Stathmin, a gene regulating neural plasticity, affects fear and anxiety processing in humans†‡
Burkhard Brocke and Klaus-Peter Lesch contributed equally to this work.
How to Cite this Article: Brocke B, Lesch K-P, Armbruster D, Moser DA, Müller A, Strobel A, Kirschbaum C. 2010. Stathmin, a Gene Regulating Neural Plasticity, Affects Fear and Anxiety Processing in Humans. Am J Med Genet Part B 153B:243–251.
Abstract
The identification of biological mechanisms underlying emotional behavior is crucial for our understanding of the pathogenesis of mental disorders. Besides genes modulating neural transmission and influencing amygdala reactivity and anxiety-related temperamental traits a different plasticity regulating genes affect interindividual differences in emotional regulation. Recently it has been demonstrated that stathmin, a regulator of microtubule formation which affects long-term potentiation (LTP), controls learned and innate fear responses in rodents, but its role in human emotion regulation is unknown. We hypothesized that in humans the gene coding for stathmin (STMN1), which is highly expressed in the lateral nucleus of the amygdala and associated thalamic and cortical structures, influences behavioral responses to fear and anxiety stimuli by way of two common single nucleotide polymorphisms (rs182455, SNP1; rs213641, SNP2). These polymorphisms are located within or close to the putative transcriptional control region. We used the acoustic startle paradigm and a standardized laboratory protocol for the induction of fear and psychosocial stress in 106 healthy volunteers to investigate the impact of stathmin gene variation on two fear- and anxiety-controlling effector-systems of the amygdala. We found that STMN1 genotype interacting with individuals' gender significantly impacts fear and anxiety responses as measured with the startle and cortisol stress response. We therefore conclude that STMN1 genotype has functional relevance for the acquisition and expression of basic fear and anxiety responses also in humans. © 2009 Wiley-Liss, Inc.
INTRODUCTION
The identification of neurogenetic mechanisms underlying emotional dysregulation is crucial for our understanding of the pathogenesis and treatment of affective and anxiety disorders [Nemeroff and Owens, 2002]. While some of the neural circuitries involved in emotional processing are well understood, little is known about the underlying molecular mechanisms.
With regard to neural circuitries, there is converging evidence that thalamo-cortico-amygdala circuitries are critically involved in the processing of fear stimuli [LeDoux, 2000a]. The amygdala has a key role in this circuitry because the pathways processing innate (unconditioned) and learned (conditioned) fear stimuli converge in the lateral nucleus of the amygdala (LA). The LA projects to the central nucleus of the amygdala (CE), the outputs of which control the expression of different fear responses, including endocrine responses (projections to the hypothalamus), and the startle response (projections to the nucleus reticularis pontis caudalis, PnC) [Davis, 2000; LeDoux, 2000a; Phelps and LeDoux, 2005].
With regard to underlying molecular mechanisms, the main research focus so far has been on the role of genetic variation in neural transmission [Hariri et al., 2002; Heinz et al., 2005; Pezawas et al., 2005; Canli et al., 2005a,b, 2006]. A different type of molecular mechanisms involved in cognitive-affective processing is targeted by genes regulating cellular microstructures underlying neural plasticity. Recently, stathmin, a regulator of microtubule formation which is highly expressed in the LA and in associated thalamic and cortical structures [Peschanski et al., 1993; Shumyatsky et al., 2002], has been identified to be crucially involved in fear processing in mice [Shumyatsky et al., 2005]. Shumyatsky et al. 2005 demonstrated that stathmin knockout mice exhibited deficits in spike-timing-dependent long-term potentiation (LTP) as determined by whole-cell recordings from amygdala slices. Furthermore, the knockout mice, exhibiting normal neuronal morphology, also showed decreased memory in amygdala-dependent fear conditioning and recognized less danger in innately aversive environments.
This suggests that differential stathmin expression might impact fear- and anxiety-related behavior also in humans. We focused on two presumably functional single nucleotide polymorphisms (SNPs) of the human stathmin gene (STMN1), located within or close to the putative transcriptional control region, rs182455 (SNP1) and rs213641 (SNP2). Unlike other STMN1 polymorphisms out of currently about forty documented SNPS, these SNPs have relatively high minor allele frequencies and are thus likely to contribute to interindividual differences in neural plasticity, amygdala reactivity, acquisition and expression of fear behavior, and ultimately anxiety. In addition SNP1 is probably a constituent of the upstream regulatory region of STMN1, whereas SNP2 is located in the 5′ UTR of the alternatively transcribed exon 1c. While the actual functional relevance of STMN1 variants has not yet been tested on the level of gene expression and protein function, polymorphisms in regulatory regions frequently affect gene expression. For example several polymorphisms in the promoter region of the gene encoding for the serotonin transporter have demonstrated to impact human brain affective processing [Hariri and Holmes, 2006]. Finally, each of the STMN1 variations alters the recognition motif and thus the potential binding of a transcription factor: SNP1 is next to a consensus sequence of a glucocorticoid responsive element and SNP2 may modify the binding of nerve growth factor- (NGF-) induced protein C (NGFIC/EGR4, http://www.genomatix.de). Both steroidal hormones and NGF have been shown to modulate STMN1 expression and protein function [Takekoshi et al., 1998; Giachino et al., 2004].
To examine the role of STMN1 variation in amygdala-relayed emotion regulation in humans, we tested the assumption that STMN1 variation impacts on the neural circuitries involved in the processing of innate and learned fear stimuli resulting in individual differences in behavioral measures of fear and anxiety. We focused on two fear- and anxiety-controlling effector systems which are impacted by LA–CE connections: projections to the PnC mediating the startle reflex and projections to the hypothalamus mediating the activity of the hypothalamic-pituitary-adrenal (HPA) stress axis and cortisol response.
To investigate influences of STMN1 variation on the startle response, we used an emotionally modulated acoustic startle paradigm, which allows the measurement of both innate and learned fear responses [Davis and Whalen, 2001]. In this paradigm, sudden high-intensity noise bursts evoke a startle response which can be measured by electromyographic recordings from the orbicularis oculi muscle. The primary acoustic startle response (ASR) is relayed via the PnC to spinal motoneurons, which elicit the ASR. Because the startling stimuli are aversive and able to induce states of fear or anxiety [Leaton and Cranney, 1990], the ASR can be regarded as a measure of innate fear. Furthermore, the ASR can be potentiated by presenting the startle stimulus in the presence of a cue which has previously been paired with an unconditioned fear stimulus (fear-potentiated startle, FPS), a process which is crucially modulated by the amygdala and more specifically, by CE activity [Davis et al., 1993; LeDoux, 2000b]. In addition, the startle response is inhibited in the presence of pleasant stimuli (pleasure-attenuated startle, PAS). Hence, FPS is a valid measure of amygdala-modulated conditioned fear responses.
To test influences of STMN1 variation on the second effector system of the amygdala, the CE-controlled activity of the HPA-system, we used the Trier Social Stress Test [TSST; Kirschbaum et al., 1993], a standardized laboratory protocol for the induction of psychosocial stress in humans. The two key TSST components for activation of the HPA axis are threat to the social self of the exposed individual along with a high degree of uncontrollability. This activation of the HPA axis reliably leads to an increase in cortisol level [Dickerson and Kemeny, 2004].
Based on the involvement of stathmin in LTP induction, we hypothesized that STMN1 variation primarily impacts on the expression of learned fear and anxiety behavior (i.e., FPS in the startle paradigm, cortisol response in the TSST). For the unmodulated ASR as an expression of innate fear, there were no hypotheses suggestive because innate fear needs no previous experience. However, reduced microtubule dynamics as a possible result of inefficient stathmin expression might also reduce excitatory postsynaptic potentials involved in the transmission of unconditioned stimuli and thus lead to an impaired signal-to-noise ratio of the transmission process [Bauer and LeDoux, 2004; Rodrigues et al., 2004]. Therefore, we assumed that STMN1 variation is also associated with the unmodulated ASR.
When investigating the impact of genetic polymorphisms on complex outcomes like emotional regulation it is reasonable to assume that other factors might also play a role, either as independent parameters or interacting with genetic variations. Probably one of the most influential factors is sex since functional and structural differences in the brain as well as neurochemical sexual dimorphisms have been reported between males and females [Cahill, 2006]. Therefore, additionally, we expected a modulating sex effect.
METHODS
Subjects
Participants included 59 female and 59 male students of the University of Dresden. Of these, 113 participants were successfully genotyped for STMN1 SNP1, while STMN1 SNP2 was genotyped in 110 participants. From the remaining participants, seven participants had to be excluded during data preprocessing due to excessive EMG artifacts or because of virtually no startle responses, leaving 54 female and 52 male adults for the final STMN1 SNP1 sample and 53 female and 52 male adults for the final STMN1 SNP2 samples (mean age for both samples 23.8 years, SD = 2.6, range 19–31 years). All participants underwent a semistructured screening for psychiatric or neurological disorders or treatment. All participants were non-smokers and none of the female participants used hormonal contraceptives. Since in premenopausal women, salivary cortisol responses differ in the luteal and the follicular phase [Kirschbaum et al., 1999], women's cycles were controlled. Participants were informed about the aims of the study, consented in the procedure and were paid 40 Euros. The study design was approved by the Ethics Committee of the German Psychological Association.
Materials and Design
In the startle paradigm used here, acoustic startle probes were delivered alone and during viewing of emotional pictures. To elicit a startle response, a single 50 msec burst of white noise (95 dB SPL with an instantaneous rise time) was presented binaurally over Eartone A3 Audiometric Insert Earphones (Aearo Company, Indianapolis, IN). Pictorial stimuli consisted of 48 affective pictures. Forty color pictures, consisting of 16 unpleasant, 12 neutral and 12 pleasant scenes, were selected from the International Affective Picture System (IAPS) [Lang et al., 1999] on the basis of their affective valence and arousal ratings by the normative sample. Eight additional unpleasant black and white pictures displaying angry or fearful faces were chosen from a standard set of pictures of facial affect [Ekman and Friesen, 1976]. The picture series comprised 12 different semantic contents: 3 pleasant, 3 neutral, and 6 unpleasant. The pleasant pictures included attractive men, attractive women, and erotic couples whereas the unpleasant set included attacking humans, vicious animals, mutilated bodies, contamination, angry, and fearful faces. The neutral set included pictures of buildings, landscapes, and kitchen objects. Each of the 12 contents included 4 different exemplars. Digitized versions of the pictures were displayed on a 17-inch computer screen. Each picture was presented for 6 s and the pictures were grouped in four blocks of 12 pictures. Each block consisted of 3 pleasant, 3 neutral, and 6 unpleasant content pictures. During picture viewing, an acoustic startle probe was administered at 0.5, 2.5, or 4.5 sec after picture onset on 9 of these 12 trials. The timing of the startle probes was balanced across content categories. One picture in each of the 12 content categories was presented without a startle probe and used as filler stimuli and was not included in the startle data evaluation. Pictures were organized such that not more than three pictures of the same affective valence and not more than three pictures with the same startle onset time could occur consecutively. Otherwise, stimulus order was pseudo-randomized. Finally, in each block, three acoustic startle probes were delivered in the intertrial interval (ITI) to measure the baseline startle response and to further decrease the predictability of the startle stimulus.
Affective Rating
Evaluative judgments of pleasure and arousal were measured using the Self-Assessment Manikin (SAM) [Lang, 1980]. The SAM valence scale shows a graphic figure with expressions ranging from happy to unhappy, and the SAM arousal scale displays a graphical representation of a figure with expressions ranging from calm and relaxed to excited. Ratings of valence and arousal were made on nine-point-scales.
Physiological Data Collection and Reduction
The eyeblink component of the startle response was measured by recording EMG activity over the orbicularis oculi muscle beneath the left eye, using two Ag–AgCl electrodes with 4 mm inner diameter. A ground electrode was attached to the left mastoid. Impedance level was kept below 10 kΩ. The raw EMG signal was amplified by a SynAmps amplifier (NeuroScan Inc., El Paso, TX), sampled at 1,000 Hz, filtered (30–200 Hz band pass), rectified and integrated. Responses to startle probes were defined as EMG peak in a time window from 20 to 140 msec after probe presentation. Trials with excessive EMG artifacts were excluded.
TSST Psychosocial Stress Protocol
The Trier social stress test (TSST) was employed for the induction of psychosocial stress. This standardized laboratory stressor consists of a free speech and a mental arithmetic task in front of an audience and has been shown to result in significant endocrine, cardiovascular, immune, and subjective responses [Kudielka et al., 2007]. Including an introduction and a preparation phase, the total procedure takes approximately 15 min. The TSST has been found to elicit the strongest and most reliable cortisol responses to laboratory stress compared with other protocols [Kirschbaum et al., 1993].
Cortisol Analysis
Salivary cortisol samples were obtained using “Salivettes” (Sarstedt; Rommelsdorf, Germany) and were kept at −20°C until analysis. Samples were collected repeatedly immediately before onset of the stress sessions as well as 2, 10, 20, and 30 min after cessation of stress. Salivary cortisol samples were prepared for biochemical analysis by centrifuging at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary free cortisol concentrations were determined employing a chemi-luminescence-assay (CLIA) with high sensitivity of 0.16 ng/ml (IBL; Hamburg, Germany). Intra and interassay coefficients of variation were below 8%.
Procedure
After a telephone interview regarding basic inclusion criteria (e.g., age, health, or medication) participants were scheduled for two laboratory sessions on separate days. On the first session, the participant was informed about the study goals and protocol, and after giving written informed consent, carbon monoxide (CO) was measured in the participants breath with a Breath CO Monitor (Micro-Smokerlyzer; Bedfont Scientific Ltd., Rochester, Kent, England) to confirm that the participant was a non-smoker. Subsequently, participants reclined in a comfortable chair and the EMG electrodes were attached. The participant was instructed that a series of affective pictures would be presented and that each picture should be viewed for the entire time it was on the screen. In addition, the participant was told that occasional noises heard over the earphones could be ignored. Then the series of 48 pictures was presented for 6 sec each. Between each picture, a fixation cross was displayed for a randomly generated variable interval, ranging from 11 to 24 sec, in order to clear any emotion associated with the previous image. After the picture series was finished, the sensors were removed and participants were familiarized with the SAM rating procedure. All pictures were presented in the same order a second time. Participants were told to view each picture as long as they needed to evaluate emotional valence and arousal, and then to press the button to turn off the picture and turn on the ratings. After picture offset, participants rated their subjective experience along the dimensions of valence and arousal, using the computerized version of the Self-Assessment Manikin rating method [Lang, 1980].
A second session was scheduled on a separate day for the induction of psychosocial stress by the TSST. Since the circadian variation in cortisol levels is relatively small in the late afternoon, all TSST sessions started between 15:00 and 17:00 hr. After arrival, a basal sample of whole saliva was taken (by chewing on cotton rolls, salivettes). Following a rest period of 30 min, participants were introduced to the TSST (2 min). They then prepared their speech (3 min) and completed a short questionnaire (2 min). Afterwards, psychosocial stress was induced by exposing the participants to the Trier Social Stress Test (TSST), consisting of a 5 min free speech in a simulated job interview, and another 5 min of a mental arithmetic task in front of an evaluative panel of two individuals. Further saliva samples were taken immediately before and 2, 10, 20, and 30 min after the stress paradigm. A final saliva sample was obtained for later DNA extraction using ORAgene self-collection kits (DNA Genotek, Ottawa, Ontario, Canada). Participants were subsequently debriefed, paid for participation and thanked.
Genotyping
For genotyping, DNA was isolated from saliva using the Oragene DNA Extraction kits and protocol. SNP1 (rs182455) is in STMN1's putative regulatory region at position −1,615 bp upstream of the transcription start site of STMN1's exon 1a (http://thr.cit.nih.gov/molbio/proscan/), SNP2 (rs213641) is in the alternatively transcribed 5′ UTR of STMN1 designated exon 1c (+289 bp). The distance between SNP1 and SNP2 is 2,627 bp, with nearly perfect linkage disequilibrium. Genotyping was performed using the ABI PRISM SnaPshot chemistry (PE Applied Biosystems, Foster City, CA), a primer-extension methodology based upon allele-specific nucleotide incorporation. Primers for PCR and extension oligos were designed by FastPCR (http://www.biocenter.helsinki.fi/bi/Programs/fastpcr.htm) and Autodimer (http://www.cstl.nist.gov/biotech/strbase/AutoDimerHomepage/AutoDimerProgramHomepage.htm), respectively. Detailed information on primers and reagents as well as PCR and extension reactions is available upon request.
For statistical testing, T allele carriers of the STMN1 SNP1 (T/C and T/T genotypes = T+ group; N = 55, 26 male, age mean 23.5 ± 2.5 years) were compared to C/C homozygotes (T− group; N = 51, 26 male, age mean 24.1 ± 2.7 years) and G allele carriers of the STMN1 SNP2 (T/G and G/G genotypes = G+ group; N = 55, 26 male, age mean 23.5 ± 2.5 years) were compared to T/T homozygotes (G− group; N = 50, 26 male, age mean 24.1 ± 2.7 years).
Statistical Analysis
All analyses were performed using SPSS for Windows 12.0 (SPSS Inc., Chicago, IL). In the sample of the 106 participants who passed data preprocessing, the 48 startle variables (18 for unpleasant, 9 for neutral, 9 for pleasant, and 12 for baseline startle condition) were log-transformed because of the highly skewed distribution of the raw startle variables and the resulting deviation from the normal distribution (Kolmogorov–Smirnov tests, P < 0.20). The average startle magnitudes in the four conditions (baseline, unpleasant, neutral, pleasant) were computed, were tested for univariate normality (Kolmogorov–Smirnov tests, P ≥ 0.433) and were then entered into a repeated measures analysis of variance with condition as a within-subjects factor, and stathmin genotype and gender as between-subjects factors. Greenhouse-Geisser corrected degrees of freedom were used where appropriate.
To analyze the cortisol response, a difference score was computed between cortisol concentrations 20 min after cessation of the stress paradigm and cortisol concentrations immediately before onset of the TSST. The resulting variable was tested for univariate normality (Kolmogorov–Smirnov test, P ≈ 0.20) and was then entered into an univariate analysis of variance with Stathmin genotype and gender as between-subjects factors.
Pairwise linkage disequilibrium between the two SNPs was assessed using 2LD [Zhao, 2004]. Haplotype analyses were performed using the WHAP program developed by S. Purcell and P. Sham (Institute of Psychiatry, London, UK; URL: http://www.genome.wi.mit.edu/∼shaun/whap/). WHAP estimates haplotype frequencies using an expectation maximization algorithm and performs tests of global haplotype-trait association for both dichotomous and quantitative traits. It also permits tests of haplotype-specific associations by comparing each of a specific haplotype versus all other haplotypes.
RESULTS
Genotype Frequencies
The frequencies for STMN1 SNP1 genotypes were 48.1% (n = 51) for C/C, 39.6% (n = 42) for T/C, and 12.3% (n = 13) for T/T; and for STMN1 SNP2 genotypes were 47.6% (n = 50) for T/T, 40.0% (n = 42) for T/G, and 12.4% (n = 13) for G/G. The genotypes of both SNPs were in Hardy–Weinberg equilibrium (P ≥ 0.351). The two SNPs were in nearly perfect linkage disequilibrium (D′ = 1.0, χ2 = 201.0, df = 1, P < 0.0001).
Affective Picture Ratings
As some of the valence and arousal ratings were not normally distributed (Kolmogorov–Smirnov tests, P < 0.20), the medians of the valence and arousal ratings for the different picture categories were compared with each other using the nonparametric Wilcoxon tests for paired samples. The median valence ratings for unpleasant, neutral, and pleasant pictures were 2.81, 5.80, and 6.21, respectively, and the median arousal ratings were 3.95, 1.57, and 3.40, respectively. All two-way comparisons for valence, and for arousal, respectively, were highly significant (all P ≤ 0.001).
STMN1 SNP1 genotype groups did not differ in valence and arousal ratings, although the female T− group showed higher arousal ratings for unpleasant pictures (nonparametric Kruskal–Wallis tests, P = 0.036; all other P ≥ 0.262). Similarly, while STMN1 SNP2 genotype groups did not differ in valence and arousal ratings, the female G− group showed a tendency towards higher arousal ratings for unpleasant pictures (nonparametric Kruskal–Wallis tests, P = 0.061; all other P ≥ 0.216).
STMN1 Genotype Impact on Acoustic Startle and Emotional Startle Response Modulation
SNP1
Analysis of variance for the whole sample (N = 106) showed a significant condition main effect (F2.7, 270.5 = 55.94, P ≤ 0.000, η2 = 0.35). Within-subjects contrast analyses revealed that the presentation of pleasant pictures resulted in significant pleasure attenuation of the startle (PAS; pleasant vs. neutral condition: F1,102 = 122.27, P ≤ 0.001, η2 = 0.55), whereas the presentation of unpleasant affective pictures did not result in significant fear potentiation of the startle (FPS; unpleasant vs. neutral: F1,102 = 0.01, P = 0.922, η2 ≤ 0.00). However, with regard to a subset of the neutral pictures (“kitchen objects”), a significant within-subjects contrast appeared (FPS; unpleasant vs. kitchen objects: F1,102 = 11.69, P = 0.001, η2 = 0.103). Also compared to baseline, the startle response was higher in the unpleasant condition (F1,102 = 5.71, P = 0.019, η2 = 0.053) and again it was significantly enhanced in the neutral condition (F1,102 = 5.84, P = 0.017, η2 = 0.054). Figure 1A shows mean startle magnitudes in the four conditions for the whole sample.
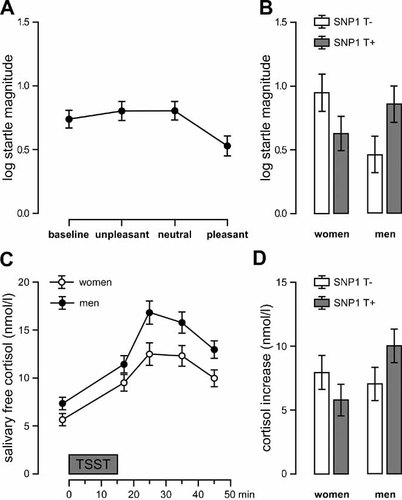
STMN1 SNP1, the acoustic startle response and the cortisol response. (A) log startle magnitudes and standard errors of means in the four conditions in the whole sample (N = 106); (B) log startle magnitudes and standard errors of means in the female T− group (N = 25) and T+ group (N = 29) as well as in the male T− group (N = 26) and T+ group (N = 26); (C) cortisol response and standard errors of means in the female group (N = 54) and in the male group (N = 52) and (D) cortisol increase and standard errors of means in the female T− group (N = 25) and T+ group (N = 29) as well as in the male T− group (N = 26) and T+ group (N = 26).
There were no genotype-specific differences in FPS or PAS as indicated by the absence of a significant condition × STMN1 SNP1 interaction effect (F2.7, 270.5 = 0.59, P = 0.60, η2 = 0.006) and no significant condition × gender × STMN1 SNP1 interaction effect (F2.7, 270.5 = 0.56, P = 0.62, η2 = 0.005). However, there was a significant STMN1 SNP1 × gender interaction effect on average startle magnitudes across conditions (F1,102 = 6.34, P = 0.013, η2 = 0.058) with the female T− group showing stronger overall startle magnitudes than the male T− group (F1,49 = 5.56, P = 0.022, η2 = 0.102). The difference between the male and female T+ group did not reach significance. Table I presents the mean startle magnitudes and standard errors of means for the four conditions. Figure 1B illustrates the genotype differences at the level of gender group comparison in the T− and the T+ group.
| Sample | N | Condition | ||||
|---|---|---|---|---|---|---|
| Baseline | Unpleasant | Neutral | Pleasant | |||
| Total | 106 | 0.74 (0.07) | 0.80 (0.07) | 0.80 (0.07) | 0.53 (0.08) | |
| T− | Female | 25 | 0.97 (0.14) | 1.05 (0.15) | 1.06 (0.15) | 0.72 (0.16) |
| Male | 26 | 0.50 (0.14) | 0.52 (0.15) | 0.55 (0.14) | 0.28 (0.16) | |
| T+ | Female | 29 | 0.66 (0.13) | 0.72 (0.14) | 0.72 (0.13) | 0.41 (0.15) |
| Male | 26 | 0.84 (0.14) | 0.95 (0.15) | 0.91 (0.14) | 0.73 (0.16) | |
- T− = C/C genotype; T+ = T/C and T/T genotype.
SNP2
Due to the nearly perfect linkage disequilibrium, the results for SNP2 were mainly the same as for SNP1. Particularly, there was a significant STMN1 SNP2 × gender interaction effect on average startle magnitudes across conditions (F1,101 = 4.87, P = 0.030, η2 = 0.046) with the female G− group showing larger overall startle magnitudes than the male G− group (F1,48 = 4.90, P = 0.032, η2 = 0.093) but no condition × genotype or condition × gender × genotype effects (all P ≥ 0.60).
STMN1 Genotype Effect on Cortisol Response
SNP1
Analysis of variance showed a significant main effect of the TSST on the salivary cortisol response (F2.0, 206.9 = 78.70, P ≤ 0.000, η2 = 0.44). As illustrated in Figure 1C, there was also a significant effect of gender on the overall cortisol response (F1,102 = 5.05, P = 0.027, η2 = 0.47) with men showing stronger cortisol responses. However, there was no significant main effect of gender on the cortisol difference score (P = 0.200).
Furthermore, analysis of variance showed no significant main effect of STMN1 SNP1 on overall saliva cortisol response and the cortisol difference between before and after the TSST (P = 0.303 and P = 0.756, respectively). However, univariate analysis of variance revealed a gender x genotype interaction effect for STMN1 SNP1 (F1,102 = 3.93, P = 0.050, η2 = 0.037) on the cortisol increase with the male T+ group showing stronger cortisol increase than the female T+ group (F1,53 = 5.61, P = 0.022, η2 = 0.096). The difference between male and female T− group did not reach significance. Table II gives the mean cortisol response 2 min before and 20 min after the TSST as well as the mean difference score and standard errors of means for the different genotypes. Figure 1D illustrates the genotype differences at the level of the gender group comparison.
| Sample | N | Condition | |||
|---|---|---|---|---|---|
| 2 min before TSST | 20 min after TSST | Difference | |||
| Total | 106 | 6.43 (0.46) | 14.04 (0.79) | 7.67 (0.65) | |
| T− | Female | 25 | 4.79 (0.95) | 12.70 (1.60) | 7.91 (1.33) |
| Male | 26 | 6.78 (0.93) | 13.80 (1.56) | 7.02 (1.30) | |
| T+ | Female | 29 | 6.31 (0.88) | 12.07 (1.45) | 5.75 (1.23) |
| Male | 26 | 7.77 (0.93) | 17.76 (1.56) | 9.99 (1.30) | |
- T− = C/C genotype; T+ = T/C and T/T genotype.
SNP2
With regard to STMN1 SNP2 univariate analysis of variance revealed a similar trend towards a gender × genotype interaction effect (F1,101 = 3.298, P = 0.072, η2 = 0.032) on the difference score between the saliva cortisol level after and before the TSST. Again, there was no significant main effect of SNP2 on the overall saliva cortisol response and the cortisol increase after the TSST (P = 0.128 and P = 0.646, respectively).
Haplotype Analyses
There were only two common SNP1–SNP2 haplotypes present in the sample, C–T with a frequency of 0.68, and T–G with a frequency of 0.31. A third very rare haplotype (C–G, frequency 0.01, i.e., one individual) was found in the female subsample only. Hence, haplotype association tests were skipped, as they could not provide additional information.
DISCUSSION
Inactivation of the gene coding for stathmin in mice (stmn1) has been shown to moderate the amygdala-dependent acquisition and expression of learned and innate fear behavior patterns [Shumyatsky et al., 2002, 2005]. Here we demonstrate for the first time that an analogous role may be attributed to stathmin in fear-related behavior in humans. Our results suggest that STMN1 variation influences the expression of two forms of fear and anxiety related behavior: the mediation of the startle response and the mediation of the stress-induced HPA activation respectively. Thus our study identified two behavioral systems and underlying neural mechanisms as correlates of STMN1 genotype. These systems are supposed to be prominently involved in the development of anxiety disorders and depression. The findings provide suggestive evidence for the functional significance of the STMN1 variants investigated and they may contribute to a better understanding of fear and anxiety regulation at the molecular level.
Startle Response
Female carriers of the SNP1 T− allele exhibited significant stronger startle response across all conditions than the male T− group, a result that was mirrored when analyzing STMN1 SNP2, which was in nearly perfect linkage disequilibrium with SNP1. However, we could not confirm our hypothesis that carriers of the candidate variants of the STMN1 gene show a differential enhancement of the startle response during viewing unpleasant pictures. This means, that female T− and G− carriers exhibited a stronger startle response even in the baseline condition when the amygdala complex is assumed to be not involved in startle modulation according to the standard model of the acoustic startle circuit. This is an intriguing result and parallels the findings of Brocke et al. 2006 and Armbruster et al. 2009, which revealed a significant impact of variants of the serotonin transporter gene already upon baseline startle. These findings are also consistent with results of a recent twin study [Anokhin et al., 2007] where the absolute startle magnitude showed high heritability (59–61%) while there was no genetic influence on the startle modulation across emotional valence conditions although in animal studies genetic influence on the affect modulated startle also has been reported [Anisman et al., 2000]. However, the reported effects of human STMN1 variation interacting with gender have not been replicated as yet.
Because the startling stimuli of the ASR, sudden high-intensity noise bursts, are aversive and able to induce a state of fear [Leaton and Cranney, 1990], parallel processing of startling stimuli in the thalamo-amygdala and thalamo-cortico-amygdala pathways in addition to the primary startle circuitry is suggestive [LeDoux, 2000b]. If the response signal within the usual time window (20–140 msec) integrates several serial projections from different brain nuclei to the PnC [Lingenhöhl and Friauf, 1994], an early involvement of the amygdala complex in the processing of startling stimuli seems possible. While in the model of a primary acoustic startle circuit, the PnC is the most important brainstem site for the evocation of the ASR, other brain nuclei than the PnC also play a role in mediating the ASR [Yeomans and Frankland, 1995; Koch, 1999]. This view is supported by two findings: (a) excitatory postsynaptic potentials (EPSP) recorded intracellular from PnC neurons show multiple peaks that occur at constant latencies [Lingenhöhl and Friauf, 1994] which suggests excitatory input from multiple afferents; (b) field potentials specifically related to the ASR were recorded in the basolateral amygdala suggesting that the amygdala is to some extent also involved in the modulation of the ASR [Ebert and Koch, 1997].
Moreover, although amygdala lesions have been demonstrated to block FPS [Funayama et al., 2001] there is also some, albeit inconclusive evidence for an impaired overall startle response following amygdala lesion [Angrilli et al., 1996; Kettle et al., 2006]. Hence, we cannot rule out the possibility that the amygdala complex is possibly not only involved in the emotional modulation, but also in the processing of startling stimuli per se and that the observed association of STMN1 variation with the overall startle response is mediated by differential amygdala activity. Moreover, as suggested above the STMN1 genotype and the resulting reduced microtubule dynamics might additionally impact the overall startle response by way of reduced EPSPs involved in US transmission and leading to impaired signal-to-noise ratio of the transmission process [Rodrigues et al., 2004; Bauer and LeDoux, 2004]. Furthermore, the LA, the basal nuclei of the amygdala and, in accordance with recent findings the CE [Wilensky et al., 2006], are believed to be a site of memory storage in fear learning [Fanselow and LeDoux, 1999] and LTP is involved in the reconsolidation of fear memories which is required after reactivation during retrieval [Nader et al., 2000]. Therefore STMN1 variation influencing microtubule dynamics and LTP might even differentially impact reconsolidation of innate fear stimuli.
A further result of the study was a significant condition main effect associated with a remarkable effect size (η2 = 0.35). However, while compared to the neutral condition startle responses of the total group were attenuated as expected when processing pleasant affective pictures, they were not potentiated in the unpleasant condition. Yet, with regard to a subset of the neutral pictures (“kitchen objects”), a significant enhancement of the startle response of the total group appeared in the unpleasant condition and the same was the case, when we compared the unpleasant condition with the baseline condition. Contrary to expectations, the startle response was significantly enhanced in the neutral condition (total set) compared to baseline as well. However, this result converges with findings from other studies [Vrana et al., 1988; Lang et al., 1990; Bradley and Lang, 2000; Bradley et al., 2001; Ruiz-Padial and Vila, 2007]. It has often been observed that different sub-categories of neutral pictures produce very heterogeneous effects on the magnitude of the startle response, sometimes surmounting the magnitude of the startle potentiation through categories of unpleasant pictures [Bradley et al., 2001]. Furthermore it has been shown that the amygdala is also activated when the situation can be described as uncertain or ambiguous which may be the case with some of the neutral pictures [Davis and Whalen, 2001; Rosen and Donley, 2006]. It may be assumed that the processing of such neutral pictures gives rise to additional projections from the associative cortex to the CE and then to the PnC [Davis and Whalen, 2001].
Cortisol Response
Male carriers of the T+ allele of the STMN1 SNP1 exhibited a significantly larger cortisol increase from baseline to peak cortisol levels in response to stress induction than female T+ carriers, and again the result was mirrored when analyzing STMN1 SNP2. These findings support our assumption that genetic variation of STMN1 modulates fear and anxiety related behavior through genetically driven differential activity of the LA–CE connection and its impact on the hypothalamus and the HPA-system in a sex-dependent manner. It is widely accepted that during psychological stress, the diurnal cortisol secretory pattern is overridden by signals to the hypothalamus that originate in the limbic system [Lovallo, 2006]. However, the LA projects to a variety of additional brain areas that are involved in fear and anxiety, and the stress response might also be induced by pathway other than that involving the LA–CE connection and directly related effector systems. In this context, additional indirect regulation of the HPA-axis by the LA and CE through connections with the bed nucleus of the stria terminalis (BNST) is of particular interest [Davis, 2006; Rosen and Donley, 2006], because in the latter stathmin is also highly expressed [Peschanski et al., 1993].
Taken together, the results revealed a reaction pattern showing an inverse reactivity of the stathmin genotypes in the two sexes. Concerning startle responses the female T− group showed stronger reactions than the male T− group whereas in the TSST the male the T+ group showed stronger cortisol responses than the female T+ group. This inverse reactivity of the sexes might be due to the variation of a glucocorticoid response element close to SNP1 locus (see Introduction) and parallels other findings showing a gender specific reactivity of the HPA-axis system [Kirschbaum et al., 1999]. However, functionality of the GRE binding site and its link to sex effects has not been demonstrated experimentally as yet.
In sum, the present data provide first evidence of a possible impact of human STMN1 variation in interaction with individuals' gender on fear and anxiety-related behaviors as assessed with the startle and cortisol stress response. However, in contrast to investigating animals, the reported effects of human STMN1 variation interacting with gender have not been replicated as yet.
As Cahill 2006 pointed out, sex differences at the level of brain organization and function are neither small nor unreliable. Since there is mounting evidence that males and females differ in brain regions involved in emotional regulation as well as in several neurotransmitter systems the influence of sex as an additional factor as well as a factor interacting with other variables has to be taken into account and became visible in the present results. Our data provide supporting evidence for the notion that STMN1 variation and sex impact two well-established behavioral systems and neural and molecular mechanisms underlying the acquisition and expression of fear and anxiety. In addition, the findings provide some evidence that the STMN1 genotype has functional relevance for the regulation of basic fear and anxiety responses also in humans. This approach, suggesting a novel plasticity regulating molecular mechanism, may also contribute to a better understanding of human fear behavior at the molecular level. Though the reported effects of human STMN1 variation interacting with gender have not been replicated as yet, they get some support from the intriguing and replicated findings about stathmin1 impacting the acquisition and expression of innate and learned fear behavior in mice.
Acknowledgements
The authors would like to thank N. Steigerwald for technical assistance in genotyping, G. Arnold for analyzing cortisol samples, and U. Buhss for excellent work in processing and analyzing the EMG data. The authors are indebted to T. Hensch for helpful comments during the preparation of this article. Supported by the European Commission (NEWMOOD LSHM-CT-2003-503474), Bundesministerium für Bildung und Forschung (IZKF 01 KS 9603), and the Deutsche Forschungsgemeinschaft (SFB 581, KFO 125/1-1, KI 537/20-2).




