Genome scan in sibling pairs with juvenile-onset mood disorders: Evidence for linkage to 13q and Xq†
Please cite this article as follows: Wigg K, Feng Y, Gomez L, Kiss E, Kapornai K, Tamás Z, Mayer L, Baji I, Daróczi G, Benák I, Osváth VK, Dombovári E, Kaczvinszk E, Besnyő M, Gádoros J, King N, Székely J, Kovacs M, Vetró Á, Kennedy JL, Barr CL. 2009. Genome Scan in Sibling Pairs With Juvenile-Onset Mood Disorders: Evidence for Linkage to 13q and Xq. Am J Med Genet Part B 150B:638–646.
Abstract
Mood disorders (bipolar and depressive disorders) in children and adolescents are associated with significant morbidity and mortality. Twin and family studies, for the most part, indicate higher familiality and heritability for mood disorders that onset in childhood/adolescence than those that onset in adulthood. To identify the genetic contribution to mood disorders that onset in childhood/adolescence, we performed a genome scan on 146 nuclear families from Hungary containing an affected proband and affected siblings. In total, the pedigrees contained 303 affected children: 146 probands, 137 siblings with a first episode of mood disorder before 14.9 years of age, and 20 siblings with onset of their first episode after 14.9 years of age but before the age of 18. The results of the genome scan using 405 microsatellite markers did not provide evidence for linkage at the recommended genome wide significance level for any novel loci. However, markers on two chromosomes, 13q and Xq, provided evidence for linkage in regions previously identified as linked to bipolar disorder in multiple studies. For the marker on chromosome 13q the peak non-parametric multipoint LOD score was at the marker D13S779 (LOD = 1.5, P = 0.004). On chromosome Xq, evidence for linkage was observed across a large region spanning two regions previously linked to bipolar disorder; Xq24 to Xq28, with a peak at marker TTTA062 (LOD 2.10, P = 0.0009) in Xq28. Results for these regions exceed the recommended P-value for a replication study of P < 0.01 and thus provide evidence for these two loci as contributing to mood disorders with juvenile onset. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Mood disorders are complex, impairing disorders with high prevalence and are associated with high mortality [Kessler et al., 2003]. Along with ischaemic heart disease and cerebrovascular disease, depression is one of the top three causes of disease burden in developed countries and fourth worldwide [Murray and Lopez, 1997]. Twin studies indicate moderate heritability of depressive disorders with a recent meta-analysis of five studies indicating heritability of 31–42% with shared environmental influences accounting for very little (<5%) of the variance [Sullivan et al., 2000]. The largest twin study of depression in adults (15,493 twin pairs) confirms these estimates, indicating heritability of 38% [Kendler et al., 2006]. Higher heritability (∼70%) has been indicated in a large, clinically ascertained sample compared to community based samples [McGuffin et al., 1996]. Higher heritability is also estimated for bipolar disorder (60–85%) [Faraone and Tsuang, 2003; Smoller and Finn, 2003].
Family studies support non-independence of depressive and bipolar disorders. A summary of family studies published from 1960 to 2001 calculated the age-corrected morbid risk for first-degree relatives of bipolar probands to be higher for unipolar disorder (14.1%) as well as bipolar disorder (8.7%) compared to controls (0.7% bipolar, 5.2% unipolar). Summary of studies of first-degree relatives of families with a unipolar proband indicate a modest morbid risk for bipolar disorder (2.2%) compared to the risk for unipolar disorder (17.9%) but higher than controls [Smoller and Finn, 2003]. In addition to family studies, limited twin studies support overlapping genetic influences with one twin study of depression and mania estimating a genetic correlation of 0.65 [McGuffin et al., 2003].
Family studies show the highest relative risk for mood disorders in families of probands with early age of onset and/or recurrent episodes compared with the risk in the general population [Rice et al., 1987; Strober et al., 1988; Moldin et al., 1991; Strober, 1992; Todd et al., 1993; Kovacs et al., 1997; Neuman et al., 1997; Kovacs and Devlin, 1998; Schurhoff et al., 2000; Kessler et al., 2001; Faraone et al., 2003]. We note that early-onset was defined differently across the studies. Twin studies of depressive symptoms in children/adolescents provide a wide range of heritability estimates dependent on age, instrument, and informant [Thapar and McGuffin, 1994; Todd and Botteron, 2001; Rice et al., 2002; Thapar and Rice, 2006]. The majority of studies have identified higher heritability of depression symptoms in child and adolescent twins, with a number of studies reporting heritability in the range of 60–80% [Thapar and McGuffin, 1994; Eaves et al., 1997; Hudziak et al., 2000; Happonen et al., 2002; Scourfield et al., 2003; Thapar and Rice, 2006]. Currently there are no published twin studies for bipolar disorder with childhood/early-adolescent onset but the increased familiality seen in family studies indicates that this phenotype is likely to be more heritable. Because of the indication of higher heritability and familiality with early-onset probands, a number of studies have focused on these types of families for genetic linkage and association studies [Zubenko et al., 2003; Holmans et al., 2004; Adams et al., 2005; Burcescu et al., 2005; Camp et al., 2005; Burcescu et al., 2006].
The increased familiality and heritability estimates for early-onset mood disorders indicate increased genetic loading but it is not known if this indicates a distinct genetic subtype [Kovacs, 1996; Kaufman et al., 2001]. Similarities exist in the clinical picture of child, adolescent, and adult onset cases although the relative frequency of specific symptoms has been found to differ by age [Ryan et al., 1987; Carlson and Kashani, 1988; Mitchell et al., 1988; Roberts et al., 1995; Kovacs, 1996]. Biological correlates identified in adult patients are less evident, or less consistently observed, in studies of children and adolescents [Kaufman et al., 2001]. Support of a genetic subtype has been suggested from some family studies that indicate that early-onset bipolar cases have more severe symptoms, more psychotic features, greater rates of comorbidity and a poorer response to lithium [Strober et al., 1988; Schurhoff et al., 2000; Spencer et al., 2001; Faraone and Tsuang, 2003; Faraone et al., 2003]. Thus, the study of families selected with early-onset cases may identify unique genetic risk factors contributing to early age of onset.
Only recently have linkage studies on mood disorders focused on age of onset as a phenotype [Zubenko et al., 2003; Holmans et al., 2004; Camp et al., 2005; McGuffin et al., 2005; Holmans et al., 2007; Levinson et al., 2007]. These previous studies indicate that early-onset depression provides an informative phenotype for analyses of mood disorders with novel loci identified that appear to replicate across samples (e.g., 15q [Holmans et al., 2007]) as well as replication of regions previously linked to bipolar disorder (e.g., 18q [Camp et al., 2005; McGuffin et al., 2005]). However, the definition of early-onset was defined over a wide age range for probands (≤25–31) even up to middle age for the age of onset for relatives (<41 years) [Zubenko et al., 2003; Holmans et al., 2004; Camp et al., 2005]. It is not known if the genetic contribution to early-onset as defined in the previous studies will differ genetically from onset in childhood/adolescence and further if juvenile-onset families represent a genetic subtype with unique genetic influences as has been previously indicated. Thus far there has been no reported genome scans on samples with onset of mood disorders restricted to childhood or adolescence.
In this study we performed a genome scan using 146 affected sibling pair families from Hungary with a proband with onset of a mood disorder (depressive or bipolar disorder) before 14.9 years of age. The affected siblings of the proband were selected to meet the same criteria; however, 20 of the 157 affected siblings that were included were unaffected at 14.9 years of age but developed a depressive episode before the age of 18.
MATERIALS AND METHODS
Families
This study was part of a larger multidisciplinary program project to identify risk factors in mood disorders that onset in childhood [Forbes et al., 2006; Liu et al., 2006; Perez-Edgar et al., 2006; Shaw et al., 2006; Silk et al., 2006; Kapornai et al., 2007; Kiss et al., 2007]. The families were recruited from 23 mental health facilities across Hungary. The Interview Schedule for Children and Adolescents-Diagnostic Version (ISCA-D) was used for the diagnosis of the probands and siblings for this study. The ISCA-D is an extension and modification of the ISCA [Sherrill and Kovacs, 2000]. Child psychiatrists or psychologists conducted the psychiatric assessments after completion of 3 months of training. Interviewers were trained to reach an average of 85% symptom agreement with “gold standard” ratings (provided by experienced trainers) on five consecutive videotaped interviews. Routine follow-up training sessions were held to minimize drift.
The probands were between the ages of 7 and 14.9 years of age when they were first evaluated for this study. The majority of the subjects (87%) were in a current episode of depression at their first assessment. Children were interviewed about their symptoms and parents were interviewed about their child. Approximately 1 month later both parents and children were interviewed separately again. Information on symptoms was obtained both for current (1 month before the interview) and past symptoms (prior to 1 month or alternatively prior to the last episode). The final diagnosis was based on the consensus diagnosis of two independent child psychiatrists.
Written informed consent for adults and assent for children was obtained from all participants as required by the Institutional Review Boards of the University of Pittsburgh, The University of Toronto, and in Hungary. Additional details of the diagnostic assessment, interviewer training, interrater reliability, and inclusion/exclusion criteria for the families enrolled in this study can be found in previous publications [Liu et al., 2006; Kapornai et al., 2007; Kiss et al., 2007].
Genotyping
DNA was extracted from blood samples using a standard high salt extraction method [Miller et al., 1988]. The microsatellite panel of markers was genotyped by Prevention Genetics (http://www.preventiongenetics.com/). A panel of 405 markers (Marshfield screening set 16) was genotyped at an average intermarker distance of 9.4 cM and with an average heterozygosity of 0.75.
Statistical Analysis
Genotyping errors were checked by first identifying Mendelian errors using the PedStats program (http://www.sph.umich.edu/csg/abecasis/PedStats/). Further data checking was performed using the error option of Merlin (multipoint engine for rapid likelihood inference, http://www.sph.umich.edu/csg/abecasis/Merlin/) [Abecasis et al., 2002] to identify potential double recombinants as a sensitive check for genotyping errors. All Mendelian errors and double recombinants were either resolved or removed from the analyses. We used the non-parametric multipoint statistics (NPL) [Kruglyak et al., 1996] and LOD score calculations proposed by Kong and Cox 1997 incorporated in Genehunter Plus and the modified Lander–Green algorithm that uses sparse inheritance trees incorporated in the program Merlin [Abecasis et al., 2002]. Graphs of the linkage results (Zlr) were created using easyLINKAGE [Lindner and Hoffmann, 2005]. Allele frequencies were calculated from the founders. The map distances were determined by Prevention Genetics using the July 2003 human reference sequence (NCBI Build 32), produced by the International Human Genome Sequencing Consortium (http://genome.ucsc.edu/cgi-bin/hgGateway). Map distances and information on this mapping panel are available from the Marshfield Genetics web site (http://research.marshfieldclinic.org/genetics/GeneticResearch/screeningsets.asp). For the markers mapped in UCSC, we verified the position of the markers relative to each other using the March 2006 human reference sequence (NCBI Build 36.1).
RESULTS
The analyses for this study were based on the genotypes of 146 families with 303 affected children. All of the probands met DSM-IV criteria for a mood disorder (depressive disorder or bipolar disorder), with the onset of the first episode by 14.9 years of age. Of the 157 affected siblings 137 met the same criteria of the probands with onset before 14.9 years of age and 20 siblings were unaffected at 15 but developed an episode of depression before the age of 18. Of the 303 siblings, 301 were full siblings and 2 were half-siblings. Eight of the families had three affected children and one family had four affected children. One family had two affected children and a half-sibling who was affected (the parents of the half-siblings were unavailable). DNA was available for both parents for 85 of the families and a single parent for the remaining 61 families. Of the children in the study, 52% were boys and 48% were girls. This is in agreement with the previous studies indicating that preadolescent males and females are either affected equally, or with a slight excess of males [Hankin et al., 1998; Kovacs et al., 2003]. There were 35 male–male pairs and 31 female–female pairs with the remainder being mixed sex pairs.
At the time of diagnosis, 2.3% of the children met the criteria for bipolar disorder, three probands, and six siblings. There were no sib pairs with both children diagnosed with bipolar disorder. Of the 20 siblings that had onset after the age of 15, two siblings had bipolar disorder. We did not exclude these known cases with bipolar disorder because, as discussed above in detail, family and twin studies indicate that bipolar and depressive disorders share substantial genetic overlap [McGuffin et al., 2003]. Further, based on previous longitudinal studies, we predict that ∼15–30% of the children who are currently diagnosed with a depressive disorder will develop bipolar disorder as they enter young adulthood [Strober and Carlson, 1982; Kovacs et al., 1994; Kovacs, 1996, 1997; Geller et al., 2001]. Because it is not possible to predict which of these children will develop bipolar disorder they cannot be excluded.
Using the non-parametric analyses we did not identify any markers with a LOD score >3.63 for genome wide significance for a genome scan of affected sibling pairs as recommended by previous guidelines [Lander and Kruglyak, 1995]. Results across all the chromosomes are shown in Figure 1 (Zlr shown by distance in cM according to the Prevention Genetics map distances). We identified seven markers with nominal evidence for linkage (P < 0.01; see Table I).
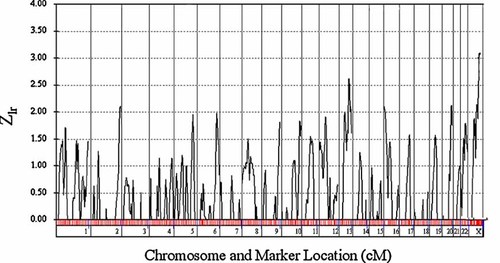
Zlr plot (Genehunter Plus) results for all chromosomes.
| Location | Locus | Positiona | MARKER | Mb | Merlin | Genehunter Plus | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z mean | P-value | LOD | P-value | Zlr | npLOD | P-value | |||||
| 2q37.2 | n/a | 235,684,273–235,684,431 | GATA23A02 | 236.31 | 1.94 | 0.030 | 1.06 | 0.0140 | 2.0431 | 0.6378 | 0.0432 |
| 12q12 | D12S1301 | 42,348,809–42,349,071 | GATA91H06M | 42.35 | 2.05 | 0.020 | 1.04 | 0.0140 | 1.9135 | 0.6497 | 0.0417 |
| 13q32.3 | D13S779 | 100,301,949–100,302,305 | ATA26D07 | 99.20 | 2.28 | 0.011 | 1.50 | 0.0040 | 2.6204 | 1.1183 | 0.0116 |
| Xq25 | n/a | 120,705,649–120,705,884 | GATA165B12 | 119.58 | 2.46 | 0.007 | 1.58 | 0.0030 | 2.0387 | 0.4498 | 0.0757 |
| Xq27.3 | n/a | 145,513,600–145,513,642 | TATC043 | 144.37 | 2.26 | 0.012 | 1.36 | 0.0060 | 2.5306 | 1.1948 | 0.0095 |
| Xq27.3 | DXS998 | 146,415,590–146,415,818 | 224ZG11 | 146.30 | 2.33 | 0.010 | 1.49 | 0.0040 | 2.6896 | 1.3323 | 0.0067 |
| Xq28 | n/a | 153,184,723–153,184,773 | TTTA062 | 153.05 | 2.48 | 0.007 | 2.10 | 0.0009 | 2.9895 | 1.4498 | 0.0049 |
- a Marker position according to UCSC March 2006 assembly.
On chromosome 13q, we identified the marker D13S779 with a multipoint non-parametric LOD score peak of 1.50 (P = 0.004; Fig. 2). This region of chromosome 13q, including this specific marker, has been linked to bipolar disorder in numerous studies [Detera-Wadleigh et al., 1999; Kelsoe et al., 2001; Liu et al., 2001; Shaw et al., 2003; McGuffin et al., 2005], using the criteria of P < 0.01 for significance, evidence for linkage in a replicated linkage region applies.
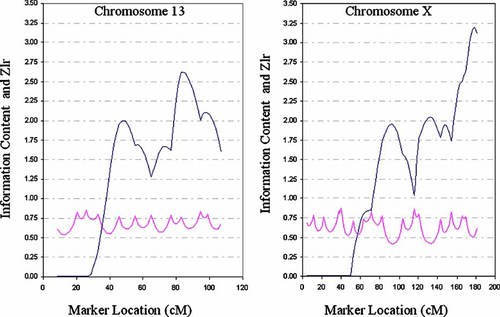
Zlr plot (Genehunter Plus) and information content for chromosomes 13 and X.
The most significant results from the genome scan were located across a broad region on chromosome Xq (Fig. 2) with the peak multipoint LOD score at the marker TTTA062 (npLOD score 2.10, P = 0.0009). This is in agreement with previous genome scans for bipolar disorder that indicate linkage to two regions of Xq; Xq24 [Pekkarinen et al., 1995] and Xq28 [Berrettini, 1998; Massat et al., 2002].
Two other markers provided multipoint non-parametric LOD scores >1, with peaks at the markers GATA23A02 on 2q37.2 and D12S1301 on 12q12 (Table I). For 2q37, the linkage study of Zubenko et al. 2002 for early-onset depression identified linkage to the marker D2S427 in 2q37.1 (reported as 2q35-36), located at ∼232 Mb (UCSC) and our peak marker, GATA23A02 located at ∼236 Mb [Zubenko et al., 2003]. Although only supported by this linkage report previously, further study of this region may be warranted. For the 12q12 region, there is no support from previous linkage studies; thus, in the absence of corroborating evidence, we conclude that this marker may represent a false-positive finding.
DISCUSSION
This is the first genome scan for families with both probands and siblings having onset of a mood disorder in childhood/adolescence. Previous genome scans have defined early-onset depressive disorders using a cut-off of onset of first episode before the age of 25 or 30 [Zubenko et al., 2003; Holmans et al., 2004; Camp et al., 2005], with relaxed criteria in some studies for the affected relatives (≤40). Family studies indicate that early age of onset identifies a more familial form of mood disorders but the age at which early versus late onset should be delineated is not clear. For example, one study found increased risk for bipolar disorder among relatives if the age of first affective symptoms for the probands was under 12 years of age (mean for the sample) compared to 13 or later [Pauls et al., 1992]. Recent data indicate that the age of onset distribution for bipolar disorder is a mixture of three Gaussian distributions with peaks at 16.9, 26.9, and 46.2 years of age with some support for differences in clinical characteristics for these groups (psychotic symptoms during affective episode, family history of affective illness, number of suicide attempts) [Bellivier et al., 2001]. Thus our study having restricted the age of onset to childhood and adolescence could potentially identify unique genetic risk factors contributing to this phenotype. Instead we found support for two previously reported regions.
Previous genome scans of bipolar disorder have implicated a number of chromosome regions with the most highly supported linkage results on chromosomes 1q31-32, 4p16, 6pter-p24, 6q16-25, 9p22.3-21.1, 10p14, 10q21-26, 12q23-24, 13q31-32, 14q24.1-32.12, 18p11, 18q21-23, 21q22, 22q11-13, and Xq24-28 [Badner and Gershon, 2002; Baron, 2002; Segurado et al., 2003; Craddock and Forty, 2006]. Linkage results for chromosome 13q provide evidence for two regions, a proximal (∼13q14) and a distal region (∼13q32-33) [Stine et al., 1997; Detera-Wadleigh et al., 1999; Kelsoe et al., 2001; Liu et al., 2001; Shaw et al., 2003]. The distal linkage region flagged in our study, 13q32-33, has shown linkage in several genome scans and selected linkage studies for mood disorders [Detera-Wadleigh et al., 1999; Kelsoe et al., 2001; Shaw et al., 2003] and supported by meta-analyses [Badner and Gershon, 2002]. Notably, Detera-Wadleigh et al. 1999 identified significant evidence for linkage (LOD 3.5) to 13q32 for the phenotype of bipolar I, bipolar II with major depression, schizoaffective disorder, and recurrent unipolar disorder. Fine mapping of this region identified the most significant evidence for linkage within the interval bounded by D13S779 and D13S225 [Liu et al., 2001]. Liu et al. 2003 identified a modest LOD score of 2.20 at the marker D13S779 for bipolar disorder. The genome scan of Kelsoe et al. 2001, and their follow-up study with the additional pedigrees and marker typing [Shaw et al., 2003], provided evidence for linkage to 13q32-33. The genome scan of recurrent major depression provided evidence for linkage at 13q31.1-q31.3 with the peak at D13S775, located 6.7 Mb from D13S779 [McGuffin et al., 2005]. A number of linkage studies have supported the Xq region as containing a susceptibility gene for bipolar disorder. The first studies were based on observations of X-linked inheritance in some families [Rosanoff et al., 1935], followed by evidence for linkage to Xq28 in the region of color blindness and glucose-6-phosphate-dehydrogenase (G6PD) deficiency (maximum LOD score 9.17 assuming heterogeneity) [Baron, 1977; Baron et al., 1987]. Follow-up studies, using more informative molecular genetic markers and some updated diagnoses, reduced the significance of that linkage finding (maximum LOD <3) [Baron et al., 1993; Baron, 2001]. Based on these findings, the chromosome Xq28 region became the focus of several linkage studies with some studies supporting this region [see for summary of studies Berrettini, 1998; Massat et al., 2002].
Chromosome Xq became the focus of study again after a genome scan of a large family with bipolar disorder from Finland indicated evidence for linkage to a 19 cM interval of Xq24-q27.1, with the highest LOD score for the marker DXS994 (130.15 Mb UCSC) [Pekkarinen et al., 1995]. However, this region was located proximal to the previous linkage region reported on Xq28. The region was followed up with increased density of marker typing in the large family as well as in 40 additional families. The marker with the best result in that study was DXS1047 (128.90 Mb UCSC) on Xq25 located 1.2 cM proximal to DXS994 [Ekholm et al., 2002]. Notably the study of select chromosomes using the NIMH Genetics Initiative pedigrees identified evidence for linkage in the first 97 families to the markers DXS1047 (Xq25) and GATA31E08 (DXS2390) at Xq27.1 [Stine et al., 1997]. Our relatively broad linkage peak supports linkage across the region of Xq24-28, identified in previous linkage regions.
Our previous association studies of the nuclear families (sib pairs and trios) from Hungary, that included these sibling pair families, identified association to several genes including brain derived neurotrophic factor (BDNF; 11p14.1) [Strauss et al., 2005], neurotrophic tyrosine kinase receptor 3 (NTRK3; 15q25.3) [Feng et al., 2008], arginine vasopressin (AVP, 20p13) [Dempster et al., submitted], and the arginine vasopressin 1b receptor (AVPR1B; 1q32.1) [Dempster et al., 2007]. No evidence was found for linkage to these regions in this genome scan. This is not particularly surprising given that for the association studies we were able to use our collection of trios (currently >600) in addition to these sibling pair families, a much larger sample. For the study of each particular gene we used the currently available families ranging in number from 258 nuclear families for studies published in 2005 [Strauss et al., 2005] to 603 for studies completed in 2007 [NTRK3 Feng et al., 2008]. In addition to the larger sample of families available for association studies, linkage generally has reduced power to identify susceptibility genes of minor effect for common risk alleles compared to methods that rely on linkage disequilibrium [Risch and Merikangas, 1996]. However, in some circumstances linkage can be a more powerful approach, for example, when there is low linkage disequilibrium between the tested markers and the risk alleles, and allelic heterogeneity with risk alleles residing on different haplotypes.
We have followed up the linkage results using association studies of key candidate genes in the entire sample of families. In the chromosome 13q region we looked at association of the G72/G30 gene, which shows association to bipolar disorder in multiple studies [Hattori et al., 2003; Chen et al., 2004; Detera-Wadleigh and McMahon, 2006; Williams et al., 2006]. We found evidence for association to three markers within this gene that had been previously reported to be associated with bipolar disorder 2008. Although, after correction for multiple testing, the results were no longer significant, this gene should be considered for further study as a candidate to juvenile-onset mood disorders based on the prior evidence.
Within the Xq region, two genes have previously been reported as associated with bipolar disorder [Ekholm et al., 2002; Zubenko et al., 2003]. We have looked at these two genes: GABRA3 and GPR50, but neither has provided significant evidence of association in our entire sample of families [Feng et al., 2007; Feng et al., unpublished work].
Previous genome scans for early-onset mood disorders have implicated a number of chromosomal regions with some overlap between studies. Of note is the chromosome 15q region that has provided some evidence for linkage in samples of families with early-onset depression [Holmans et al., 2004; Camp et al., 2005] and one with recurrent depression [McGuffin et al., 2005]. In our study, we found no evidence for linkage to this region although an association study of the NTRK3 gene in this region provides evidence for association (allele frequency of the associated alleles ∼0.40–0.50) in our larger sample [Feng et al., 2008]. Instead, the two regions we found with evidence for linkage were regions that have been identified in previous genome scans for bipolar disorder in samples where the probands were unselected for age. This was an unexpected finding given that, based on previous longitudinal studies, ∼20–30% of these children is expected to develop bipolar disorder [Strober and Carlson, 1982; Kovacs et al., 1994; Kovacs, 1996; Kovacs, 1997; Geller et al., 2001], and thus would represent a minority of cases in our sample.
Sampling variation could be a factor with over-representation of families linked to a specific region included in the genome scan by chance. Alternatively, the pedigrees linked to the chromosome 13q and Xq regions in previous studies may represent a population more similar to ours, either in ethnicity, or in clinical features. Interestingly, the linkage study of the X chromosome by Baron et al. 1990, indicated that the X-linked families had an earlier age of onset (4–10 years), a higher ratio of bipolar illness to unipolar illness, as well as a more recurrent and severe form of unipolar illness.
In summary, our genome scan of juvenile-onset mood disorders points to two chromosomal regions previously identified as linked to bipolar disorder, 13q and Xq, supporting continuity for child and adult genetic risks at least for these two loci. Age specific genetic risk factors may still be identified with larger samples with increased power.
Acknowledgements
The National Institute of Mental Health Program Project grant, MH 56193 and the National Alliance for Research on Schizophrenia and Depression, supported this work.




