate case–control analysis, data for age at first admission was lacking [Jönsson et al., 2008]. All individuals in the study were unrelated and of Caucasian origin. Detailed descriptions of the samples have previously been reported [Hansen et al., 2007; Jönsson et al., 2008; Kähler et al., 2008].
| Sample | Na | Gender (women) (%) | Age of onsetb |
|---|---|---|---|
| Case–control | |||
| Denmark (DK) | 406 (375) | 42.4 | 27.2 ± 8.9 |
| Norway (NO) | 159 (120) | 46.5 | 27.6 ± 8.7 |
| Sweden (SE) | 255 (222) | 37.2 | 26.3 ± 7.2 |
| Affected sib-pairs | |||
| China (CH) | 243 (223) | 49.4 | 23.2 ± 6.6 |
- a Broad (narrow, excluding schizoaffective and schizophreniform disorders) definition of schizophrenia.
- b Mean ± standard deviation.
The Danish Scientific Committees, the Danish Data Protection Agency, the Norwegian Scientific-Ethical Committees, the Norwegian Data Protection Agency, the Ethical Committee of the Karolinska Hospital, the Stockholm Regional Ethical Committee, and the Swedish Data Inspection Board all approved the study. All participants had given informed consent prior to inclusion in the study.
Chinese Affected Sib-Ship Sample
The clinical sample was made up of 243 affected individuals from 114 families where at least two siblings were affected with schizophrenia (n = 223), schizophreniform (n = 7), or schizoaffective disorder (n = 13) according to DSM-III-R by using a Chinese version of the Structured Clinical Interview for DSM-III-R Disorders (SCID) (Table I). The majority of families (n = 102) contained two affected siblings. In addition, ten families with three affected siblings, one family with four, and one family of five affected siblings were included in the sample. Age at onset was determined as the age of first episode of symptoms according to participants' medical record. All of the participants were Han Chinese. Further descriptions of the Chinese sample have been reported in a previous linkage study [Cai et al., 2001]. The study was approved by the Ethical Committee of West China Hospital, and all participants gave written informed consent.
SNP Selection and Genotyping
Two functional MTHFR SNPs, namely rs1801131 (C677T) and rs1801133 (A1298C) previously associated with schizophrenia were selected for genotyping in the Scandinavian sample. Genomic DNA was extracted from whole blood samples, and the two SNPs were genotyped at the SNP Technology Platform in Uppsala, Sweden (www.genotyping.se), using the Illumina BeadStation 500GX and the 1536-plex Illumina Golden Gate assay (Illumina, Inc., San Diego, CA). The sample success rate was 99.74% and 99.88% for rs1801131 and rs1801133, respectively, and the reproducibility of the genotyping was 100% according to duplicate analysis of 2.6% of the genotypes.
For the Chinese samples the C677T-polymorphism was genotyped by cleavage with restriction enzyme as previously described [Deng et al., 2002]. In short, white blood cells were isolated using lymphocyte secretion medium, and genomic DNA was extracted with phenol–chloroform. Polymerase chain reaction (PCR) was performed on an automated DNA thermal cycler. The PCR forward and reverse primers were 5′-CAAAGGCCACCCCGAAGC-3′ and 5′-AGGACGGTGCGGTGAGAGTG-3′, respectively. The PCR products were digested with HinfI, and PCR fragments were run on 2% agarose gels and stained with ethidium bromide.
Mendelian consistency was examined and sib-ships with inconsistent genotypes were excluded from the analysis. In total 243 affected individuals from 114 families (each with at least two affected siblings) were successfully genotyped. For the majority of families (n = 82) both parents were successfully genotyped, but for 27 families only one parent was successfully genotyped, and for five sib-ships no parental genotypes were available.
Statistical Analysis of Scandinavian Samples
Hardy–Weinberg (HW) equilibrium was tested using Fisher's exact test as implemented in PEDSTATS [Wigginton and Abecasis, 2005]. The amount of linkage disequlibrium (D′ and R2) between the two SNPs, and haplotype frequencies were determined with Haploview 4.0 [Barrett et al., 2005]. The fixation index (FST) was calculated for each SNP separately with FSTAT (version 2.9.3.2), grouping individuals by country of origin.
The effect of country of origin, gender, and MTHFR polymorphisms on the age of onset of schizophrenia was examined with survival analysis. The survival function was estimated with the Kaplan–Meier (product-limit) method in Proc Liftest (SAS/STAT® software, version 9.1.3, SAS institute, Inc., Cary, NC). The heterogeneity of survival functions were tested with the non-parametric k-sample test (country, gender, genotype) or the trend test (genotype, ordered by number of minor allele) using the log-rank weight function, which gives similar weights to all time points. Cox proportional hazard regression (Proc Phreg) was used to estimate the proportional hazard rate associated with rs1801133 and gender. In the analysis the effect of rs1801133 on age of onset was modeled as a time-dependent function of genotype, stratifying with respect to gender. That is, in accordance with the Kaplan–Meier curves the effect of one T allele was assumed to be zero before the age of 38, and the effect of two T alleles was assumed to zero before the age of 24. To estimate the hazard ratio between men and women, age of onset was modeled as a time-dependent function of gender, with no effect of time before the age of 24. The between countries heterogeneity in the effect of rs1801133 and gender on age of onset was tested by including the interaction terms between the rs1801133 genotypes and country, and between gender and country in the proportional hazard regression models, respectively.
Statistical Analysis of Chinese Sample
Mendelian consistency was checked with PedCheck [O'Connell and Weeks, 1998]. The association between C677T-polymorphism and age of onset in the family-based sample was assessed with UNPHASED (version 3.1.2) [Dudbridge, 2008]. The software calculates the retrospective likelihood of all the observed genotypes in a nuclear family, given the traits of the children. The approach is robust to population structure, and produces a valid analysis for multiple offspring in the presence of linkage. In the analysis age of onset was treated as a quantitative variable, with no assumptions of the variable being normally distributed, and gender was used as a confounder.
In addition, we tested the heterogeneity in survival curves between C677T genotypes in families where both parental genotypes were known, by means of transmission disequilibrium. Kaplan–Meier curves for the three genotypes were generated and the Chi-square statistic for the non-parametric k-sample test was calculated with Proc Lifetest. The observed Chi-square statistic was then contrasted against random expectations conditioned on the parental genotypes, by simulating random transmissions of parental alleles and recalculating the Chi-square statistic for each of 1,000 simulations. For this test families where both parents were homozygous were regarded as non-informative and excluded from the analysis.
The proportional hazard rate associated with carrying one or two copies of the C677T T allele was estimated from all affected individuals with Cox proportional hazard regression (Proc Phreg). In the analysis age of onset was modeled as a function of family and C677T genotype, stratifying with respect to gender. The Kaplan–Meier curves suggested that the effect of the genotype clearly depended on time. And thus, the effect of the C677T locus on onset rate was modeled as being zero before a threshold age and constant thereafter. The model fit was evaluated for a suit of threshold ages (between 0 and 30), and a threshold age of 20 yielded the best fitting model (i.e., a minimum of the −2 log likelihood of the model).
RESULTS
Scandinavian Samples
Both rs1801131 and rs1801133 were in Hardy–Weinberg equilibrium (P = 0.23 and 0.80, respectively). The two MTHFR polymorphisms were in complete linkage disequilibrium (D′ = 0.99), in the sense that the haplotype consisting of both mutant alleles was completely missing among the genotyped individuals. However, the information content in the two markers was only partly overlapping with a correlation coefficient (R2) of 0.21. We found no evidence of stratification between countries with a fixation index (FST) of 0.001 and 0.002 for rs1801131 and rs1801133, respectively.
The functional MTHFR SNP rs1801133 (C677T) significantly affected the age at onset of schizophrenia in a dose-dependent manner (plog-rank = 0.0024, Fig. 1A). That is, individuals that carried one copy of the mutant T allele showed an accelerated rate of disease onset after an age of 38 years, (n = 342, hazard rate = 2.4, confidence interval [CI95] = 1.5–3.9, pk-sample = 0.007, ptrend = 0.0005), whereas individuals carrying two copies of the T allele showed an accelerated rate of onset 14 years earlier (n = 76, hazard rate = 1.7, CI95 = 1.2–2.3, P = 0.0015). Thus, all individuals with the TT genotype in the sample were affected before 40 years of age, and all individuals with the CT genotype were affected before 51 years of age (Fig. 1A). These effects of the TT and the CT genotype were similar across the three samples, as indicated by non-significant interaction effects between rs1801133 genotypes and country of origin (P = 0.60 and 0.65, respectively). We found no evidence of rs1801131 affecting the age of schizophrenia onset (pk-sample = 0.61, ptrend = 0.48). Combined rs1801133 and rs1801131 heterozygotes have been reported to have lower MTHFR enzyme activity than single heterozygotes for the rs1801133 polymorphism [Weisberg et al., 1998; Chango et al., 2000]. We therefore analyzed the combined effect of the two polymorphisms with regard to age of onset. Combined heterozygotes tended to have an earlier age of onset than subjects heterozygote for the rs1801133 but homozygote for rs1801131 polymorphism, but this difference was not significant (P = 0.11).
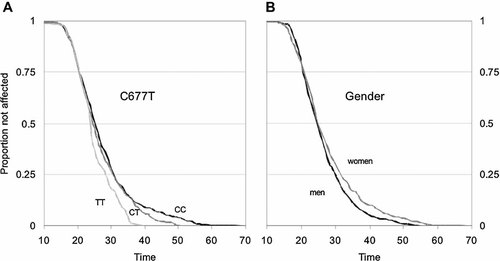
Kaplan–Meier survival curves, describing the proportion of patients who have not yet been affected by schizophrenia, as a function of age. A: Age of onset as a function of the number of methylenetetrahydrofolate reductase (MTHFR) C677T (rs1801133) genotype (P = 0.0015). The black line represents the CC genotype (n = 401), dark gray the CT genotype (n = 342), and light gray the TT genotype (n = 76), respectively. B: Age of onset as a function of gender (P = 0.007). The black line represents men (n = 479) and gray line women (n = 341).
The average onset of schizophrenia was approximately 1.5 years later in women than men (P < 0.013, Fig. 1B). This was not due to a general shift towards earlier onset in men, as the proportion of patients that were affected before 24 years of age was similar across gender. However after 24 years of age, men had an accelerated rate of onset, and the hazard ratio between men and women for this age segment was 1.25 (CI95 = 1.04–1.49). The onset of schizophrenia was similar in the three samples originating from Denmark, Norway, and Sweden with no significant difference in survival distributions (plog-rank = 0.16, data not shown).
Chinese Affected Sib-Ship Sample
The marginal genotype frequencies for MTHFR C677T were 0.37, 0.46, and 0.17 for the CC, CT, and TT genotypes, respectively, in the 114 examined families. Age of onset was significantly associated with the C677T polymorphism within families (P = 0.023), and both genotypes containing the mutant T allele were associated with an earlier age of onset. The additive values of the CT and TT genotypes were −0.09 and −0.06, respectively, with largely overlapping confidence intervals (CI95: −0.16 to −0.019, and −0.16 to 0.03). Thus onset of schizophrenia occurred on average 6.8 months earlier in individuals carrying the T allele than in their siblings that were homozygous for the wild-type allele (P = 0.008). We used gender as a confounder in the analysis. However there was no sign of gender affecting the average onset age of schizophrenia within families of multiple affected siblings (P = 0.99).
The association between the C677T genotype and age of onset was also examined through survival analysis. The Kaplan–Meier plot and proportional hazards regression suggested that the MTHFR C677T T allele had little or no effect on early onset, but that after 20 years of age carriers of the T allele had an accelerated onset rate. That is, the survival curves of the CT and TT genotypes large overlapped with that of the CC genotype until an age of 20 years, but then clearly diverge (Fig. 2). The heterogeneity of survival curves was tested through transmission disequilibrium in 60 families with both parental genotypes known and at least one heterozygous parent, and the difference was statistically significant (P = 0.028, based on 1,000 transmission simulations). The hazard rate associated with carrying the mutant T allele was estimated to be 2.1, assuming no effect prior to the age of 20 and a proportional hazard rate there after (CI95 = 1.12–4.07, P = 0.021). That is, individuals carrying the mutant T allele showed an approximately doubled rate of disease onset after the age of 20, as compared to their siblings who were homozygous for the wild-type C allele.
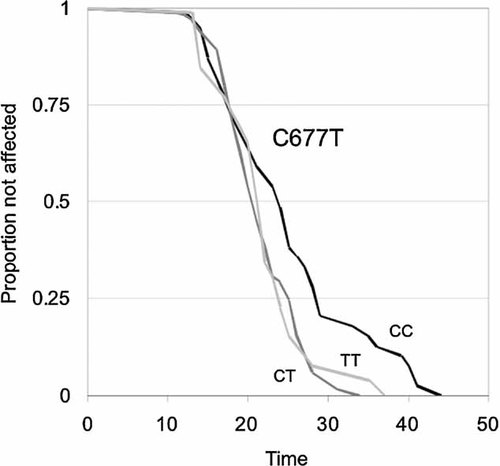
Kaplan–Meier survival curves, describing the proportion of siblings who have not yet been affected by schizophrenia, as a function of age and methylenetetrahydrofolate reductase (MTHFR) C677T (rs1801133) genotype (P = 0.028, from 1,000 transmission simulations). Sixty sib-ships were both parents were genotyped and at least one parent was heterozygous, were included in analysis. The black line represents the CC genotype (n = 39), dark gray the CT genotype (n = 62), and light gray the TT genotype (n = 26), respectively.
DISCUSSION
The present study shows a significant association between the MTHFR C677T polymorphism and age of onset in schizophrenia. The mutant T allele was associated with earlier age of onset in unrelated schizophrenia patients from three Scandinavian samples, and in Chinese high-risk families with multiple affected siblings. In both sporadic and familial cases the association was not apparent among early onset patients, but was primarily manifest in individuals with an onset age above the typical (median).
In the Scandinavian sample we observed a dose-dependent response in age of onset to the numbers of MTHFR 677T alleles after the age of 24 years: MTHFR 677T homozygotes showed an accelerated rate of onset compared to CT and CC carriers after age 24, with all patients in this subgroup affected before age 40 years. MTHFR C677T heterozygotes, however, showed an accelerated rate of onset first after 38 years of age, with all individuals affected before an age of 51 years. In the Chinese high-risk families no such dose–response pattern was evident. Instead both homozygous and heterozygous MTHFR 677T carriers showed signs of an accelerated rate of onset, primarily after the age of 20. It is possible that the discrepancy between the two sample sets reflects that the effect of the locus is influenced by other loci, which vary in the different populations, or with cultural or environmental differences between Scandinavia and China. However, the sample sizes were limited, and in particular the number of familial homozygous MTHFR 677T carriers was low. Thus, we cannot rule out that the difference in estimated response to the C677T genotypes between unrelated Scandinavian and Chinese familial patients was due to chance alone. Still, despite the dissimilarities between the Scandinavian and Chinese samples, with different ethnicities, ascertainment routes, definition of age at onset, and different genotype assays, where the more recent technology used in the Scandinavian samples may eliminate some of possible errors obtained in assays where manually reading of gels is needed, the results indicated a surprisingly similar pattern of association.
There was no evidence of association between another MTHFR polymorphism, A1298C, and age of disease onset. For this polymorphism, the evidence for association with schizophrenia has been questioned [Jönsson et al., 2008], whereas the C677T polymorphism has been associated with schizophrenia in all meta-analyses so far performed [Lewis et al., 2005; Muntjewerff et al., 2006; Zintzaras, 2006; Gilbody et al., 2007; Allen et al., 2008; Jönsson et al., 2008; Shi et al., 2008].
The MTHFR C677T functional polymorphism and related parameters, such as deficiency of folate and cobalamin as well as elevated plasma homocysteine levels, have been extensively studied in relation to different etiologically complex chronic diseases. Results concerning connection of this polymorphism with elevated risk for different diseases have often been inconsistent. Notwithstanding, relationships between this genetic MTHFR variant and age of onset have been reported in numerous studies. For example, it has been suggested that the MTHFR C677T polymorphism modifies effects of other independent risk factors for coronary artery disease (CAD) towards an earlier age of onset, as family history and homocysteine levels were associated with earlier onset of CAD only in MTHFR 677TT individuals [Mager et al., 2005]. The MTHFR C677T polymorphism is not considered a risk factor for ischemic stroke, but it is possible that the 677TT genotype could be a minor vulnerability factor in patients younger than 60 years [Linnebank et al., 2005]. In a Brazilian study no significant overall association was reported between MTHFR C677T genotypes and colorectal cancer, but 677TT and CT genotypes were more common in patients below 50 years compared with those at an older age [Lima et al., 2007]. A significant excess of MTHFR 677CT and TT genotypes was observed among breast cancer cases diagnosed before the age of 40 years [Campbell et al., 2002]. In a Taiwanese case–control study there was no overall significant association between the MTHFR C677T polymorphism and Parkinson disease, but there was a trend for association between the T allele and early age at onset, and this trend was significant in those with an age of onset above the average [Lin et al., 2007]. Our findings, indicating that the MTHFR 677 T-allele predispose to an earlier age of onset in schizophrenia patients unaffected before the typical (median) age of onset, is consistent with these findings.
Disturbances in DNA methylation are assumed to play role in schizophrenia etiology [Mill et al., 2008], because such disturbances may interfere with the normal time- and tissue-specific activation of particular genes. In an animal model, both heterozygous and homozygous MTHFR knockout mice displayed decreased DNA methylation in brain and ovaries with heterozygotes showing values in-between homozygous mutants and the wild-type genotype. The mild enzyme deficiency in humans that has been correlated to the MTHFR 677T allele is similar to that observed in the heterozygous MTHFR knockout mice [Chen et al., 2001]. Studies of glioblastoma multiforme, the most common primary brain tumor, indicated that low functioning MTHFR C677T variants is an important contributory factor to genomic hypo-methylation characteristic in some types of this tumor [Cadieux et al., 2006]. Individuals with MTHFR 677TT genotype showed diminished DNA methylation in peripheral blood mononuclear cell DNA, compared with carriers of the 677CC genotype, and this effect was particularly pronounced when folate levels were low [Friso et al., 2002]. Thus, it may be speculated that deviant DNA methylation is one possible mechanism by which aberrant one-carbon metabolism influences age of onset in schizophrenia.
The concept that schizophrenia begins in younger ages in men than in women is well established since Kraepelin's time [Kraepelin, 1919; Angermeyer and Kuhn, 1988; Häfner et al., 1993]. However, recent studies from Asian countries partly challenge this view [Gangadhar et al., 2002], suggesting an overall gender equality. The inability to replicate the European findings has been discussed in terms of different mortality rates among infants and less drug abuse, which may affect the genders differently. Interestingly, a recent community-based rural Indian study reported an earlier age of onset in women than men before 30, whereas this relationship was reversed after the age of 30 [Venkatesh et al., 2008]. In our Scandinavian sample, onset of schizophrenia appeared approximately 1.5 years earlier in men than women. However, this gender difference was evident only among individuals, who became affected after the age of 25 years. The present results with a significant earlier mean onset among men only after a certain age is thus consistent with the Indian report [Venkatesh et al., 2008]. Given the association between the MTHFR 677T allele and elevated homocysteine concentrations, in this context it is of interest that several studies have reported elevated homocysteine blood levels among primarily young men diagnosed with schizophrenia [Levine et al., 2002; Applebaum et al., 2004; Adler Nevo et al., 2006].
Recent studies of relationships between age of onset and various cofactors demonstrate that gender differences in age of onset are not detectable in all groups of patients with schizophrenia. Some reports have claimed that only in paranoid schizophrenia, (which has the latest age of onset among all classical schizophrenia subtypes), significant gender onset differences could be found, with earlier onset ages for men [Beratis et al., 1994; Salokangas et al., 2003]. It has also been suggested that gender differences in age at onset of the disorganized subtype, with the earliest onset among the schizophrenia subtypes, is inverted with earlier onset in female patients [Beratis et al., 1994]. Another study suggested that gender differences in age of onset, with younger ages for men, were significant in a subgroup of neuroleptic-responsive patients with paranoid schizophrenia only [Meltzer et al., 1997]. Other authors suggest the existence of two schizophrenia subgroups defined by an onset before and after 28 years [Schurhoff et al., 2004]. Thus it is possible that the observed lack of modifying effects of gender and MTHFR 677T on patients with an early onset partly reflects variation in the etiopathogenic processes underlying the development of schizophrenia.
In conclusion, the MTHFR 677T-allele is associated with an accelerated rate of onset in schizophrenia patients characterized by a moderate or late onset age. This finding was replicated in two independent samples representing both unrelated mainly sporadic cases from a Scandinavian population and familial cases from a Chinese high-density sample.
Acknowledgements
We thank patients for their participation and express our gratitude towards health professionals who facilitated our work. This study was financed in China by grants to HD from the National Nature Science Foundation of China (30670757), in Denmark by grants to TW from the Copenhagen Hospital Corporation Research Fund, the Danish National Psychiatric Research Foundation, the Danish Agency for Science, and Technology and Innovation (Centre for Pharmacogenetics), in Norway from the Research Council of Norway (147787, 167153), the South-Eastern Norway Health Authority (123/2004), Oslo University Hospital, and University of Oslo, and in Sweden from the Swedish Research Council (2006-2992, 2006-986, 2008-2167), the regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet, Wallenberg Foundation, and the HUBIN project. We thank Alexandra Tylec, Agneta Gunnar, Monica Hellberg, and Kjerstin Lind for technical assistance. We also thank Kristina Larsson, Tomas Axelsson, and Ann-Christine Syvänen at the SNP Technology Platform in Uppsala for performing the genotyping. The SNP Technology Platform is supported by Uppsala University, Uppsala University Hospital, and by the Knut and Alice Wallenberg Foundation.




