Linear clinical progression, independent of age of onset, in Niemann–Pick disease, type C†
How to Cite this Article: Yanjanin NM, Vélez JI, Gropman A, King K, Bianconi SE, Conley SK, Brewer CC, Solomon B, Pavan WJ, Arcos-Burgos M, Patterson MC, Porter FD. 2010. Linear Clinical Progression, Independent of Age of Onset, in Niemann–Pick Disease, Type C. Am J Med Genet Part B 153B:132–140.
Abstract
Niemann–Pick disease, type C is a neurodegenerative, lysosomal storage disorder with a broad clinical spectrum and a variable age of onset. The absence of a universally accepted clinical outcome measure is an impediment to the design of a therapeutic trial for NPC. Thus, we developed a clinical severity scale to characterize and quantify disease progression. Clinical signs and symptoms in nine major (ambulation, cognition, eye movement, fine motor, hearing, memory, seizures, speech, and swallowing) and eight minor (auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, psychiatric, and respiratory problems) domains were scored. Data were collected from 18 current NPC patients and were extracted from records of 19 patients. Both patient cohorts showed a linear increase in severity scores over time. Cross-sectional evaluation of current patients showed a linear increase in the severity score. Longitudinal chart review of historical data demonstrated that although age of onset varied significantly, the rate of progression appeared linear, independent of age of onset, and similar in all patients. Combining the data from both cohorts, disease progression could be modeled by the following equation: Ŝt0+x = Ŝt0 + 1.87x; where Ŝt0 is the initial score and Ŝt0+x is the predicted future score after x years. Our observation that disease progression is similar across patients and independent of age of onset is consistent with a biphasic pathological model for NPC. This scale may prove useful in the characterization of potential biomarkers, and as an outcome measure to monitor disease progression in NPC patients. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Niemann–Pick Disease, type C (NPC, OMIM #257220) is an autosomal recessive lysosomal storage disorder characterized by intracellular accumulation of cholesterol and glycosphingolipids [Pentchev et al., 1987; Zervas et al., 2001a,b]. NPC has a wide clinical spectrum with a variable age of presentation that ranges from fetal onset with ascites to progressive dementia in adults [Yerushalmi et al., 2002; Imrie et al., 2007; Sevin et al., 2007]. Hepatosplenomegaly and liver dysfunction are common in infancy [Kelly et al., 1993; Imrie et al., 2007], but the major clinical problems are neurological. Neurological features are progressive, and include ambulatory impairment, cerebellar ataxia, dementia, dysarthria, dysphagia, psychotic episodes, seizures, and supranuclear vertical gaze palsy. The incidence has been estimated to be 1/120,000–150,000 [Vanier and Millat, 2003]. Mutations of two genes, either NPC1 or NPC2, cause NPC (reviewed by Vanier and Millat 2003). Mutation of NPC1 underlies about 95% of cases. NPC1 maps to chromosome 18q11-12, and encodes an integral membrane protein that functions in intracellular cholesterol transport and homeostasis [Ory, 2004]. Mutation of NPC2 also causes NPC. NPC2 maps to chromosome 14q24.3 and encodes a 132 amino acid lysosomal protein that binds cholesterol [Naureckiene et al., 2000].
There is no definitive therapy for NPC. After Pentchev et al. 1985, 1987 found that cholesterol transport was impaired in NPC, a therapeutic trial combining a low cholesterol diet with cholesterol-lowering drugs was attempted. However, there was no apparent impact on the neurological symptoms [Patterson et al., 1993; Patterson and Platt, 2004]. Similarly, hepatic transplantation was unsuccessful in ameliorating the progression of neurological symptoms [Gartner et al., 1986]. Substrate reduction therapy using miglustat (N-butyldeoxynojirimycin, Zavesca), an inhibitor of glucosylceramide synthase, improved horizontal saccadic eye movement velocities in a subset of patients in a randomized trial after one year of therapy [Patterson et al., 2007]. In the same study, dysphagia improved and a trend toward improved ambulation was observed in the treated patients. The long-term efficacy of miglustat therapy is still being investigated.
A number of additional potential therapeutic interventions have been suggested by recent work performed in NPC mice. These include treatment with neurosteroids [Griffin et al., 2004], liver X receptor (LXR) and pregnane X receptor (PXR) agonists [Langmade et al., 2006; Repa et al., 2007], or cyclodextrin [Liu et al., 2008]. However, the absence of a universally accepted clinical outcome measure or surrogate marker is a major impediment to the design and implementation of a therapeutic trial for NPC. Variable onset and progression of individual symptoms further compound this problem. In this article, we describe the development of a clinical progression scale and validate it by applying it to both a cross-sectional study of current NPC patients, and a longitudinal study based on chart review of prior patients. Notably, both cohorts show similar linear phenotypic progression that appears to be independent of age of onset. This clinical progression scale may prove useful as a tool to characterize potential biomarkers, and as an outcome measure to monitor long-term disease progression in NPC patients as well as response to therapies.
METHODS
Participants and Procedures
This study was approved by the National Institute of Child Health and Human Development Institutional Review Board. Consent and, when appropriate, assent were obtained. A clinical severity scale was developed to ascertain clinical symptoms in nine major and eight minor clinical areas (Table I). Four of the domains (ambulation, fine motor skills, speech, and swallowing) were modified from the disability scale developed by Iturriaga et al. 2006. The scoring of each domain was designed to allow a score to be derived from a comprehensive clinical evaluation. A Likert-like scale was used to assign nine major domain scores of 0–5 and eight minor domain scores of 0–2. Clinical experience was used to weight the various scales. Summation of all 17 domains yielded total possible scores that range from 0 to 61, with a higher score indicating more severe clinical impairment. A comprehensive medical history form was developed to document both the clinical history of current patients and to serve as a guide in extraction of data from medical records. To be scored, seizures, cataplexy, and narcolepsy had to be definitive and not questionable. For the swallowing domain, one point was scored if the patient had a history of cough while eating. Additional points were added if the patient had intermittent or consistent dysphagia with either liquids or solids. Hearing loss refers to sensorineural hearing loss and not hearing loss secondary to conductive defects. The diagnosis of NPC was established by either biochemical testing or mutation analysis. All patients have NPC1 by either molecular or complementation group testing. Two patient groups were studied. The first group consisted of 18 NPC patients (current cohort) who were enrolled in an observational study at the National Institutes of Health Clinical Center (NIH CC) between August 2006 and September 2007 (Table II). The second patient cohort consisted of 19 NPC patients (historical cohort) for whom we had sufficient medical records to generate at least three scores at different time points. Medical records were reviewed for 36 patients followed at the NIH CC by other investigators between 1972 and 2005. Of the 36 previous NIH CC patients with a diagnosis of NPC, 16 patients had 3 or more NIH admissions with adequate documentation to generate a severity score. Of the current patients, patient 1 had previous NIH admissions for which records were available and patients 13 and 15 had sufficient outside medical records from which we could derive longitudinal data.
- *Score is additive within these two subsections.
- **PTA, pure-tone average—reported on the audiogram.
| Patient | Gender | Age at presenting symptom | Presenting symptom | Age at 1st neurologic symptom | 1st neurologic symptoma | Age at diagnosis | Age (years) at 1st NIH evaluation | Score at 1st NIH evaluation |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 18 months | Splenomegaly | 9 years | Vertical gaze palsy | 9 years | 21 | 35 |
| 2b | M | 6 months | Splenomegaly | 5 years | Fine motor limitation | 5 years | 7 | 5 |
| 3b | F | 8 months | Splenomegaly | 2 years | Clumsy, possible VGP | 8 years | 13 | 33 |
| 4 | M | Neonate | Jaundice, splenomegaly at 3 months | 2–4 years | Clumsy, dysarthria | 3 months | 5 | 11 |
| 5 | M | 12 months | Splenomegaly | 3 years | Fine motor limitation | 8 years | 10 | 16 |
| 6 | M | 18 months | Hepatosplenomegaly | 3 years | Dysarthria | 2 years | 16 | 18 |
| 7 | F | 2 months | Splenomegaly | 18 months | Clumsy | 7 years | 11 | 22 |
| 8 | F | 6 months | Splenomegaly | 2–3 years | Clumsy | 2 years | 4 | 22 |
| 9 | M | 2 weeks | Jaundice, splenomegaly at 3 months | 2 years | Abnormal gait | 6 years | 7 | 7 |
| 10 | M | 18 months | Splenomegaly | 6 years | Fine motor, coordination problems | 2 years | 11 | 18 |
| 11 | M | 3 months | Hepatosplenomegaly | 2 years | Clumsy, speech delay | 2 years | 4 | 5 |
| 12 | F | Neonate | Hepatosplenomegaly | 12 months | Clumsy | 9 months | 4 | 12 |
| 13 | M | 8 years | Learning delay | 8 years | Learning delay, hearing loss | 21 years | 32 | 39 |
| 14 | M | 5 months | Splenomegaly | n/a | None reported | 2 years | 3 | 3 |
| 15 | F | 39 years | Hearing loss | 39 years | Hearing loss | 48 years | 51 | 24 |
| 16 | M | Neonate | Jaundice, splenomegaly | n/a | None reported | 3 years | 4 | 2 |
| 17 | M | 3 weeks | Hepatomegaly, Jaundice | 2 years | Clumsy | 9 months | 6 | 7 |
| 18 | F | 5 years | Hepatosplenomegaly | 8–12 years | Clumsy, hearing loss | 24 years | 25 | 20 |
- a Reported by parents.
- b Siblings.
Statistical Analysis
To model the variation of the total score per individual over time as a function of age, we fitted linear mixed models (LMM). Different LMM models were fitted: random intercept and fixed shape (model 1); fixed intercept and random shape (model 2); random intercept as well as random shape (model 3) (all with order 1 auto regressive correlation structure—AR(1)); and random intercept as well as random shape (model 4) with no within-individual correlation. In all models, we used the age of the individuals over time as a covariate. Gender was not included as a covariate due to the limited population size. Patient age was computed by subtracting the date of birth (DOB) from the evaluation date. The best model was selected using likelihood ratio test (LRT). The LRT is a statistical test of the goodness-of-fit between various models. Statistical modeling was done with R [R Development Core Team, 2008] and nlme packages [Pinheiro et al., 2007]. For determination of inter-rater reliability, three independent reviewers scored 16 records. Cronbach's α and Cohen's κ coefficient were calculated [Cronbach, 1951; Cohen, 1960] Data in the text, unless otherwise specified, are presented as the mean ± standard error of the mean.
RESULTS
We developed a clinical severity scale (Table I) in order to characterize clinical progression and to index potential biomarkers to disease severity. Clinical signs and symptoms in nine major domains (scored 0–5: ambulation, cognition, eye movement, fine motor, hearing, memory, seizures, and speech, swallowing) and eight minor domains (scored 0–2: auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, psychiatric, and respiratory problems) were evaluated.
We initially used this scale to characterize 18 NPC patients enrolled in an NIH observational study. Patient demographics are shown in Table II. Age at initial NIH evaluation ranged from age 4 to 51 years with a mean and median age of 12.9 and 8.5 years, respectively. The male to female ratio was 11:7. The most common presenting symptom in this cohort was splenomegaly (15/18, 83%) with a mean age at presentation of 3.4 years. Four patients (22%) presented with neonatal jaundice; however, in three of these cases, splenomegaly was noted by 3 months of age. Two patients (13 and 18) were noted to have learning difficulties by age 8 and 18 years, respectively. The initial symptom in the single adult onset case (patient 15) was hearing loss. Of note, hearing loss was a prominent clinical issue in the three oldest patients. A significant delay in diagnosis of NPC was observed for the majority of patients. Excluding the one patient who had an older affected sibling (patient 2), of the 16 cases initially presenting with jaundice or splenomegaly, the diagnostic delay ranged from 3 months to 19 years with a mean and median of 4.3 and 1.8 years, respectively. The diagnostic delays for patients 13, 15, and 18 were, respectively, 13, 9, and 19 years.
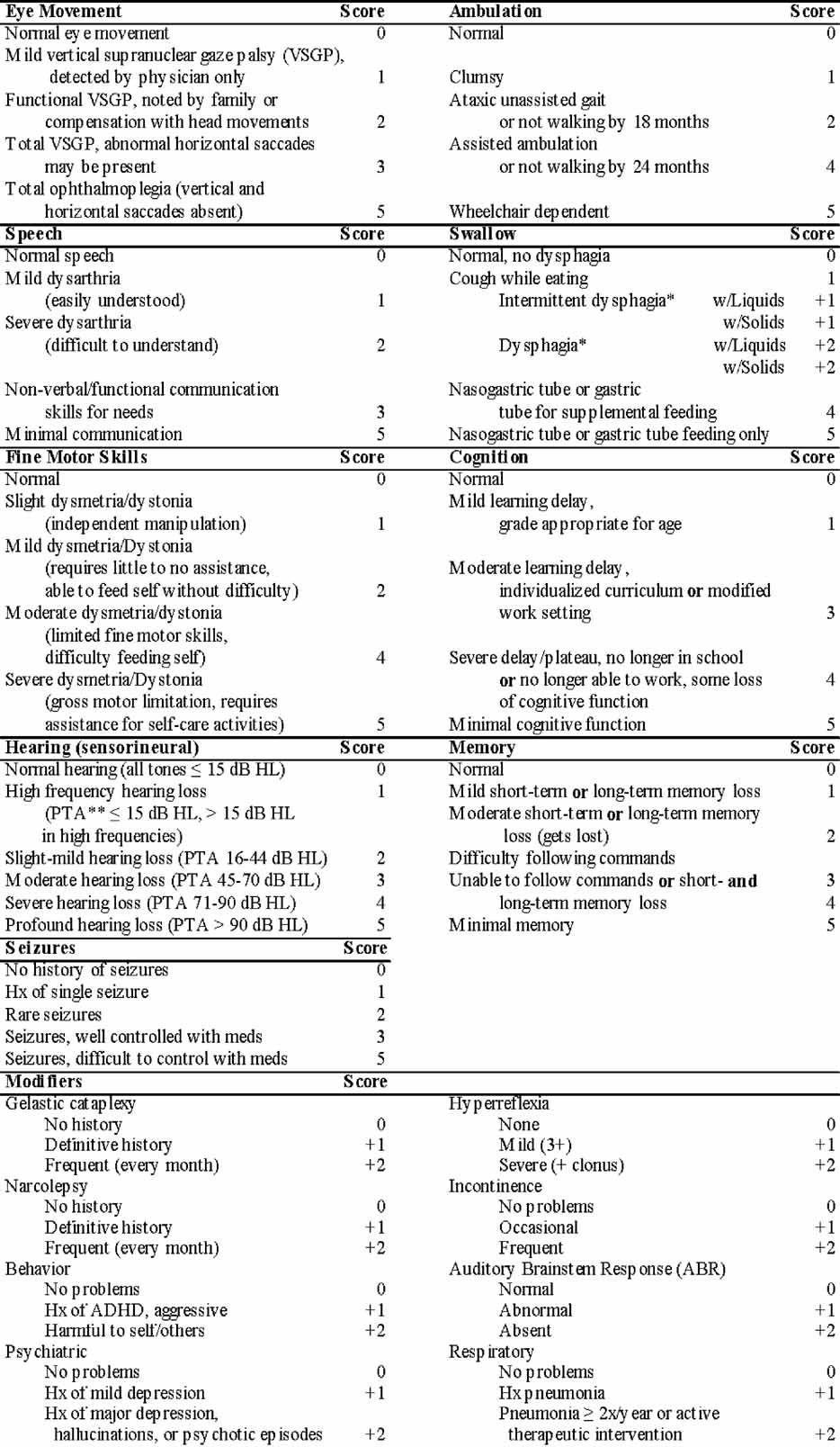
When we scored these current patients using our severity scale, as one would expect for a progressive disorder, severity scores increased with age. However, the increase in the severity score as a function of age was nonlinear (Fig. 1A). In contrast, severity scores increase in a linear manner (r2 = 0.64, P < 0.0001) when plotted against the time interval between the age at initial presentation and our first evaluation. The slope of the linear regression line suggests a mean rate of progression of 1.4 ± 0.3 points per year after appearance of the presenting symptom (Fig. 1B). Because the neurological onset is insidious and often difficult to accurately determine retrospectively, for Figure 1B, we defined the onset of the presenting symptom (Table II) as time zero. Four of the patients had total scores less than or equal to five. These signs and symptoms give an indication of the earliest clinical neurological manifestations of this disorder. Eye movement, hearing, speech, and ABR abnormalities were noted in three of the four patients. The eye movement abnormality was only apparent to the parents in one of the cases. Hearing deficits were confined to high frequency hearing loss, and did not cause functional impairment. One of these low scoring patients had mild deficits in cognition and memory. In this cohort, seven patients were being treated off-label with miglustat at the time of their initial evaluation. No obvious clustering was apparent (Fig. 1B), and the mean rate of progression (1.4 ± 0.3) was not significantly altered when patients on miglustat were excluded from the analysis.
NPC clinical progression. A: Clinical severity scores for the current cohort as a function of age. B: The change in the clinical severity score between onset of symptoms and the first NIH evaluation. Patients on off-label miglustat use are indicated by open circles. C: Longitudinal severity scores in a cohort of 19 historical patients. D: The progression slope was determined by linear regression of each of the curves depicted in (C). E: Contribution of the major domains to the total score for the current cohort and the historical cohort. For the historical cohort, we compared early findings (0–5 years after onset) to late findings (>6 years after onset).
This clinical progression scale was also applied to a chart review of 19 NPC patients. Age at initial evaluation ranged from age 2 to 38 years with a mean and median age of 14.7 and 14.0 years, respectively. The male/female ratio was 12:7. Severity scores versus age for individual patients are depicted in Figure 1C. The initial score for these patients corresponds to the initial NIH evaluation. The majority of these patients were manifesting neurological symptoms by the time they were referred to the NIH. Interestingly, once symptoms appear, the rate of progression appears to be independent of age of onset (P = 0.44, r2 = 0.04) and remarkably similar across patients (Fig. 1D). The progression slope for individual patients ranged from 0.8 to 5.1 with a mean slope of 1.9 ± 0.2 points per year. Excluding the single patient with very rapid progression (greater than 3 standard deviations above the mean) yields a mean progression slope of 1.7 ± 0.1 points per year.
Figure 1E shows the proportion of the total score that each domain contributes for both the current and historical cohort. Data from the historical cohort were divided into early (0–5 years after onset) and late (≥6 years after onset). The contribution of each domain to the total score is similar in both cohorts and no major difference was observed comparing early and late scores. We next analyzed the average severity scores from both cohorts to determine the degree of correlation between the total score and the individual domains. Although there was a statistically significant relationship between age and the total score (P = 0.006), the correlation coefficient was not as high (r2 = 0.44) as seen with other variables. In addition, age correlated relatively poor (r2 < 0.6) with the individual domains. Significant correlations were observed between the total score and all major domains except hearing. The strongest correlations (r2 > 0.8) with the total score were with swallowing (0.90), ambulation (0.83), memory (0.83), and cognition (0.82). Between individual components, strong correlations (r2 > 0.8) were only observed between ambulation and both fine motor (0.85) and swallowing (0.83). Inter-rater reliability was analyzed by determination of Cronbach's α and Cohen's κ. For three independent raters, Cronbach's α was 0.845 (95% CI = 0.735–0.897), 0.844 (95% CI = 0.729–0.893), and 0.850 (95% CI = 0.760–0.892), and the global Cronbach's α, calculated by combining all the measures from the three raters, was 0.846 (95% CI = 0.798–0.877). It is generally accepted that Cronbach's α values over 0.70 indicate acceptable sensitivity and validity of a scale. Using the total score measured by each rater, the weighted Cohen's κ coefficient showed a significant agreement between raters (κ = 0.888, 95% CI = 0.802–0.975, P < 0.0001).
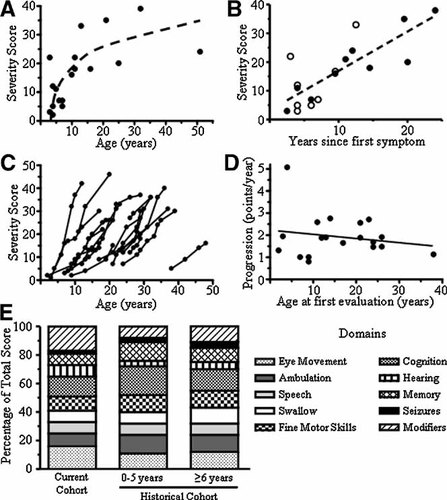
To model the variation of the total score per individual over time as a function of age, we combined data from both cohorts (Fig. 2) and tested different LMMs using LRT. A random intercept, random shape model with no within-individual correlation best fit the data with the population progression curve described by the following equation: Ŝ = −10.7 + 1.87 (age). The standard error of the slope is ±0.18, and the 95% CI for the slope is 1.52–2.23. For a given subject, future scores can be predicted by the following equation: Ŝt0+x = Ŝt0 + 1.87x; where Ŝt0 is the initial score and Ŝt0+x is the predicted future score after x years.
Statistical modeling. To model phenotypic progression in NPC, data from all patients were combined. Patients ranged in age from 2 to 51 years (mean = 18.6, SD = 11.5), number of visits ranged from 1 to 8 (mean = 3.4, SD = 2.1), and the total scores ranged from 1 to 46 (mean = 18.6, SD = 10.7). The figure shows the individual data points, the model curves for individual patients (dashed lines), and the predicted population curve (heavy solid line).
DISCUSSION
A major issue impairing the development and implementation of a therapeutic trial for NPC is the lack of well defined and accepted outcome measures. Identification of biomarkers in NPC would be of great assistance; however, for a biomarker to be used as a surrogate for disease progression, its relationship to disease severity and progression needs to be defined. Thus, a validated phenotypic severity scale would provide a useful tool to correlate quantitative biochemical, imaging, or clinical data with disease severity and progression. While several phenotypic scales have previously been proposed for NPC, these previous staging systems are limited in their usefulness to correlate with quantitative data. Higgins et al. 1992 developed a five stage classification system that included both clinical and neurophysiological findings, and Klarner et al. 2007 developed a five stage classification system based on clinical symptoms. Both of these scales classify patients into broad categories and produce a step-wise progression of disease severity. Iturriaga et al. 2006 developed a four domain “disability scale” for NPC patients. The four clinical domains included in this scale were ambulation, language (speech), manipulation (fine motor), and swallowing. This scale differentiated between classical and variant cases; however, no longitudinal data were presented and the dynamic range (4–18) is limiting. To address the limitations of these previous severity scales, we expanded the Iturriaga disability scale to include additional clinical findings frequently observed in NPC (cognition, eye movement, hearing, memory, and seizures), and accounted for less frequent clinical findings by inclusion of a series of “modifier” domains (auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, psychiatric, and respiratory status). Using this scale, scores can range from 0 to 61, thus providing a greater dynamic range. To characterize and validate this scale, we applied it in a cross-sectional study of current patients and in a longitudinal review of medical records.
When we applied this severity scale to a historical cohort of NPC patients, we observed that progression in individual patients appeared to be linear and that the rate of progression was independent of age of onset. This was somewhat surprising since we initially expected that the rate of progression would be inversely related to age of onset. Supporting our observation in the historical cohort, we also observed a linear increase in phenotypic severity in our current cohort of patients. This observation has several important implications. First, although the accrual of individual symptoms will vary, patients and families can be given anticipatory guidance with respect to the expected rate of progression. Second, the similar rate of progression allows for data from juvenile and adult onset patients to be combined and statistically modeled. The ability to combine data from patients of variable age of onset will facilitate recruitment for clinical trials. Third, given sufficient time, one could use this severity score directly as an outcome measure. However, its greatest utility will likely lie in the ability to index other quantitative biochemical, imaging, or clinical data. As such it will be useful in validating the relationship of potential biomarkers to disease severity and progression.
Several caveats need to be considered when applying the results reported in this paper. First, our patient populations did not include patients with the perinatal or infantile variants of NPC. Thus, until studied, the conclusions reached in this study should not be applied to those patients. Second, the number of adult-onset patients in this study is limited. Replication of our results in a larger cohort of adult patients would strengthen our conclusion that disease progression is similar in both classical and adult onset patients. Third, a small percentage of patients may have a more aggressive disease progression as illustrated by the patient in our historical cohort with a progression rate of 5.1 points/year. Review of this patient's chart did not reveal any obvious distinguishing clinical features; thus care needs to be exercised in providing anticipatory guidance. Fourth, due to limited data available with respect to rate of accrual of mild neurological symptoms early in the course of this disease, one should not assume that mildly affected patients will accrue additional neurological symptoms at the rate predicted for this population of patients. To accurately define progression of this stage of the disease, a prospective study of asymptomatic/mildly affected patients will need to be performed. Fifth, data from both of our cohorts are confounded by potential therapeutic interventions. Some patients in the historical cohort were enrolled in a clinical trial to aggressively reduce cholesterol [Patterson et al., 1993], and approximately half of the patients in our current cohort are on varying doses of miglustat. Although our analysis did not reveal any differences between the miglustat treated and untreated group, due to variable dosing and length of treatment, one cannot conclude from our data that miglustat therapy is not effective. In addition, many NPC patients are treated with a number of dietary supplements. Although no obvious effects were observed, these interventions could introduce variability and need to be acknowledged as confounding factors if one attempts to use these data as a historical control.
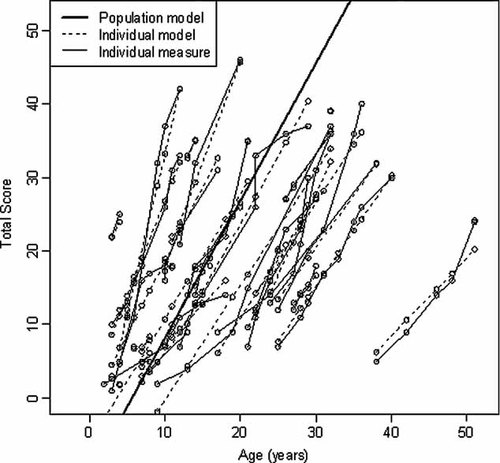
The observation that the rate of disease progression is independent of age of onset is interesting from both a clinical and biological perspective. From a biological perspective, this may provide insight into the pathological mechanisms underlying this disorder. In many neurodegenerative disorders later onset forms have a more benign course and slower progression. In those cases, one can propose a model in which a single integrated pathological process results in progressive neuronal dysfunction which occurs at a rate proportional to the rate of lysosomal accumulation. Reactive processes such as microglial activation are integrally linked with the intracellular lipid accumulation that occurs in NPC deficient neurons. Symptoms are observed after given thresholds of neuronal dysfunction or loss are reached (Fig. 3A). In this model, patients who have more rapid intracellular accumulation of lipids will show earlier onset and more rapid phenotypic progression (Fig. 3, inset). This model does not adequately explain our observation that phenotypic progression is similar in most patients, and independent of the age of onset. An alternative model is shown in Figure 3B. In this case, at least two independent pathological phases are proposed. The first pathological phase involves an initiating stimulus that varies in its severity. The initiating stimulus, likely intracellular accumulation of lipids due to the NPC1 deficiency, results in neuronal “toxicity.” Like the first model this phase occurs at differing rates. However, in this model the first pathological phase does not lead directly to irreversible neuronal dysfunction or loss. Instead, this model proposes that once a “toxicity threshold” is reached, then a second pathological process, such as microglial activation, is initiated. This second pathological process results in a constant rate of irreversible neuronal loss or dysfunction. Similar to what was proposed in the first model; individual symptoms are observed after given thresholds of impaired neuronal function are reached. This second model is consistent with our observation of a variable age of onset with subsequent similar phenotypic progression occurring at a similar rate in all patients. If this two phase model is correct, elucidating the biological factors that lead to the variability of progression to the second phase will be important. Genotypic differences that encode NPC1 proteins with different levels of residual protein function are likely a major contributor to this variability. However, two factors preclude analysis of this with our current data set. First, NPC1 demonstrates a high degree of allelic heterogeneity with over 240 different mutations described [Runz, 2009], and the most common mutation only accounts for approximately 15–20% of identified alleles [Vanier and Millat, 2003]. Since many mutations are “private”; except for siblings, it is difficult to identify groups of patients with the same genotype. Second, a biochemical assay to quantify residual NPC1 function does not exist. This precludes grouping patients with different mutations but similar residual NPC1 activity. A much larger cohort of NPC1 patients will be necessary to make these types of correlations. It is likely that modifier genes, such as apolipoprotein E genotype [Saito et al., 2002], and potentially environmental factors also contribute to the observed variability in age of onset.
Theoretical modeling. Two hypothetical pathological models are presented. In the first model (A), a single pathological process leads to progressive neuronal loss or dysfunction. Neurological symptoms occur once given thresholds appear. The rate of neuronal loss determines both age of onset and the rate of clinical progression (inset). This model predicts that later onset would be associated with slower clinical progression. In the second biphasic model (B), we propose that one pathological process occurs at variable rates that results in neuronal toxicity. Once a given “toxicity threshold” is crossed, a second reactive pathological process results in neuronal loss or dysfunction. The rate of the second process is constant, and like in the first model symptoms occur once given thresholds of neuronal loss are crossed. In this model, age of onset is determined by the first process; whereas, appearance of symptoms will subsequently occur at a similar rate in different patients.
Previous studies testing therapeutic interventions in the NPC1 mutant mouse model support the idea that this hypothetical initial pathological process is amendable to therapeutic intervention. Griffin et al. 2004 showed that a single injection of 7-day-old (P7) NPC1 mutant mice with allopregnanolone significantly delays the onset of overt symptoms, but injections on days P10, P17, and P23 were less effective. Liu et al. 2008 reported that single P7 injection of cyclodextrin, a carrier used to solubilize allopregnanolone and which itself can extract hydrophobic molecules such as cholesterol from cellular membranes, can significantly prolong lifespan in NPC1 mutant mice.
From a clinical perspective, the alternative models potentially impact therapeutic approaches. The timing of therapeutic intervention may be critical. Substrate reduction therapy, such as inhibition of glycosphingolipid synthesis with miglustat, may be effective in the presymptomatic first phase, but less so after a “toxicity threshold” is reached. In mouse studies, miglustat significantly delayed onset of symptoms and death when therapy was started presymptomatically at 3.5 weeks of age [Zervas et al., 2001]. In contrast, efficacy in a human trial, where all participants had neurological symptoms and signs at enrollment, appeared to be more limited [Patterson et al., 2007]. In contrast to human trials, treatment of NPC1 mutant mice is often initiated prior to physical symptoms being present; thus it may be difficult to extrapolate therapeutic efficacy to patients who are typically symptomatic at the time of diagnosis. Similarly, anti-inflammatory therapy designed to impact reactive processes, such as microglial activation, may be time dependent and more likely to have an impact once patients enter the second phase.
In summary, we have developed a clinical severity scale that quantifies the major symptoms of NPC. We have validated it in both a cross-sectional study of current NPC patients and a longitudinal chart review of a historic cohort. This scale will be a useful benchmark against which to correlate disease biomarkers with disease progression and as a long-term outcome measure for therapeutic trials.
Acknowledgements
This work was supported in part by the intramural programs of NICHD, NHGRI, NIDCD, and ORD. The Ara Parseghian Medical Research Foundation and Dana's Angels Research Trust have provided funding to support N.Y. We would like to acknowledge previous NINDS investigators and fellows whose work on NPC provided the medical records that were reviewed to obtain the historical data. We would also like to thank Dr. Steven Walkley and Dr. Daniel Ory for critical review of the manuscript and Christopher Zalewski for assistance with audiological evaluations. Finally we would like to express our gratitude to the parents, guardians, and patients who have participated in these studies.




