Meta-analysis of brain-derived neurotrophic factor p.Val66Met in adult ADHD in four European populations†‡
B. Franke, S. Johansson, J. Haavik, A. Reif, M. Bayés, and B. Cormand contributed equally to this work as senior members of the International Multicentre Persistent ADHD CollaboraTion (IMpACT).
How to Cite this Article: Sánchez-Mora C, Ribasés M, Ramos-Quiroga JA, Casas M, Bosch R, Boreatti-Hümmer A, Heine M, Jacob CP, Lesch K-P, Fasmer OB, Knappskog PM, Sandra Kooij JJ, Kan C, Buitelaar JK, Mick E, Asherson P, Faraone SV, Franke B, Johansson S, Haavik J, Reif A, Bayés M, Cormand B. 2009. Meta-Analysis of Brain-Derived Neurotrophic Factor p.Val66Met in Adult ADHD in Four European Populations. Am J Med Genet Part B 153B:512–523.
Abstract
Attention-deficit hyperactivity disorder (ADHD) is a multifactorial, neurodevelopmental disorder that often persists into adolescence and adulthood and is characterized by inattention, hyperactivity and impulsiveness. Before the advent of the first genome-wide association studies in ADHD, genetic research had mainly focused on candidate genes related to the dopaminergic and serotoninergic systems, although several other genes had also been assessed. Pharmacological data, analysis of animal models and association studies suggest that Brain-Derived Neurotrophic Factor (BDNF) is also a strong candidate gene for ADHD. Several polymorphisms in BDNF have been reported and studied in psychiatric disorders but the most frequent is the p.Val66Met (rs6265G > A) single nucleotide polymorphism (SNP), with functional effects on the intracellular trafficking and secretion of the protein. To deal with the inconsistency raised among different case–control and family-based association studies regarding the p.Val66Met contribution to ADHD, we performed a meta-analysis of published as well as unpublished data from four different centers that are part of the International Multicentre Persistent ADHD CollaboraTion (IMpACT). A total of 1,445 adulthood ADHD patients and 2,247 sex-matched controls were available for the study. No association between the p.Val66Met polymorphism and ADHD was found in any of the four populations or in the pooled sample. The meta-analysis also showed that the overall gene effect for ADHD was not statistically significant when gender or comorbidity with mood disorders were considered. Despite the potential role of BDNF in ADHD, our data do not support the involvement of p.Val66Met in the pathogenesis of this neuropsychiatric disorder. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder that often persists into adolescence and adulthood and is characterized by inattention, hyperactivity and impulsiveness and causes significant social, educational and psychological problems in childhood and adulthood. Heritability of ADHD has been estimated at 39–91% and it is considered a complex disorder with genetic and environmental risk factors [Faraone et al., 2005]. The genetic causes of ADHD are still unknown, but so far genetic research has mainly focused on candidate genes of the dopaminergic and serotoninergic systems [Comings et al., 2000; Ribasés et al., 2009]. In addition to these neurotransmission pathways, neurotrophic factors, which participate in development, survival and functional maintenance of neurons, may be involved in neuroplasticity changes that take place in the central nervous system and may also contribute to the genetic predisposition to ADHD. Among these, Brain-Derived Neurotrophic Factor (BDNF) is a strong candidate gene. Psychostimulant and antidepressant drugs commonly used for ADHD treatment modulate the expression of BDNF and its specific receptor NTRK2 [Meredith et al., 2002; Chase et al., 2007]. In addition, heterozygous Bdnf knockout mice display hippocampal-dependent learning deficiencies, aggressiveness, anxiety, and hyperactive locomotor behavior when compared with wild-type littermates, which interestingly can be rescued in an enriched environment [Linnarsson et al., 1997; Kernie et al., 2000; Rios et al., 2001; Chourbaji et al., 2004]. Chronic infusion of Bdnf into the substantia nigra of animal models alters locomotion activity, whereas its intracerebroventricular administration decreases locomotion, motility and rearing [Martin-Iverson et al., 1994; Kobayashi et al., 1997]. Finally, several association studies have tested the possible involvement of BDNF in ADHD, although the results are controversial. Some of these studies focus solely on BDNF or a few candidate genes (Table SI; [Friedel et al., 2005; Kent et al., 2005; Brookes et al., 2006; Lee et al., 2007; Schimmelmann et al., 2007; Xu et al., 2007; Conner et al., 2008; Lanktree et al., 2008; Oades et al., 2008]), whereas others are GWAS [Neale et al., 2008; Sonuga-Barke et al., 2008; Lasky-Su et al., 2008a,b; Anney et al., 2008b].
The BDNF gene is located at 11p13-14, is organized in 13 exons, and encodes a 247 amino acid precursor peptide (pro-BDNF) that is proteolytically cleaved to form the mature protein of 153 amino acids. Several polymorphisms of BDNF have been reported and studied in a number of psychiatric disorders. The most frequent is the rs6265 (p.Val66Met) SNP located within the pro-BDNF region, which goes along with increased BDNF serum concentrations in Met allele carriers [Lang et al., 2009]. Egan et al. 2003 demonstrated that although it is located in the pro-BDNF polypeptide and does not alter the intrinsic biological activity of the mature protein, the rs6265G > A sequence variant impairs the intracellular processing and secretion of the mature neurotrophin. In addition, as it was recently shown that mice also secrete proBDNF, especially while the brain is developing, these data point towards an impact of p.Val66Met on neuronal development, especially in the hippocampus [Yang et al., 2009].
To deal with the inconsistency raised among different case–control and family-based association studies regarding the contribution of BDNF p.Val66Met to ADHD, we aimed to perform a meta-analysis of published as well as unpublished case–control data from four different centers (Germany, The Netherlands, Norway and Spain) integrated in the International Multicentre persistent ADHD CollaboraTion (IMpACT), which focuses its interest on adulthood ADHD.
MATERIALS AND METHODS
Patients and Controls
In total, 1,445 adult ADHD patients and 2,447 controls of Caucasian origin from four European countries (Spain, Germany, Norway, and The Netherlands) were recruited by members of the International Multicentre Persistent ADHD CollaboraTion (IMpACT). Consensus eligibility criteria for the current study across all sites were a diagnosis of ADHD according to the diagnostic criteria of DSM-IV (Diagnostic and Statistical Manual for Mental Disorders-IV), onset before the age of 7 years via retrospective diagnosis (which was confirmed by a family member, wherever possible), lifelong persistence and current diagnosis. Patients were extensively examined by experienced psychiatrists in adult ADHD using open and semi-structured interviews. Other psychiatric disorders were evaluated with the Structured Clinical Interview of DSM-IV for axis-I and axis-II disorders (SCID-I, SCID-II) or semi-structured interviews. Severity of ADHD in adulthood was measured by means of standards rating scales. Most of the clinical sample has been described before [Bekker et al., 2005; Kooij et al., 2005; Jacob et al., 2007; Franke et al., 2008; Johansson et al., 2008; Ramos-Quiroga et al., 2008; Ribasés et al., 2008]. Diagnosis was blind to genotype (see Table SII for a detailed description of the instruments and procedures used by the different sites).
Most controls (except for the Norwegian samples and part of the German samples, see Table SII) were screened for the presence of ADHD and those scoring high on symptoms of the disorder were excluded.
The study was approved by the ethics committee of each participating institution and informed consent was obtained from all subjects in accordance with the Helsinki Declaration.
DNA Isolation and Genotyping
Genomic DNA was isolated either from saliva using the Oragene™ DNA Self-Collection kit from DNA Genotek (DNA Genotek Inc., Ottawa, Canada) or from peripheral blood lymphocytes by the salting-out procedure [Miller et al., 1988].
Germany and Norway: genotyping of rs6265 was accomplished using the Sequenom MassArray genotyping platform (Sequenom, San Diego, CA).
Netherlands: The rs6265 polymorphism was genotyped using TaqMan-based genotyping. Genotyping was carried out in a volume of 10 µl containing 10 ng of genomic DNA, 5 µl of ABgene Mastermix (2×; ABgene Ltd., Hamburg, Germany), 0.125 µl of the Taqman assay (assay ID: Taqman assay: C_11592758_10; reporter 1: VIC-C-allele; reporter 2: FAM-T-allele; Applied Biosystems, Nieuwerkerk a/d Ijssel, The Netherlands) and 3.875 µl of H2O. Amplification was performed on a 7500 Fast Real-Time PCR System starting with 15 min at 95°C, followed by 50 cycles of 15 sec at 95°C and 1 min at 60°C. Genotypes were scored using the algorithm and software supplied by the manufacturer (Applied Biosystems).
Spain: rs6265 was genotyped with the SNPlex platform (Applied Biosystems, Foster City, CA) at the Barcelona node of the Centro Nacional de Genotipado (CeGen, www.cegen.org) as previously described [Tobler et al., 2005].
Statistical Analysis
We first assessed Hardy–Weinberg equilibrium (HWE) in the control groups from each IMpACT node using a χ2 test. To determine the best genetic model to be used we estimated the three possible odds ratios (ORs) and their 95% confidence intervals (CI): OR1 (Val/Val vs. Met/Met), OR2 (Val/Val vs. Val/Met) and OR3 (Met/Met vs. Val/Met) in the pooled sample (Table SIII). Thus, if OR1 = OR3 ≠ 1 and OR2 = 1, a recessive model is suggested; OR1 = OR2 ≠ 1 and OR3 = 1 indicates a dominant model; and OR1 > OR3 > 1 (or OR1 < OR2 < 1 and OR1 < OR3 < 1) suggests a codominant model. Prior to the pooling of the four individual studies, we compared genotype and allele frequencies among cases and controls from each separate IMpACT node using a χ2 test with the SNPassoc R package [Gonzalez et al., 2007]. To combine the individual study results, we conducted meta-analyses using the Meta R package (cran.r-project.org/web/packages/meta/index.html). We first tested for heterogeneity among studies using the Q-statistic, which is a weighted sum of the squares of the deviations of individual study OR estimates from the overall estimate. When the ORs are homogeneous, Q follows a χ2 distribution with r − 1 (r is the number of studies) degrees of freedom (df). If PQ < 0.10, the heterogeneity was considered to be statistically significant [Lau et al., 1997; Fleiss, 1981]. Inconsistency across studies was quantified with the I2 metric (I2 = Q − df/Q), which can be interpreted as the percentage of total variation across several studies due to heterogeneity. I2 takes values between 0% and 100%, with higher values denoting a greater degree of heterogeneity (0–25%: no heterogeneity; 25–50% moderate heterogeneity; 50–75%: large heterogeneity; 75–100% extreme heterogeneity) [Zintzaras and Hadjigeorgiou, 2004]. When no heterogeneity was present, the pooled OR was estimated using fixed-effects model (Mantel and Haenszel, 1959). Otherwise, random effects models (Laird and Mosteller, 1990) were applied to obtain the pooled OR. Random effects modeling assumes a genuine diversity in the results of various studies and it incorporates a between-study variance into the calculations (Whitehead, 2002). The results of the association tests are given as pooled ORs (which estimate the genotype-induced risk of adult ADHD), with the corresponding 95% confidence intervals (CIs). P < 0.05 was considered statistically significant. Additionally, we conducted other meta-analytical studies in which we split the sample by gender or by clinical subtype (combined and inattentive). Because ADHD is highly comorbid with mood disorders and BDNF has been extensively tested for association with this psychiatric entity [Gratacòs et al., 2007; Verhagen et al., 2008], we also considered ADHD patients with and without mood disorders.
RESULTS
A total of 1,445 adult ADHD patients and 2,247 controls from four IMpACT nodes were available for the meta-analysis. Table I shows the clinical description of the samples included in the study. Genotype distributions of the p.Val66Met SNP showed no significant departure from HWE when each control group as well as the pooled control sample were considered (P > 0.05). No significant differences were observed when genotype or allele frequencies of the p.Val66Met SNP were compared between ADHD patients from each country and their sex-matched unrelated controls (Table II). To determine the best genetic model we calculated the three ORs. The estimated ORs in the pooled sample were OR1 = 0.93 (95% CI: 0.66–1.31), OR2 = 0.99 (95% CI: 0.86–1.14), and OR3 = 1.07 (95% CI: 0.75–1.52). No heterogeneity was detected for OR1, OR2, or OR3 across samples (P = 0.43, P = 0.35, and P = 0.51, respectively) (Table SIII). The fact that none of the ORs significantly deviated from 1 did not allow to choose a particular genetic model based on the pattern of the odds ratios. Thus, comparisons of genotype frequencies between cases and controls were performed under different models (codominant, dominant, recessive, overdominant, and log-additive). The dominant one was eventually selected for the meta-analysis because (1) it was the one that produced lower P-values in most comparisons, (2) it produced the lowest Akaike Information Criterion (AIC) values, and (3) it was the only one to show nominal P-values in some particular comparisons (see below). Therefore, a Met66 dominant model with fixed effects was used to perform a meta-analysis, which showed a lack of a statistically significant effect of the p.Val66Met BDNF SNP on adulthood ADHD (P = 0.86; OR = 0.99 (95% CI: 0.86–1.13); Table III).
| Study | Germany | Netherlands | Norway | Spaina | Pool |
|---|---|---|---|---|---|
| Cases | |||||
| Female | 282 (46.45%) | 95 (50%) | 208 (47.7%) | 58 (23.5%) | 643 (44.49%) |
| Male | 325 (53. 54%) | 96 (50%) | 228 (52.29%) | 153 (76.5%) | 802 (55.50%) |
| Total | 607 | 191 | 436 | 211 | 1,445 |
| ADHD subtype | |||||
| Combined type | 408 (67.21%) | 172 (90.07%) | 230 (52.75%) | 139 (65.87%) | 949 (65.67%) |
| Hyperactive/impulsive type | 48 (7.90%) | 5 (2.61%) | 30 (6.88%) | 10 (4.73%) | 93 (6.43%) |
| Inattentive type | 151 (24.87%) | 14 (7.32%) | 73 (16.74%) | 62 (29.4%) | 300 (20.76%) |
| Sub-threshold | — | — | 97 (22.24%) | — | 97 (6.71%) |
| Unknown | — | — | 6 (1.39%) | — | 6 (0.43%) |
| Age (mean and SD) | 31 (10.26) | 42 (10.7) | 34 (10.6) | 35 (10.17) | 35 (4,6) |
| Mood disorders | 323 (53.21%) | 119 (62.30%) | 299 (68.57%) | 81 (38.38%) | 822 (56.8%) |
| Controls | |||||
| Female | 390 (46, 42%) | 243 (50%) | 237 (47.78%) | 116 (27.29%) | 986 (43.8%) |
| Male | 450 (53, 57%) | 243 (50%) | 259 (52.2%) | 309 (72.7%)) | 1261 (56.1%) |
| Total | 840 | 486 | 496 | 425 | 2,247 |
| Age (mean and SD) | 31 (10.4) | 62 (18.06) | 27 (7.2) | 43 (16.36) | 40 (15.7) |
- a Published data [Ribasés et al., 2008].
| Genotypesa | Allelesb | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | Genotype AA vs. AG + GG | Genotype AG + AA vs. GG | Allele A vs. allele G | |||||||||||
| AA | AG | GG | Sum | AA | AG | GG | Sum | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Germany | 25 (4.1) | 189 (31.1) | 393 (64.7) | 607 | 39 (4.6) | 284 (33.8) | 517 (61.5) | 840 | 0.45 | 0.88 (0.53–1.47) | 0.63 | 1.14 (0.92–1.42) | 0.21 | 1.12 (0.93–1.35) | 0.22 |
| Netherlands | 5 (2.6) | 67 (35.1) | 119 (62.3) | 191 | 12 (2.5) | 152 (31.3) | 322 (66.3) | 486 | 0.62 | 1.06 (0.37–3.06) | 0.91 | 0.84 (0.59–1.19) | 0.33 | 0.88 (0.65–1.18) | 0.38 |
| Norway | 22 (5.0) | 132 (30.3) | 282 (64.7) | 436 | 17 (3.4) | 155 (31.2) | 324 (65.3) | 496 | 0.46 | 1.50 (0.78–2.86) | 0.21 | 0.97 (0.74–1.26) | 0.83 | 0.93 (0.74–1.17) | 0.53 |
| Spain | 10 (4.7) | 79 (37.4) | 122 (57.8) | 211 | 18 (4.2) | 141 (33.2) | 266 (62.6) | 425 | 0.51 | 1.12 (0.51–2.48) | 0.24 | 0.81 (0.58–1.14) | 0.24 | 0.86 (0.65–1.13) | 0.28 |
| Pool | 62 (4.3) | 467 (32.3) | 916 (63.4) | 1,445 | 86 (3.8) | 732 (32.6) | 1,429 (63.6) | 2,247 | 0.77 | 1.13 (0.81–1.57) | 0.48 | 0.99 (0.86–1.13) | 0.90 | 0.98 (0.87–1.1) | 0.72 |
- a AA = MetMet; AG = MetVal; GG = ValVal.
- b A = Met; G = Val.
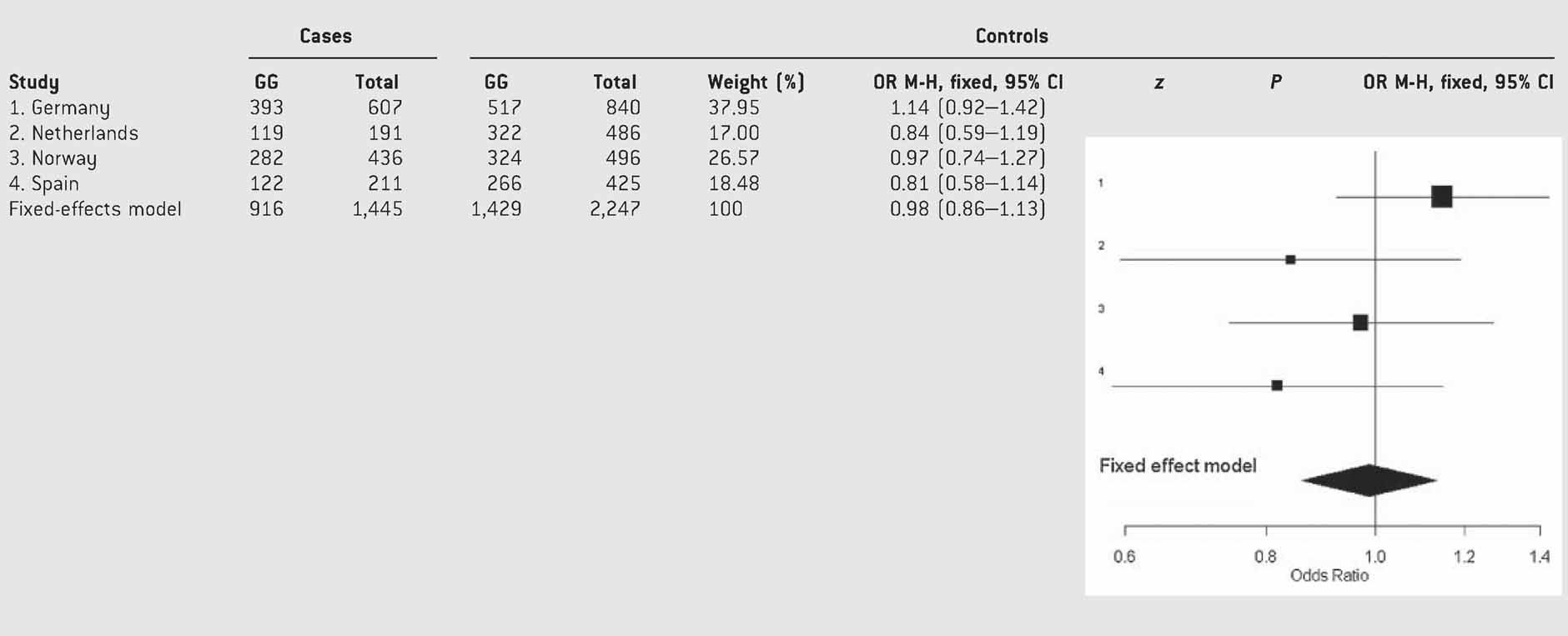
- Test of heterogeneity: Q = 3.84, df = 3, P = 0.28; I2 = 21.9% (0–88%).
We then considered the combined and inattentive subtypes separately and we did not observe statistically significant effects of p.Val66Met BDNF on ADHD subtypes in either of the four populations (Table SIV). The pooled analysis under a fixed-effects model showed no significant effect of the polymorphism on ADHD subtypes either (Table IV). The hyperactive/impulsive subtype was not studied because of the limited sample size (6.43% from the total sample; Table I).
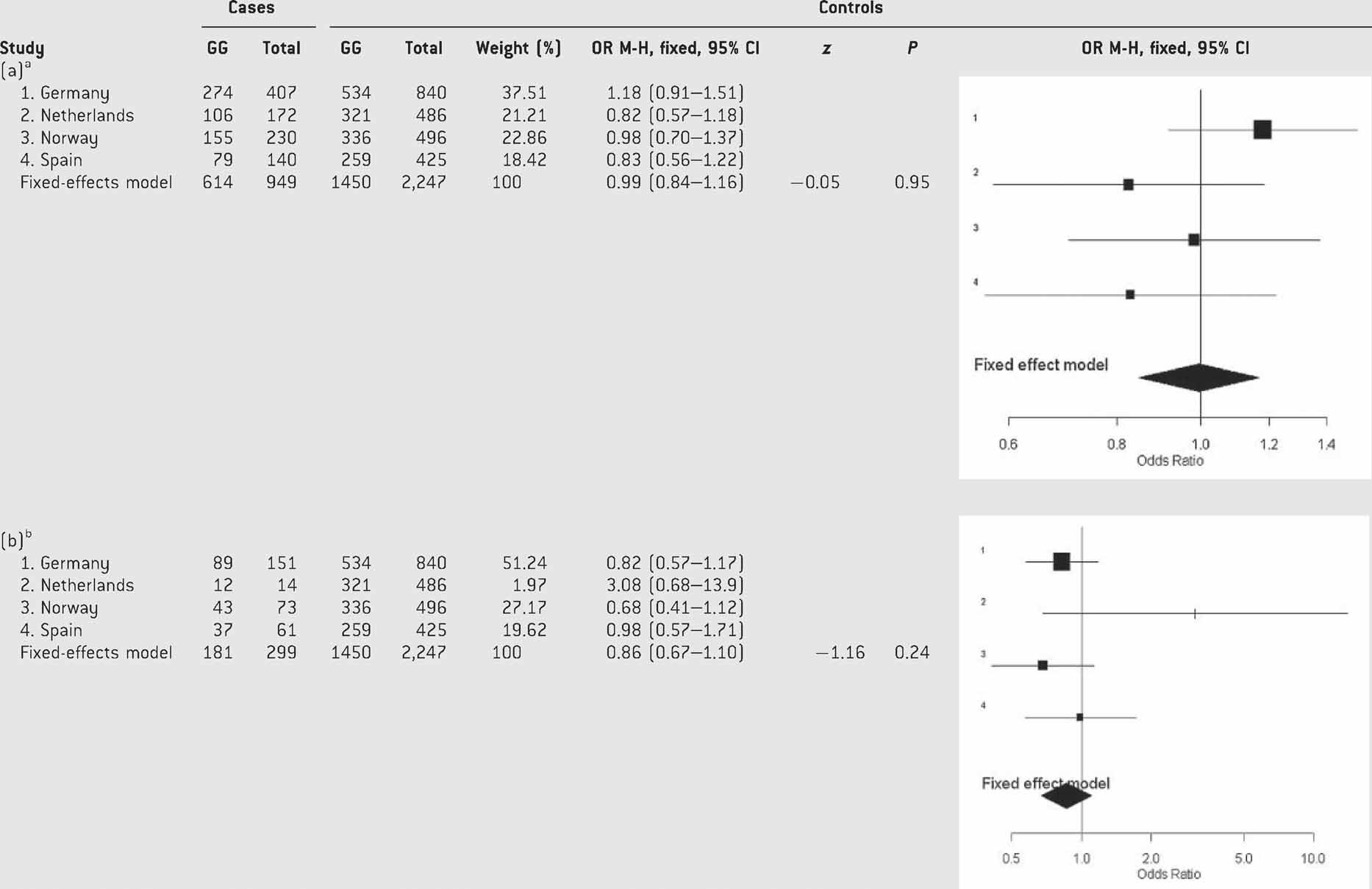
- aTest of heterogeneity: Q = 3.67, df = 3, P = 0.29; I2 = 18.3% (0–87.5%).
- bTest of heterogeneity: Q = 3.87, df = 3, P = 0.27; I2 = 22.6% (0–88.1%).
The separate analysis of males and females displayed a nominal association between p.Val66Met and ADHD in females from the Spanish cohort (P = 0.0363, OR = 0.50 (95% CI: 0.26–0.96); Table SV). Nevertheless, after having discarded heterogeneity, the pooled analysis under a fixed-effects model showed that the overall gene effect on ADHD was neither statistically significant in females (P = 0.51; OR = 0.93 (95% CI: 0.75–1.14)) nor in males (P = 0.72; OR = 1.03 (95% CI: 0.85–1.24); Table V).
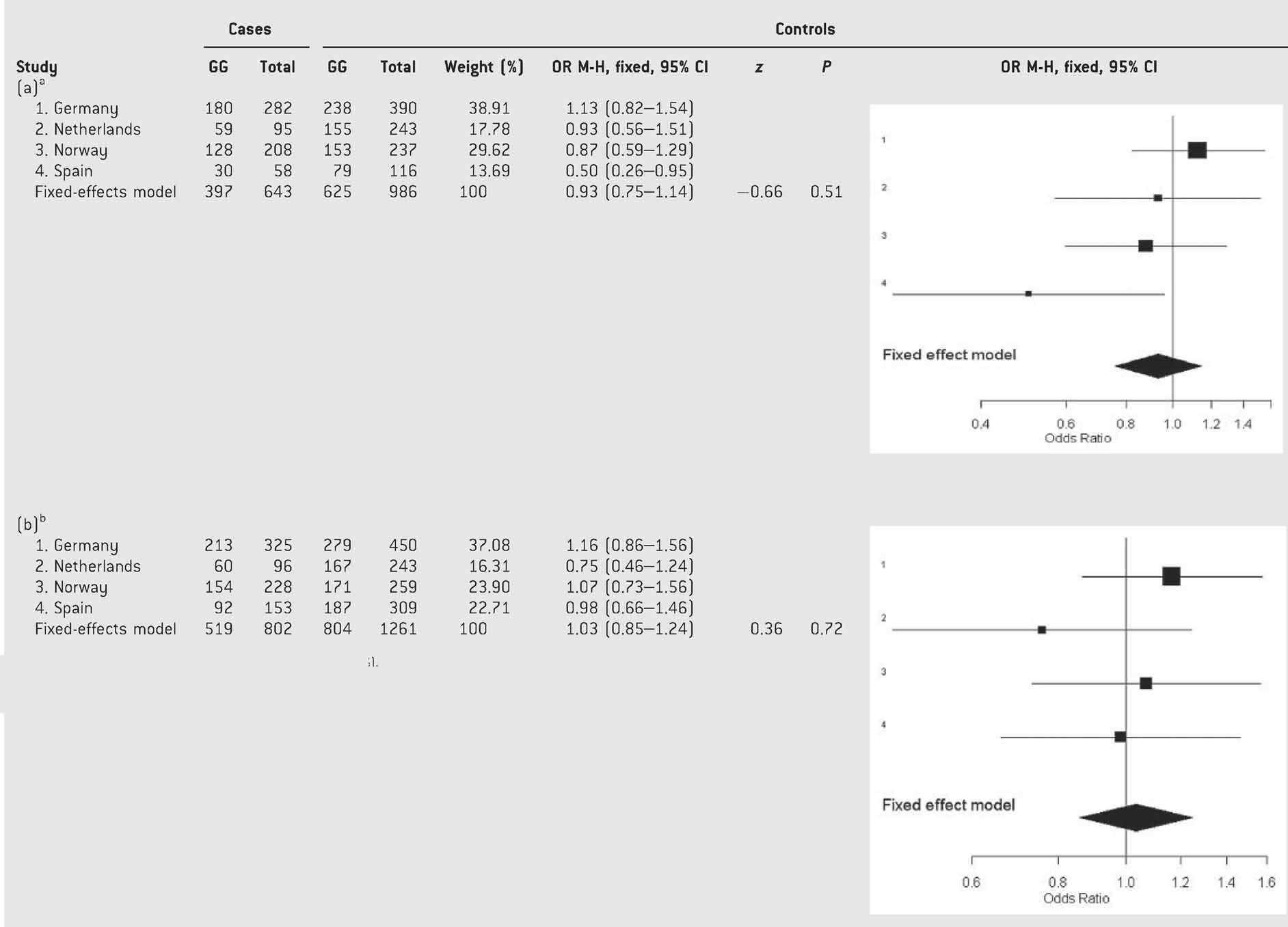
- aTest of heterogeneity: Q = 2.23, df = 3, P = 0.52; I2 = 40% (0–79.6%).
- bTest of heterogeneity: Q = 2.23, df = 3, P = 0.52; I2 = 40% (0–79.6%).
We then separated ADHD patients according to comorbidity with mood disorders and observed nominal association between BDNF and ADHD with mood disorders in some of the populations (Germany, genotypes: P = 0.035, OR = 1.33 (95% CI: 1.02–1.75) and alleles: P = 0.035, OR = 1.28 (95% CI: 1.01–1.64); Table SVI) and without mood disorders in others (Netherlands: genotypes: P = 0.047, OR = 0.60 (95% CI: 0.36–0.99); Table SVI). However, after discarding heterogeneity, the meta-analysis under a fixed-effects model showed that the overall gene effect was not significant in any of the two clinical groups considered (ADHD with mood disorders: P = 0.24, OR = 1.11 (95% CI: 0.93–1.31); ADHD without mood disorders: P = 0.20, OR = 0.88 (95% CI: 0.73–1.07); Table VI).
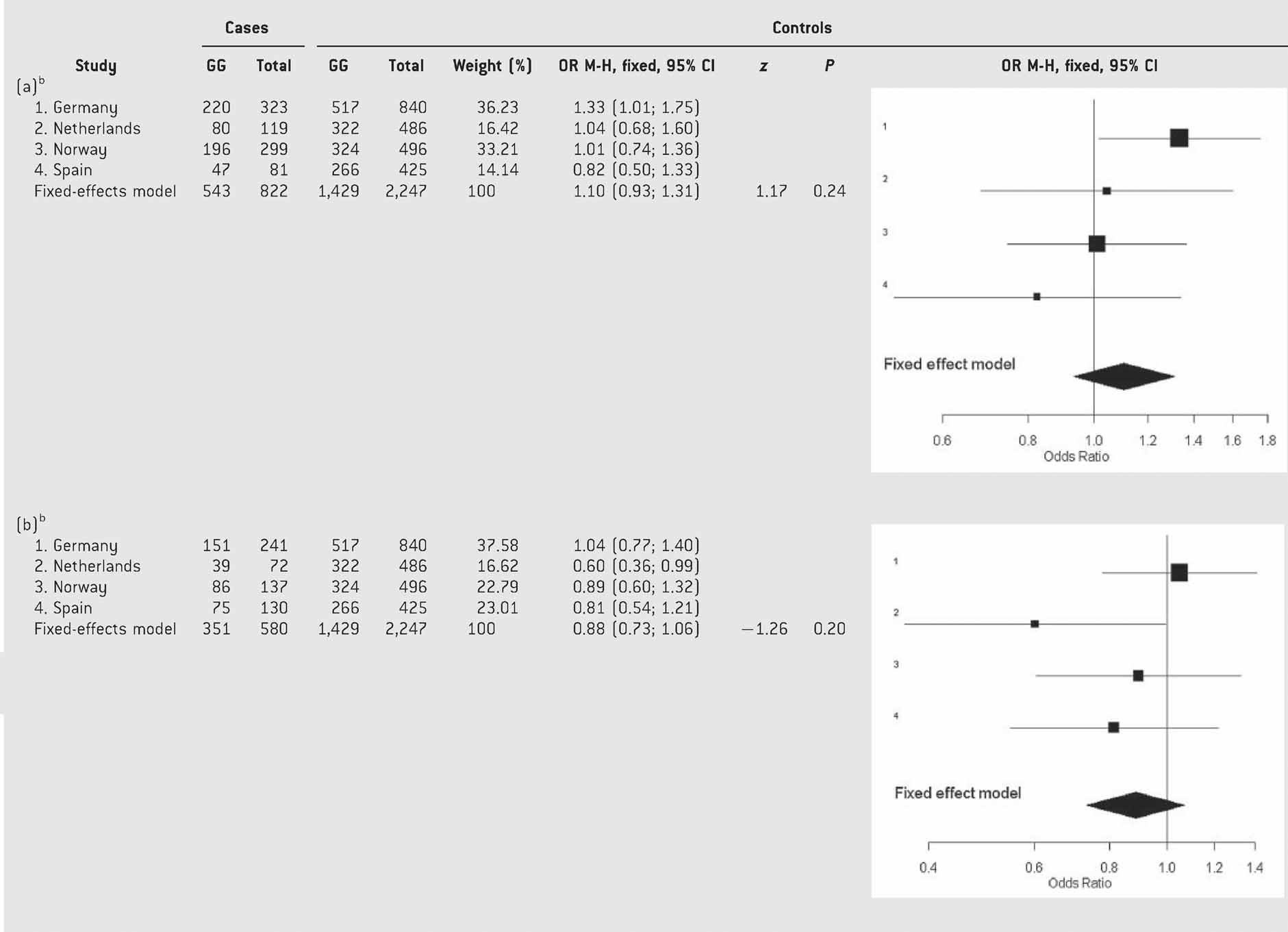
- aTest of heterogeneity: Q = 3.64, df = 3, P = 0.30; I2 = 17.6% (0–87.4%).
- bTest of heterogeneity: Q = 3.71, df = 3, P = 0.29; I2 = 19% (0–89.6%).
DISCUSSION
To our knowledge, this is the first meta-analysis performed to evaluate the association between BDNF and adulthood ADHD. No evidence of association was found between the p.Val66Met variation and this neuropsychiatric disorder. Although most of the previous studies considered childhood ADHD samples (Table SI), our results are in agreement with those described by Friedel et al. 2005 in 88 childhood ADHD patients and 96 controls, Schimmelmann et al. 2007 in 468 children from 294 families comprising one or more affected sibs, Lee et al. 2007 in 315 ADHD children from 266 nuclear families, or Brookes et al. 2006 in 674 DSM-IV combined type probands with 808 siblings, in which no association between the p.Val66Met SNP and ADHD was detected. However, other association studies have reported a possible contribution of BDNF to ADHD (Table SI). In this regard, Kent et al. 2005 demonstrated a preferential paternal transmission of the p.Val66 allele in 341 childhood ADHD trios and Xu et al. 2007 found no association between p.Val66Met and ADHD but showed evidence for a decreased transmission of the −270T/p.Val66 haplotype in two independent samples of ADHD child probands. In addition, two studies considered the BDNF gene in adulthood ADHD and while Lanktree et al. 2008 found evidence for a p.Val66 contribution, Conner et al. 2008 showed no association between this polymorphism and adult ADHD scores (Table SI). Two genome-wide association studies (GWAS) have been reported in ADHD so far, one of them in 343 adult ADHD patients and 304 controls using a DNA pooling approach [Lesch et al., 2008] and the other one in 958 affected family trios with child ADHD sibs considering either ADHD as a categorical trait or related quantitative measures such as ADHD symptoms, age at onset of ADHD symptoms or conduct problems [Neale et al., 2008; Lasky-Su et al., 2008a,b; Anney et al., 2008b]. All of them included the p.Val66Met variation (rs6265) and other polymorphic sites within the BDNF gene, but they did not achieve genome-wide significance.
- (1)
Genetic effects for common variants may be reduced and difficult to detect in association studies of small sample sizes. The present meta-analysis, that considers relatively large well-characterized clinical samples that fulfilled DSM-IV criteria for ADHD and were evaluated using a common set of diagnostic instruments, improves the statistical power to detect association (>95%) with respect to the analyses of previous datasets. Our strategy also allowed examination of heterogeneity between the different series of patients and controls included in the study. In this regard, we did not detect heterogeneity of ORs or evidence that a single study accounted for the significance or magnitude of the pooled study.
- (2)
The proportions of the different ADHD subtypes differ among the reported studies. Thus, while most patients described by Kent et al. 2005 (81%) or Xu et al. 2007 (100% UK and 78% Taiwan) were combined ADHD, a lower proportion was seen in the other studies (<70%), including the present meta-analysis (66%). If the BDNF contribution to ADHD was subtype-specific, these differences could account for the distinct results between studies.
- (3)
Gender differences among studies may also explain controversial results, as a recent study suggests that hyperactivity may be gender-specific [Kim et al., 2007]. In this regard, male BDNF conditional knock-out mice exhibit hyperactivity, whereas females display normal locomotor activity with a prominent increase in depression-like behaviors [Monteggia et al., 2007]. Although we found nominal association between BDNF and ADHD in Spanish females, the pooled analysis stratified for gender showed that the overall gene effect on ADHD was not statistically significant.
- (4)
Other studies have reported a gene-specific parent of origin effect in ADHD. In this regard, Kent et al. 2005 demonstrated a significant preferential paternal transmission of the p.Val66 allele to children with ADHD. Nevertheless, other groups did not find evidence for a preferential paternal transmission of BDNF risk alleles in ADHD [Kim et al., 2007; Anney et al., 2008a]. This aspect, however, could not be assessed in the present case–control association study.
- (5)
Genetic variants in BDNF have generally been linked to a number of other psychiatric phenotypes, that may share genetic risk factors with ADHD, such as schizophrenia, eating disorders, bipolar disorder or depression [Neves-Pereira et al., 2002; Sklar et al., 2002; Green et al., 2006; Gratacòs et al., 2007; Verhagen et al., 2008; Duncan et al., 2009]. Since comorbidity with these disorders is frequently observed in ADHD (e.g., substantial proportion of our ADHD sample showed comorbidity with mood disorders (56.8%)), in contrast to most of the previous studies we tested directly whether this comorbidity might bias association results for ADHD [Kent et al., 2005; Xu et al., 2007; Lanktree et al., 2008]. Although we observed a nominal association between BDNF and ADHD with mood disorders (in the German sample) or without mood disorders (in the Dutch sample), the pooled analysis showed that the overall gene effect was not significant in any of these two groups.
- (6)
Most of the previous studies reporting a BDNF contribution to the susceptibility to ADHD include only children samples, which may suggest a childhood-specific association between this neurotrophic factor and ADHD. Only one study reports association between BDNF and adult ADHD, although the sample size is limited [Lanktree et al., 2008]. In this regard, our group identified a childhood-specific association between ADHD and the BDNF specific receptor, NTRK2 [Ribasés et al., 2008] that might support a differential genetic component in childhood ADHD with or without remission of the phenotype across life span.
- (7)
Although it has been reported that the p.Val66Met polymorphism alters the intracellular trafficking and activity-dependent secretion of BDNF [Egan et al., 2003], this SNP may not be functionally involved in ADHD, although it is still possible that other BDNF sequence variants do contribute to the phenotype.
In conclusion, although BDNF remains as a candidate risk factor for ADHD, we found no evidence of association between the p.Val66Met SNP and adulthood ADHD using a meta-analysis strategy in large well-characterized clinical samples from four European populations. Further dissection of clinical phenotypes as well as full genetic coverage, in terms of LD, of the BDNF gene in larger cohorts of children as well as adult patients may improve our knowledge about the involvement of BDNF in the susceptibility to this complex neuropsychiatric disorder.
Acknowledgements
We are grateful to all patients and controls for their participation in the study. SNP genotyping services of the Spanish samples were provided by the Centro Nacional de Genotipado (CeGen; www.cegen.org). Genotyping of the Norwegian samples was performed at the CIGENE national technology platform supported by the Functional Genomics Program (FUGE) of the Research Council of Norway. We thank M. Nogueira, N. Gómez-Barros and M. Corrales for their involvement in the clinical assessment and to M. Dolors Castellar and A. Daví for their help in the recruitment of control subjects in Spain. We are indebted to T. Töpner, N. Steigerwald, and J. Auer for excellent technical assistance and to J. Wegener, S. Groβ-Lesch and S. Kreiker for kind help in ascertaining patients and diagnostic assessment in Germany. We thank P. Borge and S. Erdal for help with genotyping of the Norwegian samples. We thank R. Makkinje, M. Naber and A. Heister for help with genotyping in The Netherlands. The Dutch controls were derived from the Nijmegen Biomedical Study. Principal investigators of the Nijmegen Biomedical Study are L.A.L.M. Kiemeney, M. den Heijer, A.L.M. Verbeek, D.W. Swinkels and B. Franke. Financial support for the Spanish part of the study was received from “Instituto de Salud Carlos III-FIS, Spain” (PI040524, PI041267, PI080519) and “Agència de Gestió d'Ajuts Universitaris i de Recerca-AGAUR” (2005SGR00848). M. Bayés and M. Ribasés are recipients of a “Ramon y Cajal” and a “Juan de la Cierva” contracts, respectively, from “Ministerio de Ciencia y Tecnología” (Spain). The German part of the study was supported by the Deutsche Forschungsgemeinschaft (Grant RE1632/1-1 and 1-3 to A. Reif, KFO 125 to A. Reif, C.P. Jacob, and K-P. Lesch; SFB 581 to K-P. Lesch, SFB TRR 58 to A. Reif and K-P. Lesch), BMBF (01GV0605 to K-P. Lesch; IZKF 01KS9603, N-4 to A. Reif) and the EC (NEWMOOD LSHM-CT-2003-503474, to K-P. Lesch). The Norwegian part of the study was supported by the Research Council of Norway and Helse Vest. The Dutch part of the project was supported by the Hersenstichting Nederland (Fonds Psychische Gezondheid).




