Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer's disease: New data and meta-analysis†‡
Noriko Fukumoto and Takashi Fujii contributed equally to this work.
How to Cite this Article: Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai S-J, Matsushita S, Nacmias B, Comings DE, Arboleda H, Ingelsson M, Hyman BT, Akatsu H, Grupe A, Nishimura AL, Zatz M, Mattila KM, Rinne J, Goto Y, Asada T, Nakamura S, Kunugi H. 2010. Sexually Dimorphic Effect of the Val66Met Polymorphism of BDNF on Susceptibility to Alzheimer's Disease: New Data and Meta-Analysis. Am J Med Genet Part B 153B:235–242.
Abstract
Conflicting results have been reported as to whether genetic variations (Val66Met and C270T) of the brain-derived neurotrophic factor gene (BDNF) confer susceptibility to Alzheimer's disease (AD). We genotyped these polymorphisms in a Japanese sample of 657 patients with AD and 525 controls, and obtained weak evidence of association for Val66Met (P = 0.063), but not for C270T. After stratification by sex, we found a significant allelic association between Val66Met and AD in women (P = 0.017), but not in men. To confirm these observations, we collected genotyping data for each sex from 16 research centers worldwide (4,711 patients and 4,537 controls in total). The meta-analysis revealed that there was a clear sex difference in the allelic association; the Met66 allele confers susceptibility to AD in women (odds ratio = 1.14, 95% CI 1.05–1.24, P = 0.002), but not in men. Our results provide evidence that the Met66 allele of BDNF has a sexually dimorphic effect on susceptibility to AD. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Alzheimer's disease (AD) is a common neurodegenerative disease and is neuropathologically characterized by loss and atrophy of basal forebrain cholinergic neurons and the limbic structures [Mattson, 2004]. Mutations in several genes are known to cause familial AD, namely those encoding amyloid precursor protein [Goate et al., 1991], presenilin-1 [Sherrington et al., 1995], and presenilin-2 [Levy-Lahad et al., 1995]. The ε4 allele of the apolipoprotein E (APOE) gene confers susceptibility to familial and sporadic AD [Saunders et al., 1993]. However, AD is a genetically complex disorder and these genetic markers for AD cannot explain the overall genetic susceptibility. Thus, additional genes may be involved in the development of AD.
Since neurotrophins such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3) promote the development, regeneration, survival, and maintenance of function of neurons [Reichardt, 2006], polymorphisms of the genes encoding these proteins may confer susceptibility to neurodegenerative diseases. Several lines of evidence have suggested that BDNF, in particular, is an important candidate gene for susceptibility to AD. Reduced BDNF mRNA levels were observed in postmortem hippocampi and temporal cortices of patients with AD [Connor et al., 1997], and lower protein levels of BDNF in the entorhinal cortex were reported in AD [Hock et al., 2000]. Immunohistochemical and Western blotting studies revealed a selective decline of the BDNF/TrkB neurotrophic signaling pathway in the frontal cortex and hippocampus in AD [Ferrer et al., 1999].
Based on these observations, a number of genetic association studies have been performed for two polymorphisms of BDNF, Val66Met, and C270T. The non-synonymous polymorphism, Val66Met, is a functional single-nucleotide polymorphism (SNP), G to A substitution at nucleotide 196, which results in the Val66-to-Met amino acid change in the 5′ pro-region of the human BDNF protein [Ventriglia et al., 2002]. Two studies reported that the Met66 allele was significantly associated with an increased risk of AD [Saarela et al., 2006; Tsai et al., 2006], while one study reported that the Val66 allele was the risk-increasing allele [Matsushita et al., 2005]. The majority of studies, however, have found no significant association (Supplementary Fig. 1) [Ventriglia et al., 2002; Bagnoli et al., 2004; Combarros et al., 2004; Nacmias et al., 2004; Bian et al., 2005; Bodner et al., 2005; Desai et al., 2005; Lee et al., 2005; Li et al., 2005; Nishimura et al., 2005; Vepsalainen et al., 2005; Akatsu et al., 2006; Forero et al., 2006; Zhang et al., 2006; He et al., 2007; Huang et al., 2007]. The C270T polymorphism in the non-coding region of BDNF was detected by our group and found to be associated with late-onset AD [Kunugi et al., 2001]. Subsequently, two other groups reported that the T270 allele was significantly associated with an increased risk of AD [Nishimura et al., 2004; Olin et al., 2005], while one group reported the opposite [Saarela et al., 2006]. Other studies reported no significant association (Supplementary Fig. 2) [Riemenschneider et al., 2002; Bagnoli et al., 2004; Nishimura et al., 2004; Bodner et al., 2005; Desai et al., 2005; Lee et al., 2005; Matsushita et al., 2005; Akatsu et al., 2006; Tsai et al., 2006; Zhang et al., 2006; Huang et al., 2007]. These conflicting results require further investigation.
METHODS
Case–Control Study Sample
We genotyped 657 patients with AD (427 females; 73.5 years [SD] 8.7) and 525 healthy controls (305 females; 67.1 years [SD] 10.3) who were recruited around the Tokyo Metropolitan area, Japan. Diagnoses were made by neurologists according to the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [McKhann et al., 1984] for “probable AD.” The numbers of individuals with and without a family history of dementia were 211 and 425, respectively, while the remaining 21 individuals had undetermined family histories. Controls were interviewed and those who had a family history of dementia within their first-degree relatives were not enrolled in the study. All subjects were biologically unrelated Japanese individuals. After description of the study, written informed consent was obtained from every subject. The study protocol was approved from the ethics committee of the National Center of Neurology and Psychiatry, Japan.
Genotyping
The two SNPs of BDNF were genotyped using the TaqMan 5′-exonuclease allelic discrimination assay. TaqMan probes of the “Assay-On-Demand” (C__11592758_10) for Val66Met (rs6265) and TaqMan primers (forward: GGAGCCAGAATCGGAACCA; reverse: CCAGCGCTTGCCTACCT) and probes (VIC: CTCACGGGTCCCCG; FAM: CTCACGAGTCCCCG) of the “Assay-by-Design” for C270T and Universal PCR Master Mix were obtained from Applied Biosystems (Foster City, CA). Thermal cycling conditions for the polymerase chain reaction (PCR) were one cycle at 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 58°C for 1 min. After amplification, the allele-specific fluorescence was measured on ABI PRISM 7900 Sequence Detection System (Applied Biosystems). Genotype data were read blind to the case–control status. We also genotyped the subjects for the APOE gene, according to the methods of Wenham et al. 1991.
Meta-Analysis
To examine whether there was a possible sex difference in the effect of these polymorphisms on AD in a larger sample, we organized a multi-center collaborative study and performed a meta-analysis. We searched for published case–control association studies of the Val66Met or C270T polymorphism with AD in the PubMed database (National Center for Biotechnology Information; NCBI; www.ncbi.nlm.nih.gov/), using combinations of terms “BDNF,” “brain-derived neurotrophic factor,” “polymorphism,” “Val66Met,” “C270T,” “C-270T,” and “Alzheimer.” Additionally, reference lists of these and relevant articles, and the AlzGene Database (www.alzforum.org/) [Bertram et al., 2007] were referred to. As a result, 23 association studies of AD with Val66Met (Supplementary Table III) and 18 with C270T (Supplementary Table IV) were identified. Then an e-mail calling for participation in the collaborative study was sent to corresponding and first authors. Sixteen research groups for the Val66Met and 12 for the C270T responded and participated in this study. Genotype data with information on sex were combined.
Genotypes in the control groups from all research groups were in Hardy–Weinberg equilibrium. In the meta-analysis, heterogeneity, publication bias, sensitivity analysis, and Rosenthal's failsafe N were determined. Meta-analytic procedures were carried out using Comprehensive Meta-Analysis v.2.0 (Biostat, Inc., Englewood, NJ). To confirm that there was no significant difference in the allele distributions of patients and controls between the collected and the uncollected data (i.e., studies whose authors did not respond to us), Breslow–Day tests were performed using R software (R Development Core Team, 2007). With respect to Val66Met, the summary data for the Breslow–Day tests are shown in Supplementary Table V. There was no significant difference between the collected and uncollected data (χ2 = 2.0, df = 1, P = 0.15).
RESULTS
Case–Control Study
Genotype distributions for Val66Met, C270T, and APOE were in Hardy–Weinberg equilibrium for both patients and controls (data not shown). Genotype distributions for APOE were significantly different between the patients and controls as expected (P = 2 × 10−18) (Supplementary Table I). Genotype and allele distributions for Val66Met are shown in Table I. There was a trend towards an increased frequency of the Met66 allele in patients compared to controls (P = 0.063). When men and women were examined separately, the allele distribution differed between the two groups in females (odds ratio [OR] = 1.30, 95% CI = 1.05–1.60, P = 0.017), but not in males (OR = 1.02, 95% CI = 0.78–1.32, P = 0.91) (Table I).
| Genotype distribution | P-value, df = 2 | Allele distribution | P-value, df=1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |||||||||||
| n | Val/Val | Val/Met | Met/Met | n | Val/Val | Val/Met | Met/Met | Val | Met | Val | Met | |||
| Total | 657 | 218 (0.33) | 319 (0.49) | 120 (0.18) | 525 | 197 (0.38) | 249 (0.47) | 79 (0.15) | 0.18 (χ2 = 3.4) | 755 (0.57) | 559 (0.53) | 643 (0.61) | 407 (0.39) | 0.063 (χ2 = 3.5) |
| Female | 427 | 142 (0.33) | 205 (0.48) | 80 (0.19) | 305 | 122 (0.40) | 143 (0.47) | 40 (0.13) | 0.057 (χ2 = 5.7) | 489 (0.57) | 365 (0.60) | 387 (0.63) | 223 (0.37) | 0.017 (χ2 = 5.7) |
| Male | 230 | 76 (0.33) | 114 (0.50) | 40 (0.17) | 220 | 75 (0.34) | 106 (0.48) | 39 (0.18) | 0.96 (χ2 = 0.09) | 266 (0.58) | 194 (0.44) | 256 (0.58) | 184 (0.42) | 0.91 (χ2 = 0.01) |
The genotype and allele distributions for C270T are shown in Supplementary Table II. There was no significant difference in the genotype or allele distribution between the patients and controls. Also when sexes were examined separately, no significant association was found for either sex.
Meta-Analysis
With respect to Val66Met, individual studies contained 64–998 patients with AD and 45–671 controls, and the combined sample consisted of 4,711 patients and 4,537 controls (Supplementary Table III). There was no heterogeneity across studies (total: Q = 26.7, df = 21, P = 0.18; men: Q = 16.0, df = 15, P = 0.38; women: Q = 13.5, df = 15, P = 0.56). Thus, we performed the fixed effects meta-analyses (Fig. 1, Tables II and III). The meta-analysis showed no significant association between AD and the Met66 allele (OR = 1.05, 95% CI = 0.98–1.11; Z = 1.43, P = 0.15; Supplementary Fig. 1). Meta-analysis of data in men and women separately revealed a significant association in women (OR = 1.14, 95% CI = 1.05–1.24; Z = 3.05, P = 0.002; Fig. 1A), but not in men (OR = 0.97, 95% CI = 0.87–1.08; Z = −0.54, P = 0.59; Fig. 1B). In the sensitivity analysis, the association of the Met66 allele with AD remained significant after removal of any one study (Supplementary Table VI): even if our data were removed, there remained a significant association for women (residual OR = 1.11, 95% CI = 1.02–1.22; Z = 2.30, P = 0.022). The Rosenthal failsafe N for women was 31 studies. No evidence of publication bias was indicated by Egger's test (intercept = 0.80, 95% CI = −0.37 to 0.54, t = 1.46, P = 0.17).
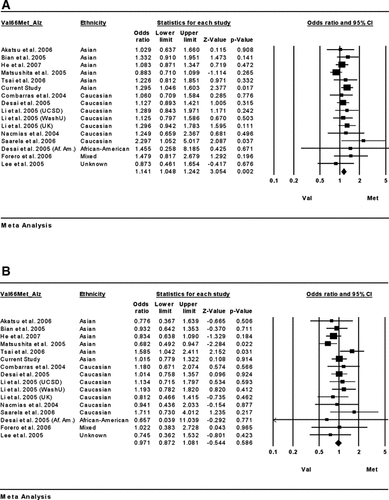
Forest plots of meta-analysis on the possible association between the Val66Met polymorphism of BDNF and Alzheimer's disease in female (A) and male (B) subjects.
| Study | Ethnicity | Genotype distribution | Allele distribution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||||||||
| n | Val/Val | Val/Met | Met/Met | n | Val/Val | Val/Met | Met/Met | Val | Met | Val | Met | ||
|
Akatsu et al. 2006 |
Asian | 58 | 16 | 36 | 6 | 86 | 30 | 42 | 14 | 68 | 48 | 102 | 70 |
|
Bian et al. 2005 |
Asian | 108 | 20 | 67 | 21 | 105 | 36 | 47 | 22 | 107 | 109 | 119 | 91 |
|
He et al. 2007 |
Asian | 318 | 92 | 152 | 74 | 332 | 97 | 170 | 65 | 336 | 300 | 364 | 300 |
|
Matsushita et al. 2005 |
Asian | 340 | 117 | 170 | 53 | 321 | 104 | 154 | 63 | 404 | 276 | 362 | 280 |
|
Tsai et al. 2006 |
Asian | 84 | 19 | 50 | 15 | 101 | 33 | 50 | 18 | 88 | 80 | 116 | 86 |
| Current study | Asian | 427 | 142 | 205 | 80 | 305 | 122 | 143 | 40 | 489 | 365 | 387 | 223 |
| Subtotal | 1,335 | 406 | 680 | 249 | 1,250 | 422 | 606 | 222 | 1,492 | 1,178 | 1,450 | 1,050 | |
|
Combarros et al. 2004 |
Caucasian | 161 | 107 | 47 | 7 | 155 | 105 | 44 | 6 | 261 | 61 | 254 | 56 |
|
Desai et al. 2005 |
Caucasian | 669 | 449 | 201 | 19 | 411 | 287 | 115 | 9 | 1,099 | 239 | 689 | 133 |
| Li et al. 2005 (UCSD)a | Caucasian | 87 | 51 | 32 | 4 | 226 | 150 | 67 | 9 | 134 | 40 | 367 | 85 |
| Li et al. 2005 (WashU)b | Caucasian | 248 | 163 | 81 | 4 | 215 | 150 | 60 | 5 | 407 | 89 | 360 | 70 |
| Li et al. 2005 (UK)c | Caucasian | 265 | 178 | 73 | 14 | 270 | 192 | 73 | 5 | 429 | 101 | 457 | 83 |
|
Nacmias et al. 2004 |
Caucasian | 58 | 36 | 19 | 3 | 61 | 39 | 22 | 0 | 91 | 25 | 100 | 22 |
|
Saarela et al. 2006 |
Caucasian | 68 | 45 | 21 | 2 | 56 | 46 | 10 | 0 | 111 | 25 | 102 | 10 |
| Subtotal | 1,556 | 1,029 | 474 | 53 | 1,394 | 969 | 391 | 34 | 2,532 | 580 | 2,329 | 459 | |
|
Desai et al. 2005 |
African-American | 46 | 42 | 4 | 0 | 33 | 31 | 2 | 0 | 88 | 4 | 64 | 2 |
|
Forero et al. 2006 |
Mixed | 73 | 51 | 20 | 2 | 115 | 90 | 23 | 2 | 122 | 24 | 203 | 27 |
|
Lee et al. 2005 |
Unknown | 61 | 31 | 28 | 2 | 38 | 20 | 14 | 4 | 90 | 32 | 54 | 22 |
| Total | 3,071 | 1,559 | 1,206 | 306 | 2,830 | 1,532 | 1,036 | 262 | 4,324 | 1,818 | 4,100 | 1,560 | |
- a UCSD sample from the University of California, San Diego.
- b WashU sample from the Washington University.
- c UK sample from Cardiff University, Wales College of Medicine and King's College London.
| Study | Ethnicity | Genotype distribution | Allele distribution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Contorls | ||||||||||
| n | Val/Val | Val/Met | Met/Met | n | Val/Val | Val/Met | Met/Met | Val | Met | Val | Met | ||
|
Akatsu et al. 2006 |
Asian | 37 | 9 | 22 | 6 | 22 | 5 | 11 | 6 | 40 | 34 | 21 | 23 |
|
Bian et al. 2005 |
Asian | 95 | 29 | 46 | 20 | 134 | 37 | 68 | 29 | 104 | 86 | 142 | 126 |
|
He et al. 2007 |
Asian | 195 | 63 | 93 | 39 | 243 | 68 | 115 | 60 | 219 | 171 | 251 | 235 |
|
Matsushita et al. 2005 |
Asian | 147 | 54 | 77 | 16 | 150 | 46 | 69 | 35 | 185 | 109 | 161 | 139 |
|
Tsai et al. 2006 |
Asian | 91 | 24 | 42 | 25 | 88 | 31 | 45 | 12 | 90 | 92 | 107 | 69 |
| Current study | Asian | 230 | 76 | 114 | 40 | 220 | 75 | 106 | 39 | 266 | 194 | 256 | 184 |
| Subtotal | 795 | 255 | 394 | 146 | 857 | 262 | 414 | 181 | 904 | 686 | 938 | 776 | |
|
Combarros et al. 2004 |
Caucasian | 76 | 42 | 31 | 3 | 63 | 38 | 23 | 2 | 115 | 37 | 99 | 27 |
|
Desai et al. 2005 |
Caucasian | 329 | 216 | 98 | 15 | 260 | 169 | 82 | 9 | 530 | 128 | 420 | 100 |
|
Li et al. 2005 (UCSD) |
Caucasian | 94 | 54 | 38 | 2 | 126 | 81 | 39 | 6 | 146 | 42 | 201 | 51 |
|
Li et al. 2005 (WashU) |
Caucasian | 140 | 88 | 45 | 7 | 134 | 87 | 45 | 2 | 221 | 59 | 219 | 49 |
|
Li et al. 2005 (UK) |
Caucasian | 72 | 46 | 26 | 0 | 89 | 56 | 28 | 5 | 118 | 26 | 140 | 38 |
|
Nacmias et al. 2004 |
Caucasian | 25 | 12 | 10 | 3 | 36 | 16 | 16 | 4 | 34 | 16 | 48 | 24 |
|
Saarela et al. 2006 |
Caucasian | 29 | 16 | 13 | 0 | 45 | 35 | 7 | 3 | 45 | 13 | 77 | 13 |
| Subtotal | 765 | 474 | 261 | 30 | 753 | 482 | 240 | 31 | 1,209 | 321 | 1,204 | 302 | |
|
Desai et al. 2005 |
African-American | 18 | 17 | 1 | 0 | 12 | 11 | 1 | 0 | 35 | 1 | 23 | 1 |
|
Forero et al. 2006 |
Mixed | 28 | 21 | 7 | 0 | 53 | 41 | 11 | 1 | 49 | 7 | 93 | 13 |
|
Lee et al. 2005 |
Unknown | 34 | 14 | 19 | 1 | 32 | 12 | 16 | 4 | 47 | 21 | 40 | 24 |
| Total | 1,640 | 781 | 682 | 177 | 1,707 | 808 | 682 | 217 | 2,244 | 1,036 | 2,298 | 1,116 | |
Meta-analysis for the C270T polymorphism was performed in the same way. Eighteen studies were identified, of which 12, including ours, participated in the meta-analysis. The individual studies contained 58–722 AD cases and 42–525 controls, and the combined sample consisted of 2,963 subjects with AD and 2,756 controls (Supplementary Tables IV, VII, and VIII). There was a significant heterogeneity between studies (total: Q = 44.7, df = 17, P < 0.01; men: Q = 18.8, df = 11, P = 0.065; women: Q = 30.2, df = 11, P < 0.01). Thus, we performed the random effects meta-analyses (Supplementary Fig. 2). Our meta-analysis did not show significant association of AD with the T270 allele (random-effect pooled OR = 1.07, 95% CI = 0.83–1.39; Z = 0.54, P = 0.59; Supplementary Fig. 2A). Also when men and women were examined separately, our meta-analysis revealed no significant association with AD in women (OR = 1.08, 95% CI = 0.70–1.67; Z = 0.37, P = 0.72; Supplementary Fig. 2B) or in men (OR = 1.19, 95% CI = 0.77–1.84; Z = 0.78, P = 0.43; Supplementary Fig. 2C).
DISCUSSION
We showed, for the first time, a significant allelic association between the Val66Met of BDNF and AD in women in our Japanese sample (P = 0.017). In contrast, we did not observe such an association in men. When the multi-center study was organized, the sexually dimorphic effect of the Val66Met on the development of AD was similarly observed in the much larger sample (4,711 patients and 4,537 controls) from 16 research centers worldwide. These results provide evidence suggesting that the Met66 allele has a risk-increasing effect on AD in women, but not in men.
The Met66-BDNF protein has been shown to be associated with reduced transport of BDNF from the Golgi region to appropriate secretory granules in neurons, compared with the Val66-BDNF protein [Egan et al., 2003; del Toro et al., 2006]. It is reasonable to assume that the Met66 is associated with lower secretion of BDNF, which could result in attenuation of the survival signal of BDNF, compared with the Val66. In accordance with this, individuals carrying the Met66 allele have been reported to have decreased brain structures (e.g., hippocampus) than those individuals who did not carry the allele [Pezawas et al., 2004; Szeszko et al., 2005; Agartz et al., 2006; Bueller et al., 2006; Ho et al., 2006; Nemoto et al., 2006; Frodl et al., 2007; Liguori et al., 2007]. Of note, we found that female individuals carrying the Met66 allele showed more widespread age-associated volume reduction in the dorsolateral prefrontal cortices than male Met66 carriers [Nemoto et al., 2006].
Several lines of evidence suggest the sexual dimorphic effects of BDNF. The study of BDNF conditional knockout mice demonstrated sexually dimorphic effects in depression- and anxiety-related behavior [Monteggia et al., 2007]. A recent sexually stratified meta-analysis reported that the Val66Met was more important in the development of major depressive disorder in men than in women [Verhagen et al., 2008]. In Parkinson's disease as well, a sex difference in the effect of BDNF was reported [Foltynie et al., 2005]. Many epidemiological studies reported higher prevalence and incidence of AD in women than in men [Fratiglioni et al., 1997]. In an animal model of neurodegenerative diseases, aged female mice were more sensitive to kainic acid-induced excitotoxicity to neurons, compared with aged males [Zhang et al., 2008]. These findings are in line with our observations of the sexually dimorphic effect of BDNF on AD. Indeed, estrogen plays an important role in the expression of BDNF. Estrogen receptors co-localize with BDNF-synthesizing neurons in the forebrain [Miranda et al., 1993] and estrogen induces BDNF expression through the estrogen response element [Sohrabji et al., 1995].
With respect to the C270T, we obtained no evidence for an association with AD in our sample alone or in the combined sample. We observed a significant heterogeneity across studies in the meta-analysis. In addition, the allele frequency of the risk allele (T270) reported in the original study [Kunugi et al., 2001] was quite low (0.03 in total), indicating the possibility of type II error due to lack of statistical power. Thus, further studies are required to draw any conclusion.
In conclusion, we provided the first meta-analytic evidence that the Met66 allele of BDNF has a sexually dimorphic effect on susceptibility to AD. Studies elucidating the molecular mechanisms underlying this association are warranted.
Acknowledgements
This study was supported by Health and Labor Sciences Research Grants (Research on Psychiatric and Neurological Diseases and Mental Health), the Program for Promotion of Fundamental Studies, in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), and Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS).




