Variation in GRIN2B contributes to weak performance in verbal short-term memory in children with dyslexia†‡
Kerstin U. Ludwig and Darina Roeske contributed equally to this work.
How to Cite this Article: Ludwig KU, Roeske D, Herms S, Schumacher J, Warnke A, Plume E, Neuhoff N, Bruder J, Remschmidt H, Schulte-Körne G, Müller-Myhsok B, Nöthen MM, Hoffmann P. 2009. Variation in GRIN2B Contributes to Weak Performance in Verbal Short-Term Memory in Children With Dyslexia. Am J Med Genet Part B 153B:503–511.
Abstract
A multi-marker haplotype within GRIN2B, a gene coding for a subunit of the ionotropic glutamate receptor, has recently been found to be associated with variation in human memory performance [de Quervain and Papassotiropoulos, 2006]. The gene locus is located within a region that has been linked to a phonological memory phenotype in a recent genome scan in families with dyslexia [Brkanac et al., 2008]. These findings may indicate the involvement of GRIN2B in memory-related aspects of human cognition. Memory performance is one of the cognitive functions observed to be disordered in dyslexia patients. We therefore investigated whether genetic variation in GRIN2B contributes to specific quantitative measures in a German dyslexia sample by genotyping 66 SNPs in its entire genomic region. We found supportive evidence that markers in intron 3 are associated with short-term memory in dyslexia, and were able to demonstrate that this effect is even stronger when only maternal transmission is considered. These results suggest that variation within GRIN2B may contribute to the genetic background of specific cognitive processes which are correlates of the dyslexia phenotype. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Dyslexia is a complex neurodevelopmental disorder, which is characterized by pronounced difficulties in learning to read and spell that cannot be explained by low IQ or other impairments such as uncorrected visual or auditory problems, gross neurobiological deficits, or inadequate schooling. The two international disease classification systems, the International Statistical Classification of Diseases (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV™) list this disorder as a disorder of reading and spelling (ICD-10), and a disorder of reading and written expression (DSM-IV), respectively. Dyslexia is a very stable developmental disorder which affects approximately 5% of all children and adolescents [Shaywitz et al., 1990, 1999; Katusic et al., 2001].
Twin and family studies have shown that genetic factors play a substantial role in the etiology of dyslexia [Stephenson, 1907; Hallgren, 1950; Gayán and Olson, 2001; Olson, 2002; Plomin and Kovas, 2005; Fisher and Francks, 2006], and many studies have aimed to identify genomic loci contributing to the development of this complex disorder. Results from molecular linkage analyses in families with dyslexia have produced evidence for nine genomic loci which have been listed as DYX1-DYX9 by the Human Gene Nomenclature Committee [for review, Schumacher et al., 2007]. These regions are likely to harbor specific susceptibility genes, since most of them have been replicated in at least two different independent samples [Schumacher et al., 2007]. Six candidate genes have been identified to date by molecular studies, which have used either large samples (DCDC2 [Deffenbacher et al., 2004; Meng et al., 2005; Schumacher et al., 2006]; KIAA0319 [Deffenbacher et al., 2004; Francks et al., 2004; Cope et al., 2005; Harold et al., 2006]; MRPL19 and C2ORF3 [Anthoni et al., 2007]), or breakpoint mapping of chromosomal translocations in Finnish families (DYX1C1 [Taipale et al., 2003]); ROBO1 [Hannula-Jouppi et al., 2005]). Although no causative mutation has yet been identified for these genes, evidence from both functional and expression data are in support of the genetic findings [e.g., Anthoni et al., 2007; Schumacher et al., 2007]. Interestingly, a very recent article suggests a first potential functional SNP for dyslexia in KIAA0319 [Dennis et al., 2009].
Various phenotypic dimensions underlie reading and spelling ability, including phonological processing, orthographic decoding, speed of processing, and memory processes [Alloway et al., 2004; Fisher and Francks, 2006; Galaburda et al., 2006; Schulte-Körne et al., 2007a,b]. Although these dyslexia-related cognitive events are often highly correlated, a recent study has revealed evidence for distinctive components within the spectrum of reading and spelling related processes [Schulte-Körne et al., 2007b]. The dyslexia candidate genes known to date have been identified on the basis of both qualitative and quantitative measures, and have been found to be correlates of different dimensions of the cognitive phenotypic spectrum [Fisher et al., 2002; Schulte-Körne et al., 2007a; Schumacher et al., 2007].
The significance of working memory (WM) in learning to read was first proposed in 1978 in an influential study by Bradley and Bryant. This study applied an odd-one-out task, that is, the child had to differentiate between the initial or last phonemes of spoken words, keep the odd word in short-term memory and then retrieve this memorized word from storage. The phonological memory task enables a prediction of reading development to be made in children of nursery age [Bradley and Bryant, 1978]. The concept of phonological memory is part of a neurocognitive model suggested by Baddeley 2003, which differentiates three components of WM: (i) a control system (central executive) that is both, modality independent and influenced by attention, and which controls and regulates the two subsidiary storage systems, (ii) the phonological loop that temporarily stores auditory information, and (iii) the visuospatial sketchpad for the memorizing and processing of visual and spatial information. The central executive is also involved in the retrieval of information from long-term memory. Several studies have demonstrated that the WM for auditory information is impaired in subjects with dyslexia [Vellutino et al., 2004; Pickering, 2006; Swanson, 2006]. A typical task used to investigate verbal WM is a digit span task which requires a subject to temporarily store digits and then retrieve them in order, both forwards and backwards, from phonological memory. This memory deficit is considered to be a major cause of the impaired reading speed observed in dyslexia subjects.
In order to investigate the genetic contribution made to the specific cognitive processes involved in dyslexia, we examined a candidate gene for human cognitive processes, GRIN2B, which has previously been implicated in human memory performance [de Quervain and Papassotiropoulos, 2006]. In this study, the authors found variations in seven genes to be associated with inter-individual differences in human memory performance. It was also shown that a so-called memory-related genetic score, based on the number of memory-associated genetic variations present in one subject, is significantly associated with both human memory performance and memory-related brain activation. All of the seven genes involved in this cluster encode proteins implicated in signalling cascades. While five of these genes presented with only one SNP in this cluster, two genes, GRIN2B and GRM3, were found to contribute to human memory performance with a multi-locus haplotype. Furthermore, a recent genome scan in dyslexia families [Brkanac et al., 2008] provided suggestive but reproducible evidence for linkage of this cognitive process to a chromosomal region on 12p12-p13 using a phonological memory phenotype. Although no candidate genes were identified, this locus coincides well with the genomic position of GRIN2B, which is thus considered to be a candidate gene for the given linkage findings.
Apart from its hypothesized involvement in memory performance, GRIN2B has also been found to be associated with attention deficit/hyperactivity disorder (ADHD) in a Canadian sample [Dorval et al., 2007]. Nine SNPs in GRIN2B were examined in 205 nuclear families in which at least one child had been clinically diagnosed with ADHD. The authors found significantly biased transmissions for four SNPs located in intron 3. Analysis of memory as a quantitative trait, however, produced no significant findings for the markers tested. Approximately 25–40% of children with dyslexia also present with symptoms of ADHD [e.g., Pennington, 2006]. The involvement of common cognitive processes, such as speed of processing [Willcutt et al., 2005; Shanahan et al., 2006] and WM [Tiffin-Richards et al., 2008] has been suggested. The comorbidity observed between these two disorders has also been shown to be partly attributable to common genetic influences [Stevenson et al., 1993; Light et al., 1995; Chadwick et al., 1999], and we therefore considered GRIN2B to be an interesting candidate gene for certain aspects of the cognitive processes involved in both of these complex disorders.
GRIN2B codes for a subunit of the N-methyl-D-aspartate (NMDA) receptor and is expressed at high levels in the frontal cortex as well as in the hippocampal pyramidal cells of the human brain [Schito et al., 1997]. It has also been shown to influence synaptic plasticity [Kutsuwada et al., 1996], and an over-expression of GRIN2B in the forebrain of mice results in an increased activation of NMDA receptors, with mice displaying a superior performance in learning and memory tasks [Tang et al., 1999]. In summary, evidence from both functional and genetic studies points towards the involvement of GRIN2B in several aspects of human cognition.
Given that children with dyslexia perform significantly poorly in auditory WM tasks, we hypothesized that GRIN2B might be a candidate gene for short-term memory as a cognitive correlate of dyslexia. We examined polymorphisms within GRIN2B in a large German sample that was comprised of 397 dyslexia patients and their parents, and analyzed the data with respect to general biased transmission. There is increasing evidence that the parental origin of genetic variation has an impact on human phenotypes as well as allelic states. A recent study of schizophrenia patients has identified the first imprinted gene, LRRTM1, to contribute to human brain asymmetry when inherited from the father [Francks et al., 2007]. On the basis of the findings of this study, it can be assumed that the mechanism of imprinting might also contribute to cognitive phenotypes, and our data was therefore also analyzed for parent-of-origin effects.
SUBJECTS AND METHODS
Subjects
All families participating in this study were of German descent and were recruited at the Departments of Child and Adolescent Psychiatry and Psychotherapy at the Universities of Marburg and Würzburg in Germany. Potential probands were referred to the clinics by parents, teachers, special educators, or health professionals on the basis of a prior diagnosis of dyslexia or observed difficulties in learning to read and spell. Written informed consent was obtained from all parents participating in the present study as well as from their child in cases where the proband was aged >12 years. The study was approved by the ethics committee of each clinical center.
Since German clinical studies of dyslexia have tended to use spelling disorder as an inclusion criterion, and since our previous findings were also based on this selection criterion [Schulte-Körne et al., 1996, 1998, 2001; Ziegler et al., 2005; Schumacher et al., 2006], spelling ability was used as the criterion for inclusion in the present study. The recruitment strategy has been described previously [Schulte-Körne et al., 2007b]. To summarize, spelling was measured using an age-appropriate spelling-test (writing to dictation), and an observed spelling score was calculated on the basis of an assumed correlation of 0.4 between the proband's IQ and spelling [Schulte-Körne et al., 2001]. Children were classified as being “affected” when there was a discrepancy of ≥1 SD between the observed spelling score and that expected from the IQ. Families were excluded from the study if the proband or a sibling had symptoms of ADHD as ascertained during a standardized clinical interview with the mother [Unnewehr et al., 1998]. Families were also excluded in cases where the proband had a bilingual education, an IQ < 85, an uncorrected disorder of peripheral hearing or vision, a psychiatric or neurological disorder with a possible impact on the development of reading and spelling ability, or an age greater than 21 years. Our study sample consisted of 397 probands, 288 of whom were boys and 109 were girls, aged between 8 and 19 (mean = 11.99, SD = 2.30) who were affected with dyslexia according to the criteria described above, and their parents.
Phenotypic Measures
Following the initial diagnosis of dyslexia (based on the spelling discrepancy score) and inclusion into the study, probands were assessed regarding their performance in different cognitive aspects of the dyslexia phenotype. A battery of psychometric tests targeted word reading fluency, phoneme awareness, phonological decoding, rapid naming, verbal short-term memory, and orthographic coding, as previously described [Schulte-Körne et al., 2007b]. In the present study, we focused on analyzing the quantitative trait “short-term memory.” To measure short-term memory, a standardized digit span test ([Tewes, 1983]—German adaptation of the WISC-R-test) was used. Probands were read increasingly long series of numbers, which had to be repeated both forwards and backwards. While the forward digit span reflects pure storage and recall in short-term memory, the backwards digit span requires the processing of information and thus represents additional aspects of WM. The distribution of the phenotypic measure “short-term memory” in the present sample was as follows: mean = 7.2, SD = 2.5.
SNP Selection and Genotyping
GRIN2B is located in reverse orientation on chromosome 12p13.1, where it spans around 470 kb. Given its large genomic size and complex LD-structure, complete tagging of the region exceeded the budgetary constraints of the study. We therefore used an intermarker distance of 20 kb to evenly cover the region of interest, including 100 kb up- and 50 kb downstream of the gene. Since both original candidate gene studies [de Quervain and Papassotiropoulos, 2006; Dorval et al., 2007] presented overlapping findings within introns 2 and 3 of GRIN2B, the density of SNPs in those regions was increased. Also included in the assay design system were the four significantly associated SNPs reported by Dorval et al. 2007 and the six SNPs forming the multi-marker haplotype reported by de Quervain and Papassotiropoulos 2006. This was successful for all SNPs except rs1805474. SNPs were selected from publicly available databases (HapMap21a, dbSNP125), and sequences were retrieved from CHIP Bioinformatics Tools (http://snpper.chip.org/). SNPs required a minor allele frequency of at least 5% in order to be considered as a potential SNP for genotyping (for the complete list of SNPs, see Supplementary Table I). A total of 66 SNPs were included in the assay.
Genotyping was performed on genomic DNA that had been extracted from blood according to standard procedures. We used the Sequenom MALDI-TOF mass-spectrometer (MassArray® system) for genotyping of the samples, and we analyzed the data using the Spectrodesigner Software package (Sequenom™, San Diego, CA). Primers were synthesized at Metabion, Germany. All primer sequences are available upon request. Only SNPs forming three distinct clusters in the Sequenom™ Typer Analysis software were included in the analysis. In ambiguous cases, representative individuals for the groups were sequenced. Duplicate testing of known DNAs was also performed, and no discrepancies were found for duplicates, that is, on one plate or interplate duplicates.
Statistical Analysis
Only SNPs presenting with a callrate of at least 95% were included in the statistical analysis. Five SNPs did not meet this criterion and were excluded from further analysis. For the remaining 61 SNPs, P values for deviation from HWE were not lower than 8.2 × 10−4 (=0.05/61), and thus no further SNPs were excluded.
SNPs that passed the above QC criteria were checked for Mendelian inheritance errors using Pedstats 0.6.3 (http://www.sph.umich.edu/csg/abecasis/PedStats/). Inconsistent genotypes were zeroed and were not taken forward for further analysis. Transmission disequilibrium tests for the disease phenotype were performed with UNPHASED 3.0.12 (http://www.mrc-bsu.cam.ac.uk/personal/frank/software/unphased/). We checked for parent-of-origin effects in qualitative phenotypes using TDTPHASE 2.404. To test for transmission disequilibrium and imprinting in quantitative traits, we used QTDT 2.6.0, with the default orthogonal model. We performed 10,000 permutations. To test for imprinting, we applied the options -om and -op when taking into account maternal only and paternal only derived alleles, respectively. The option -ot was used to test for differences between maternally and paternally transmitted alleles.
Haplotype analysis was performed with FBAT (http://biosun1.harvard.edu/∼fbat/fbat.htm). Power calculations were performed using the Genetic Power Calculator (http://pngu.mgh.harvard.edu/∼purcell/gpc/) with the option QTL Association for sibships.
The LD structure of GRIN2B was analyzed using the parental genotypes only. The genotypic information from the parents was extracted and plotted in Haploview 4.0. The LD structure is depicted graphically using the GOLD colour scheme, with colours representing D' and values representing r2.
RESULTS
Single Marker Analysis
Five SNPs did not pass our QC measures and were excluded from the statistical analysis (see Supplementary Table I). The remaining 61 SNPs were tested for association by performing transmission disequilibrium tests with dyslexia as a qualitative trait. Only one SNP showed a nominally significant P value (rs933614, P = 0.013, data not shown), which is concordant with the number of false-positives expected when using this study design. No improvement in the results was obtained by stratifying for severity or using haplotype analysis.
Analysis of the quantitative phenotype “short-term memory” in our dyslexia individuals using QTDT yielded no more than four significant association signals (rs1012586, rs2268119, rs2216128, rs2192973; Supplementary Table I). The lowest nominal P value was P = 0.0243 for rs2268119. None of the signals withstands correction for multiple testing. All four SNPs are located in intron 3 of GRIN2B, and three of them are situated in different haplotype blocks, indicating that the association signals we found represent distinct effects (Fig. 2).
We subsequently stratified our sample for severity, and analyzed the four nominally significant markers in differently affected groups. Although group size decreased with higher affection status, P values remained significant or borderline significant (Table I). In the most severe group, SD ≥ 2.5, statistical analysis for three of the four markers was not possible because the sample size provided an insufficient number of informative events. However, rs1012586 also showed a significant effect in this group (Pnom = 0.0106).
| All (n = 397) | SD ≥ 1.5 (n = 365) | SD ≥ 2.0 (n = 249) | ||||
|---|---|---|---|---|---|---|
| P-value | Effect size | P-value | Effect size | P-value | Effect size | |
| rs1012586 | 0.0401 | 0.0058 | 0.0411 | 0.0068 | 0.0587 | 0.0171 |
| rs2268119 | 0.0243 | 0.0064 | 0.0289 | 0.0073 | 0.1879 | 0.0068 |
| rs2216128 | 0.0406 | 0.0064 | 0.0158 | 0.0142 | 0.0536 | 0.0253 |
| rs2192973 | 0.0381 | 0.0080 | 0.0150 | 0.0156 | 0.0683 | 0.0205 |
- P values are given for the QTDT analysis (without imprinting) for short-term memory performance. Data are presented for nominally significant SNPs over different severity groups.
- P values are bold if ≤0.05. The most severe group (SD ≥ 2.5, n = 116) has too low number of informative individuals.
- Effect sizes are given for each of the SNPs for each of the severity groups, according to the R2 goodness of fit measure.
We calculated effect sizes for the four significant association signals across different severity groups. As shown in Table I, effect sizes range from about 1% in the overall group (n = 397) to about 2% in the more severely affected group (SD ≥ 2.0, n = 249).
Parent-of-Origin Effects
The four SNPs showing nominally significant P values in the single-marker analysis were analyzed for a possible biased parental transmission. In the overall group we observed significant effects for all four SNPs, each of them producing P values ≤0.01 when maternal transmissions only were analyzed (Pmat, Table II). For the most significant SNP rs1012586, this P value withstands correction for multiple testing (Pcorr = 0.0036). The difference between maternal and paternal transmission (Ptest) was statistically significant for all four SNPs, with P values ≤0.03.
| All (n = 397) | SD ≥ 1.5 (n = 365) | SD ≥ 2.0 (n = 249) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mata | Patb | Testc | Mata | Patb | Testc | Mata | Patb | Testc | |
| rs1012586 | 0.0001d | 0.5518 | 0.0006d | 0.00006d | 0.5062 | 0.003 | 0.0001d | 0.5586 | 0.0004d |
| rs2268119 | 0.0026 | 0.7532 | 0.0136 | 0.0060 | 0.9747 | 0.0417 | 0.0062 | 0.6833 | 0.0131 |
| rs2216128 | 0.0089 | 0.6438 | 0.0289 | 0.0037 | 0.7624 | 0.0256 | 0.0246 | 0.9828 | 0.1212 |
| rs2192973 | 0.0086 | 0.3850 | 0.0143 | 0.0039 | 0.4879 | 0.0144 | 0.0343 | 0.5745 | 0.0491 |
- Parent-of-origin effects are given across different severity groups for SNPs showing nominally significant P values in the QTDT analysis for short-term memory performance. Data are given in form of P values for maternal transmissions, paternal transmissions, and the test for differences between maternal and paternal transmissions.
- Most severe group (SD ≥ 2.5, n = 116) has a too low number of informative individuals.
- a Maternal transmissions only.
- b Paternal transmissions only.
- c Test if maternal and paternal transmissions are significantly different. Bold if P ≤ 0.05.
- d Withstands correction for multiple testing (four SNPs, nine statistics): lowest corrected value P = 0.0022.
When the sample was stratified for severity, similar results were found for all SNPs across all groups of severity, with again rs1012586 remaining significant after correction for the number of comparisons (SD ≥ 1.5, Pcorr = 0.0022; SD ≥ 2.0, Pcorr = 0.0036). The most severe group again lacked sufficient informative events to allow statistical analysis for three of the four SNPs. However, rs1012586 also showed significant maternal transmission in this group, with Pmat = 0.0013 (Ptest = 0.011).
Genotype–Phenotype Correlation
In cases where the parental genotypes at the respective SNP were informative, the phenotypes of individuals for whom a certain allele had been maternally transmitted and individuals for whom it had not were compared. For the most significant SNP, rs1012586, 113 families were included, and the G-allele was observed to be maternally transmitted in 60 individuals, and not transmitted in 53 individuals. The results for short-term memory were compared between these two groups. As shown in Figure 1, individuals with a maternally inherited G-allele presented with a significantly better result in short-term memory compared to the other group (difference in the mean for short-term memory = 0.7 SD). Similar results were obtained for the other three SNPs (Supplementary Fig. 1).
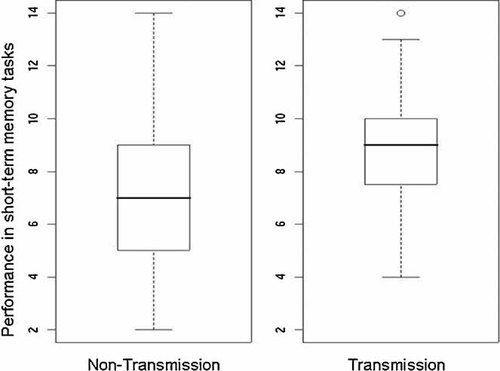
Boxplots for short-term memory performance with respect to rs1012586. For rs1012586, the performance in short-term memory tasks is shown for individuals carrying a maternally transmitted G-allele (right) versus those in whom the G-allele has not been transmitted maternally (left). For each group, the median (middle black line) and the quantiles (75%, top line; 25%, bottom line) are represented by the rectangle. The dashed vertical lines illustrate the distribution (minimum–maximum) of the performance scores. Outliers, showing more than 1.5-fold inter-quartile distance, are represented by a circle. The difference between the groups is statistically significant, with P = 0.0001.
Power Analysis
For power calculations we used the settings: singletons, “both parents” genotyped, no dominance, and a minor allele frequency of 0.35. We assumed a total QTL variance of 0.01 according to the calculated R2. For α set at 5%, this yielded a power of 29.3%.
DISCUSSION
The ability to read and spell, which is unique to humans, involves a complex and well-coordinated network of cognitive processes that must interact in a fast and efficient manner. It is widely accepted that deficits in specific cognitive functions contribute to the development of dyslexia, although the exact nature of these processes and, more particularly, the extent to which they are connected, remains elusive. Quantitative measures of different cognitive aspects are applied in research in order to dissect the complex dyslexia phenotype with respect to the genetic contribution [Marlow et al., 2001; Paracchini et al., 2007; Schulte-Körne et al., 2007b].
Several studies have shown that individuals with dyslexia have marked weaknesses in phonological WM [Baddeley and Wilson, 1993; Palmer, 2000; Swanson, 2006], and perform poorly in memory-related tasks as compared to age-matched controls. This quantitative measure is stable even in adults, and is thus considered to be one cognitive correlate within the complex dyslexia phenotype [Hulslander et al., 2004]. Although a genetic contribution to the quantitative dimensions of the dyslexia cognitive spectrum is widely assumed, no candidate gene for verbal short-term memory within the dyslexia phenotype has so far been proposed. However, recent findings suggest that loci on chromosome 15 [Berninger et al., 2008] and 12 [Brkanac et al., 2008] might be associated with phonological memory in dyslexia.
In 2006, it was shown that GRIN2B makes a general contribution to human memory performance [de Quervain and Papassotiropoulos, 2006], and a recent genome scan in dyslexia families has provided evidence that the genomic locus 12p12-p13 is likely to harbor a dyslexia candidate gene for phonological memory performance [Brkanac et al., 2008]. Given these findings, we considered GRIN2B to be a candidate gene for memory performance, and analyzed the genetic variation within this gene in a large sample of German dyslexia families. We found no evidence for associations between markers in GRIN2B and the categorical diagnosis of spelling disorder. As shown by Schito et al. 1997, GRIN2B presents with a distinct expression pattern in the human brain, with high levels being found in the frontal cortex as well as in hippocampal pyramidal cells. This suggests that this subunit of the NMDA receptor is more likely to be involved in specific cognitive processes than in compound and widespread events. When analyzing the quantitative component “short-term memory” in our entire sample, we obtained nominally significant results, which did not exceed the number of false-positives expected from this study design and the number of markers used. Following-up these signals over different severity groups confirmed a possible true contribution of GRIN2B to memory performance. Further analysis of the data was performed in order to reveal the genetic mechanism behind the observed association.
It is widely accepted that the phenotypic representation of genetic information is dependent not only on the allelic state of the genomic sequence, but also upon the sex of the parent from whom the allele is inherited. There is increasing evidence that parent-of-origin effects play a role in the human brain, behavior, and cognition [Cattanach and Kirk, 1985; Skuse et al., 1997; Isles and Wilkinson, 2000], and an imprinted gene, LRRTM1, has recently been implicated in human brain asymmetry and schizophrenia [Francks et al., 2007; Ludwig et al., in press]. To date, scant effort has been expended in the analysis of parent-of-origin effects for dyslexia candidate genes or regions. In contrast, some evidence for the preferential transmission of paternal alleles for risk genes in ADHD has been reported [Hawi et al., 2005], although these results were not replicated in an independent study [Laurin et al., 2007]. Given the general impact of imprinting mechanisms in human cognition and the high rate of comorbidity between ADHD and dyslexia, we followed up our positive signals of the single marker analysis with respect to the parental origin of the transmitted alleles. Each of the four SNPs, all of which are located in intron 3 of GRIN2B, showed statistically significant biased transmission for short-term memory when maternal transmission only was considered. Probands with alleles that had been maternally transmitted at these four SNPs displayed a significantly better performance in the respective tasks. Our results thus suggest a possible parent-of-origin effect for GRIN2B for specific cognitive processes within dyslexia families.
The design of the present study had been based mainly on previous findings implicating GRIN2B as a candidate gene for human memory performance [de Quervain and Papassotiropoulos, 2006]. We included five of the six markers which had been analyzed in that study, the sixth SNP having failed assay design. Although none of the specific markers found to be associated with memory by de Quervain and Papassotiropoulos showed significant results in the present study, the marker sets lie in close proximity to one another. As presented in Figure 2, the four markers which showed significantly biased transmission in our dyslexia sample are spread over several haplotype blocks within intron 3 and do not show high LD values with one another. It is difficult to correlate our findings on a single marker level to those found in the original study, since the results of de Quervain and Papassotiropoulos are based on individual genetic scores that were calculated for variations within multi-marker gene clusters.
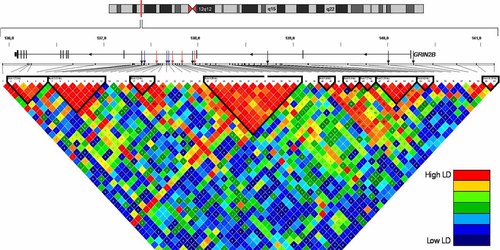
LD structure of the genomic locus of GRIN2B on chromosome 12p12. The upper part shows the genomic location of GRIN2B, including 100 kb up- and 50 kb downstream. The lower part shows the LD block structure based on 61 SNPs and the parental chromosomes. The plot is displayed by GOLD heatmap using Haploview software, with colors being based on D' values. LD blocks have been assigned using the algorithm of Gabriel et al. 2002. Red arrows indicate the SNPs that were significant in our study, while blue [Dorval et al.] and black [de Quervain and Papassotiropoulos] arrows represent significant markers from the two previous studies. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Additional support for the hypothesis that GRIN2B is involved in auditory memory performance in dyslexia has been obtained from a recent genome-scan in dyslexia families by Brkanac et al. 2008. Linkage analyses revealed suggestive, but replicated, evidence that a locus on chr.12p12-p13 contributes to phonological memory as measured by non-word repetition, with the most significant markers D12S269/AAC040Z being located only 200/500 kb away from GRIN2B. The data from that study were not analyzed with respect to parent-of-origin effects which thus precludes a direct comparison with our results. Although the functional data available so far for GRIN2B support a true effect for this gene in human cognition, it is interesting to note that, according to the UCSC genome browser (Human, March 2006, http://genome.ucsc.edu), the genomic region of GRIN2B contains several expressed sequence tags (ESTs) that do not belong to known exons. Two of them, CD514667 and AL133734, are located in the region between rs1012586 and rs2192973, and have been detected in white matter and the amygdala, respectively. It is therefore possible that the causal variant of the present association might be attributable to as yet unknown expressed sequences within the genomic region of GRIN2B.
It has already been shown that variation within introns 2 and 3 of GRIN2B contributes to ADHD susceptibility in a Canadian sample [Dorval et al., 2007]. Significant effects were demonstrated for the subdimensions “inattention” and “hyperactivity/impulsivity.” No significant P values were observed for short-term memory even though that study had applied exactly the same measures as were used in the present study (digit span forwards/backwards). These results suggest that the effect of GRIN2B on memory performance is more prominent in, if not exclusive to, children with dyslexia. An alternative hypothesis may be that the Dorval study failed to find an association with memory performance in their sample because they did not include any of the four markers found to be significantly associated in our study. It would be interesting to know whether the four significant markers from our study also show association with memory performance in the ADHD sample. It is interesting, however, that none of the significant markers from the Dorval study showed evidence for association with dyslexia in our study. One explanation might be that different variations within GRIN2B contribute to distinct cognitive processes, depending on which of these two complex disorders is considered.
Our results indicate that variation within intron 3 of GRIN2B may contribute to verbal short-term memory processes in children with dyslexia, and may partly explain the comorbidity observed between ADHD and dyslexia. It may be assumed that the variations presented here have their impact on a phenotypic level through the regulation of the expression of GRIN2B, or either of the two ESTs found within its sequence, although the true causative variant remains elusive. Given the sample size and the small effect sizes of our study, it is also possible that our results reflect a false-positive finding [Sullivan, 2007], and replication in independent samples is therefore warranted.
Acknowledgements
We thank Christine Schmael for helpful comments which greatly improved the manuscript. GSK, AW, HR, BMM, and MMN were supported by the Deutsche Forschungsgemeinschaft. MMN received support for this work from the Alfried Krupp von Bohlen und Halbach-Stiftung.




