Genome-widely significant evidence of linkage of schizophrenia to chromosomes 2p24.3 and 6q27 in an SNP-Based analysis of Korean families†
Please cite this article as follows: Hong KS, Won H-H, Cho E-Y, Jeun HO, Cho S-S, Lee Y-S, Park DY, Jang YL, Choi K-S, Lee D, Kim M-J, Kim S, Han WS, Kim J-W. 2009. Genome-Widely Significant Evidence of Linkage of Schizophrenia to Chromosomes 2p24.3 and 6q27 in an SNP-Based analysis of Korean Families. Am J Med Genet Part B 150B:647–652.
Abstract
The present study reports the results of a genome-wide SNP linkage scan for schizophrenia in the Korean population. Fifty-six multiplex schizophrenia families were analyzed. Clinical evaluations on all subjects were consistently performed by raters in a single research team. Multipoint non-parametric linkage analysis was performed, and empirical simulations were generated to determine genome-wide significance. The authors found genome-widely significant evidence of linkage for schizophrenia to chromosomes 2p24.3 (NPL Z = 3.18) and 6q27 (NPL Z = 2.90). Six other chromosomal regions, that is, 3q24, 13q12.3, 18q22.3, 20p12.2, 4p14, and 1p36.12, yielded NPL Z scores of above 2.0 for either broad or narrow phenotype classes. Although linkage to these loci has not received prominent attention in studies on Caucasian families, multiple overlaps were observed between our loci (on 2p, 3q, and 13q) and linkage peaks generated from extended families in various isolated populations. Fine mappings and the detection of candidate genes within these regions are warranted. © 2008 Wiley-Liss, Inc.
INTRODUCTION
According to genetic epidemiological studies, schizophrenia shows strong evidence of heritability [Cannon et al., 1998; Cardno and Gottesman, 2000]. However, genome-wide linkage screens of schizophrenia have produced conflicting results [Badner and Gershon, 2002; Lewis et al., 2003]. Given that epidemiological data on schizophrenia favors contributions by multiple genes each of which exert a small to moderate effect on overall disease risk, and that the genetic effects of these genes or loci may differ between ethnic groups and pedigrees [Baron, 2001; Lewis et al., 2003], controversial results from independent linkage analyses are only to be expected.
Since the introduction of genome-wide association studies using several hundred thousand SNPs and a huge number of case–control samples, this method has begun to attract considerable attention for the elucidation of complex genetic disorders [The Wellcome Trust Case Control Consortium, 2007]. However, given that even this high density of SNPs might not provide enough resolution to detect evolutionarily old causative SNPs and/or copy number variations of complex genetic disorders [Ropers, 2007] and that genome-wide association studies often use linkage information as a prior data, it is still important to obtain linkage information in different human populations.
Even though the optimal method of analyzing linkage data for a disease with a complex mode of inheritance remains uncertain, two major strategies have been utilized to increase statistical power for the detection of true linkages in schizophrenia. One involves the recruitment of a large number of families by adopting nationwide or multi-center collaborative study approaches [Arinami et al., 2005; Faraone et al., 2006; Suarez et al., 2006]. However, these approaches are vulnerable to ethnic or clinical evaluation heterogeneity problems [Baron, 2001]. Schizophrenia is a phenomenologically defined disease based on operationalized diagnostic criteria. Even though diagnostic reliability has been promoted by using the same diagnostic criteria, interview schedules, and rater training sessions, a variety of sources of unreliability exist, that is, observational variances or interpretational variances originating from differences between the internalized guidelines of clinicians established by personal experiences of psychiatric training and clinical practice [Bland and Kolada, 1988].
The other major strategy used to increase linkage analysis statistical power involves the analysis of large extended families, which are usually recruited from isolated populations [Camp et al., 2001; Lindholm et al., 2001; Bulayeva et al., 2007]. However, in general, it is difficult to identify such extended pedigrees containing large numbers of schizophrenia patients, because of the low marriage and reproduction rates of individuals who suffer from the disease [Book et al., 1978]. Moreover, affected individuals in these families may have special clinical characteristics in terms of age of onset, symptom nature, or treatment outcome, even though they all meet the diagnostic criteria of DSM or ICD. Therefore, linked loci in these families may be somewhat unique and untypical of schizophrenia patients in general. In such a case, fine mapping or the identification of associated alleles for those loci must be done using patients who may not have susceptibility genes in these loci.
Therefore, a supplementary approach using a relatively small number of families and individuals of homogenous ethnicity, ascertained using a homogenous clinical evaluation, is also warranted. Over the past 5 years, the Center for Schizophrenia Genetics at the Samsung Medical Center has identified Korean multiplex schizophrenia families and collected their genetic and clinical data. The majority of probands were recruited at the Samsung Medical Center Schizophrenia Clinic (SMC-SC), and even though some probands were referred from other hospitals, clinical interviews and diagnostic evaluations were performed by raters from the clinical evaluation team at SMC-SC in all cases. The present study reports the results of a genome-wide SNP linkage scan on these Korean families using the Illumina 6K assay (Illumina Inc., San Diego, CA). SNPs, while less informative than microsatellites, offer the advantages of having a lower mutation and genotyping error rates. Although individual SNP markers are much less informative than microsatellites and not as powerful at detecting linkage in two-point analyses, SNPs have the potential to overcome this lower informativeness when subjected to multipoint analysis [Middleton et al., 2004].
MATERIALS AND METHODS
Subjects
The recruitment of multiplex families containing two or more affected individuals among second degree relatives was performed through proband screening at the SMC-SC, the Yong-In Mental Hospital, the National Chuncheon Hospital, and a number of other hospitals in Korea. Direct interviews using the Korean version of the Diagnostic Interview for Genetic Studies (DIGS) [Joo et al., 2004] were performed by a clinical evaluation team at SMC-SC. Best estimate diagnosis for each patient was made independently by two psychiatrists (including KSH in every case) based on information obtained by direct interviews, hospital records, and interviews with relatives and/or referring psychiatrists. Final diagnoses were decided by consensus, and when consensus was not reached the cases involved were excluded from the analysis.
We limited the diagnosis of probands to schizophrenia as defined by the DSM-IV criteria. In assigning affectation status, we adopted two liability classes. For the “broad” class, affected individuals (other than probands) were diagnosed as having schizophrenia, schizoaffective disorder, or schizotypal personality disorder according DSM-IV criteria. For schizoaffective disorder and schizotypal personality disorder, only those cases maintained on long-term antipsychotics were included. The “narrow” class included those families in which all affected individuals met the DSM-IV criteria for schizophrenia. After screening for bilineal transmission of psychoses and Mendelian inconsistency (performed by evaluating more than ten microsatellite marker genotypes), 56 families with 183 members including 123 affected individuals were analyzed (Table I). Forty-six of these families had been included in previous linkage studies by the authors, that is, Kim et al. 2006 and Jang et al. 2007. This study was approved by the institutional review boards (IRBs) of Samsung Medical Center and Yong-In Mental Hospital. Informed consent was obtained from all subjects who participated in interviews and blood sampling.
| Family type | Number of families | |
|---|---|---|
| Broad class | Narrow class | |
| Two affected siblings | 14 | 10 |
| Two affected siblings + one unaffected parent | 15 | 13 |
| Two affected siblings + one unaffected grandparent | 1 | 1 |
| Two affected siblings + one unaffected sibling | 2 | 2 |
| Three affected siblings | 2 | 0 |
| Two affected siblings + two unaffected parents | 8 | 7 |
| Two affected siblings + one unaffected parent + one unaffected sibling | 2 | 2 |
| Affected aunt-niece/nephew pair + two unaffected relatives | 2 | 2 |
| Three affected siblings + one unaffected parent | 2 | 2 |
| Three affected siblings + one unaffected sibling | 1 | 1 |
| Two affected siblings + one affected parent + one unaffected parent | 1 | 1 |
| Two affected siblings + one unaffected parent + two unaffected siblings | 1 | 1 |
| Two affected siblings + one affected parent + two unaffected siblings | 1 | 1 |
| Three affected siblings + two unaffected parents | 2 | 2 |
| Two affected siblings + one affected parent + one unaffected parent + one unaffected sibling | 1 | 1 |
| Three affected siblings + two unaffected parents + one unaffected sibling | 1 | 1 |
| Total | 56 | 47 |
Genotyping
Illumina's Linkage_IV_B_Genetic_Map_v2_RevD panel was used for genome-wide SNP scanning (Illumina Inc.). This panel includes 6,008 SNP markers distributed evenly across the genome. The genetic map position of each SNP in the panel was determined by linear interpolation using NCBI build 35physical map position and a high-resolution STR genetic deCODE map. Mean and median intervals between markers were 488 kb (0.62 cM) and 315 kb (0.38 cM), respectively.
Prior to genotyping, yields of pure double strand genomic DNA samples were determined using Quant-iT™ PicoGreen® dsDNA Assay Kits (Eugene, OR). Samples were then normalized to 50 ng/µl. Genotyping reactions were performed using Illumina Bead-Station kit reagents and protocols [Fan et al., 2003]. Normalized genomic DNA (5 µl) from each sample was used as a template for Illumina Goldengate® Genotype Assays. SNP arrays were scanned using Illumina Bead Array Reader (Illumina Inc.) under the Illumina Bead Station 500G system version 2.3 [Oliphant et al., 2002].
Statistical Analysis
To eliminate some SNPs deemed to have genotyping errors or inadequate allele frequencies, we performed a pedigree-check and Hardy–Weinberg equilibrium testing on 6,008 SNP markers. Mendelian inheritance errors were first checked in each pedigree using PedCheck1.1 and 455 erroneous SNPs were excluded. We also utilized PEDSTATS for Hardy–Weinberg equilibrium testing and discarded 180 SNPs with P values <0.05. Finally, we had 5,373 (89.4% of 6,008) SNPs for analysis. Mean and median inter-marker distances between the 5,373 SNP loci were 575 kb (0.69 cM) and 339 kb (0.42 cM), respectively.
For multipoint non-parametric linkage analysis, we used MERLIN1.0.0 (Multipoint Engine for Rapid Likelihood Inference), which uses sparse inheritance trees for pedigree analysis [Abecasis et al., 2002]. MERLIN treats multiple affected individuals in families using a non-parametric method that examines all individuals in a family simultaneously, and assigns a higher score when more of them share the same allele by descent. A recent modification of MERLIN (MERLIN1.0.0) [Abecasis and Wigginton, 2005] enables it to correctly account for linkage disequilibrium from closely spaced marker sets that may inflate linkage statistics. Accordingly, MERLIN1.0.0 provides accurate solutions in dense maps containing thousands of markers. The MERLIN map file (an input file) was constructed based on deCODE genetic map distances. Marker allele frequencies were automatically calculated by the program using the genotype data of family founder members.
To evaluate the genome-wide significances of our findings, we performed an empirical simulation of genome-wide linkage analysis for 10,000 replicates of families identical to those in our sample using MERLIN. MERLIN generated simulated chromosomes using the original data structure in terms of marker informativeness, marker spacing, and missing data patterns. We investigated how many NPL Z score peaks were expected to occur in genome-wide linkage scans in the absence of linkage, and regarded several peaks in one chromosome as different local peaks if they were more than 30 cM apart. The genome-wide significance level was adjusted because we tested two phenotype classes. In this simulation, an NPL Z of 2.84 was observed at a rate of 0.024 (239/10,000) for the broad class and at a rate of 0.023 (232/10,000) for the narrow class. Therefore, these values correspond to significant thresholds for genome-wide significance, as defined by Lander and Kruglyak 1995.
RESULTS
An average minor-allele frequency of 0.28 and a mean heterozygosity of 0.34 were observed for the SNP markers used in the analysis. In the analyzed Korean population, 149 SNPs were not polymorphic. Information contents (a measure of how informative a marker or a map of markers is in a collection of pedigrees in order to extract the maximum amount of inheritance information for linkage analysis) in the current analyses were 0.57–0.93 (mean = 0.82) for the broad class and 0.58–0.96 (mean = 0.84) for the narrow class (excluding X chromosome, which showed much lower information content).
NPL Z scores over whole chromosomes are presented in Figure 1. Eight regions with NPL Z scores of greater than 2.0 were found for “broad” or “narrow” phenotype classes in eight different chromosomes (chromosomes 1, 2, 3, 4, 6, 13, 18, and 20). Detailed information on peak regions is summarized in Table II. Two chromosomal loci, that is, 2p24.3 and 6q27, reached genome-wide significance (P < 0.025, NPL Z > 2.84). The highest NPL Z peak was at 33.41 cM of chromosome 2, which corresponded to an NPL Z of 3.18. The majority of peak regions listed in Table II were supported by several SNPs (for details, see Supplementary Table I).
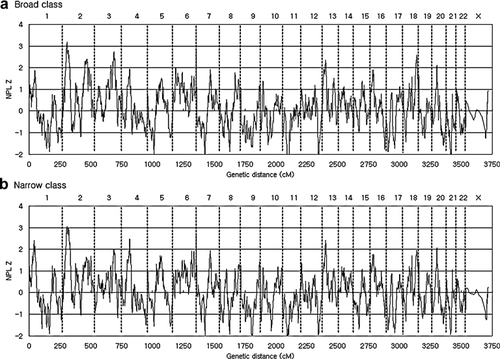
Genome-wide multipoint nonparametric linkage Z (NPL Z) scores for Korean schizophrenia families. The X-axis indicates accumulated deCODE cM distance and the Y-axis indicates NPL Z scores calculated by MERLIN. Vertical lines differentiate the chromosomes. a: Data from ‘broad’ phenotype class, (b) Data from ‘narrow’ phenotype class.
| Chromosome region | Peak SNP | Information content: broad class (narrow class) | Positiona (cM) | Position (Mb) | NPL Z (nominal P-value) | Peak regionb (Mb) | |
|---|---|---|---|---|---|---|---|
| Broad class | Narrow class | ||||||
| 1p36.12 | rs861212 | 0.87 (0.88) | 40.62 | 21.7 | 1.48 (0.07) | 2.43 (0.008) | 18.0−33.1 |
| 2p24.3 | rs1521247 | 0.87 (0.90) | 33.41 | 13.0 | 3.18 (0.0007) | 3.06 (0.0011) | 10.0−31.3 |
| 3q24 | rs1381768 | 0.87 (0.88) | 154.2 | 149.3 | 2.73 (0.003) | 2.02 (0.02) | 135.0−158.6 |
| 4p14 | rs278973 | 0.80 (0.84) | 60.32 | 40.2 | 1.96 (0.03) | 2.49 (0.006) | 24.4−56.9 |
| 6q27 | rs909475 | 0.75 (0.75) | 189.6 | 170.7 | 2.90 (0.002) | 2.71 (0.003) | 169.2−170.8 |
| 13q12.3 | rs720083 | 0.79 (0.82) | 23.84 | 29.2 | 2.36 (0.009) | 2.43 (0.008) | 24.2−31.5 |
| 18q22.3 | rs1943919 | 0.81 (0.82) | 105.45 | 69.8 | 2.59 (0.005) | 1.94 (0.03) | 62.3−72.3 |
| 20p12.2 | rs803880 | 0.81 (0.84) | 35.07 | 11.8 | 2.13 (0.02) | 2.08 (0.02) | 10.2−15.5 |
- a Genetic distance from p-terminus based on the deCODE high resolution map.
- b Peak region means the LOD-1 support interval corresponding to the local maximum LOD minus 1.
DISCUSSION
Genome-wide linkage scanning of Korean multiplex schizophrenia families using 6K SNP markers revealed eight chromosomal regions with NPL Z scores of greater than 2.0 (Table II). Genome-wide significance was attained for 2p and 6q. Highest NPL Z scores (3.18 for the broad and 3.06 for the narrow phenotype classes) in the current study occurred in the region of chromosome 2p24.3. This region precisely overlaps a schizophrenia susceptibility locus with an overall maximized heterogeneity LOD score of 4.5 (D21400–D2S1360, 29.3–39.7 cM of chromosome 2 according to the UCSC genome browser) reported by a genome-wide linkage scan of five extended pedigrees from Daghestan genetic isolates [Bulayeva et al., 2007]. A small peak LOD score for this region with a nominal P-value of less than 0.05 (P = 0.014) was also observed in schizophrenia families in an isolated Costa Rican population [DeLisi et al., 2002].
Interestingly, our linkage findings are similar to those of a genome-wide analysis of extended schizophrenia families in a Palauan population, an isolated population in Micronesia [Camp et al., 2001]. Their second highest peak with evidence of linkage and genome-wide significance was observed at 13q12–22 (LOD = 3.6 under the recessive model) which overlaps with our peak at 13q12.3 (NPL Z > 2.0). Their fourth region of interest showing suggestive evidence of linkage (3q24–28, LOD = 2.0 under the recessive model) also overlaps with our peak at 3q24 with an NPL score of 2.73. Their most positive peak was also observed on chromosome 2p, but this peak was located on 2p13–14 (NPL = 6.5 and LOD = 4.8 under the recessive model) which is around 50 cM distanced from our peak on 2p. Palauans originated from Southeast Asian [Cavalli-Sforza et al., 1994]. A Y chromosome study among Oceania populations showed that the Korean population shares a specific haplogroup with Southeast Asians [Kayser et al., 2001], which might explain the coincidence of linkage peaks found in Palauan and Korean patients.
As compared with the results of a recent meta-analysis by Lewis et al. 2003, our peaks at 20p12.2 and 18q22.3 partly overlap two bins with evidence of linkage to schizophrenia, that is, bin 20.2 (20p12.3–p11, rank 11 in weighted analysis, Pord = 0.0098) and bin 18.4 (18q22.1-qter, rank 14 in weighted analysis, Pord = 0.0103), respectively. In addition, our results are also consistent with a number of other genome-wide scans that have reported linkage signals on 13q12-13 [Shaw et al., 1998; Lindholm et al., 2004] and 1pter-p36 [Escamilla et al., 2007].
In contrast to multiple overlaps of our linkage loci of schizophrenia with those reported in isolated populations, our results are inconsistent with recent genome-wide scans in the Japanese [Arinami et al., 2005] and Taiwanese [Faraone et al., 2006] populations. Furthermore, linkage peaks in these two populations also showed no overlap. Although some results are probably false positives or negatives, one possible interpretation of this discrepancy is disease gene heterogeneity across ethnic groups even among northeastern Asian populations.
Two diagnostic classes (“broad” and “narrow”) were used in the present analysis. For chromosomal loci showing linkage evidence with genome-wide significance, higher NPL Z scores were observed when a broader category of affected status was used. This may have been due to the larger sample size of the broad group. On the contrary, some other loci generated higher NPL Z scores for the narrow group (Table II), but for these loci, significance levels did not exceed genome-wide thresholds. Further studies with larger sample sizes are needed to elucidate whether some chromosomal loci are specifically linked to the narrow phenotype class.
Previously the authors performed locus by locus linkage analysis for several chromosomal regions for which linkage findings have been replicated in schizophrenia families [Kim et al., 2006; Jang et al., 2007]. In these prior studies, we used microsatellite markers, and the subjects of these studies were also included in the present study. We observed linkage signals with nominal significance at 8p12 [Kim et al., 2006] and 1q32 [Jang et al., 2007]. However, these regions did not show further evidence of linkage in the present study, which suggests they make a low contribution to the genetic components in Korean schizophrenia patients.
In conclusion, we present genome-wide linkage scan data for schizophrenia, which was generated by analyzing Korean multiplex schizophrenia families identified using a homogenous evaluation system at a single research center. We found two regions that satisfy genome-wide criteria (P < 0.025) for linkage on chromosomes 2p24.3 and 6q27, and six more loci, that is, 3q24, 18q22.3, 13q12.3, 20p12.2, 4p14, and 1p36.12, for which NPL Z scores were higher than 2.0. Although linkage to these loci has not received prominent attention in studies on Caucasian families, multiple overlaps were observed between our loci (on 2p, 3q, and 13q) and linkage peaks generated from extended families in various isolated populations. These loci are worth pursuing, in terms of replication in other populations and the detection of candidate genes within these regions.
Acknowledgements
This study was supported by the Korean HapMap Project of the Korean Ministry of Science & Technology, and by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A040042). The study sponsors had no involvement in any stage of the study or in the decision to submit this manuscript for publication.




