Allelic variants in HTR3C show association with autism†
Please cite this article as follows: Rehnström K, Ylisaukko-oja T, Nummela I, Ellonen P, Kempas E, Vanhala R, von Wendt L, Järvelä I, Peltonen L. 2009. Allelic Variants in HTR3C Show Association With Autism. Am J Med Genet Part B 150B:741–746.
Abstract
Autism spectrum disorders (ASDs) are severe neurodevelopmental disorders with a strong genetic component. Only a few predisposing genes have been identified so far. We have previously performed a genome-wide linkage screen for ASDs in Finnish families where the most significant linkage peak was identified at 3q25-27. Here, 11 positional and functionally relevant candidate genes at 3q25-27 were tested for association with autistic disorder. Genotypes of 125 single nucleotide polymorphisms (SNPs) were determined in 97 families with at least one individual affected with autistic disorder. The most significant association was observed using two non-synonymous SNPs in HTR3C, rs6766410 and rs6807362, both resulting in P = 0.0012 in family-based association analysis. In addition, the haplotype C-C corresponding to amino acids N163-A405 was overtransmitted to affected individuals (P = 0.006). Sequencing revealed no other variants in the coding region or splice sites of HTR3C. Based on the association analysis results in a previously identified linkage region, we propose that HTR3C represents a novel candidate locus for ASDs and should be tested in other populations. © 2008 Wiley-Liss, Inc.
Autistic disorder is a severe neurodevelopmental disorder with onset in early childhood. Together with Asperger syndrome (AS) and atypical forms of autism it belongs to the autism spectrum disorders (ASDs), characterized by abnormalities in social, communication, and behavioral domains. At present, the population prevalence of ASDs is estimated to be 0.5–1% of the population, with prevalence estimates for autistic disorder around 10–20/10,000 [Fombonne, 2005; Baird et al., 2006]. A strong genetic component in autism has been established in twin studies, and family studies show a high-recurrence risk for siblings of probands with autism [Bolton et al., 1994; Bailey et al., 1995].
At least 14 genome-wide screens have been performed to identify chromosomal regions containing genes predisposing to ASDs. These studies have resulted in linkage signals on almost every chromosome [for a review, see Yang and Gill, 2007]. However, only a few regions, including 7q, 2q, 16p, and 17q have been replicated in several linkage studies, and meta-analysis provides best support for the role of 7q in ASDs [Trikalinos et al., 2006]. The putative susceptibility regions identified in the genome scans have been further studied by fine mapping and candidate gene studies at the linkage peaks. These studies have typically prioritized genes involved in neuronal development or signaling, although other positional candidates have also been investigated. To date, no confirmed susceptibility gene with common variation has been identified as a predisposing factor to ASDs. Rare, high-risk variants have been identified in X-chromosomal neuroligin 3 and neuroligin 4 in a few families with ASDs and/or mental retardation [Jamain et al., 2003; Laumonnier et al., 2004]. Rare mutations or chromosomal aberrations have been identified in SHANK3 and neurexin 1 [Durand et al., 2007; Szatmari et al., 2007], and the protein products of these genes interact with neuroligins at the synapse revealing the first molecular pathway connected to the etiology of ASDs. The role of rare chromosomal rearrangements has been well-documented in the etiology of ASDs [Vorstman et al., 2006]. In addition, both rare and recurrent copy number variants (CNVs) have recently been reported to confer susceptibility to ASDs [Szatmari et al., 2007; Kumar et al., 2008; Weiss et al., 2008]. All these results point towards a complex genetic model for genetic susceptibility to ASDs, and warrants a broad range of approaches to unravel novel genetic variants involved in the etiology of these disorders. Only one susceptibility gene, CNTNAP2 [Alarcon et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008], has been identified at a linkage peak and repeatedly shown to be involved in the etiology of ASDs. Although several high-density SNP scans are currently being performed in ASDs, the previously identified linkage peaks warrant more thorough analysis to identify novel susceptibility genes. As the molecular mode of gene action in ASDs has only been hinted at by the identification of a few rare variants, a methodical fine mapping of linkage peaks could identify novel pathways involved in the etiology of ASDs.
We have previously performed a genome-wide linkage scan for loci predisposing to ASDs in Finnish families. The most prominent linkage peak was identified at 3q25-27, with a maximum two-point LOD score of 4.31 at D3S3037 [Auranen et al., 2002]. Further, we reported that variants in neuroligin1 (NLGN1), at 3q26, are not associated with autism in Finnish families [Ylisaukko-oja et al., 2005]. In addition, we have reported a negative mutation analysis of ATP13A4 at 3q29 [Kwasnicka-Crawford et al., 2005]. Thus no evidence for the involvement of previously identified rare autism genes has been established in the nationwide study sample of Finnish autism families. In order to further characterize the role of 3q25-27 in ASDs, here we have investigated 11 functionally relevant candidate genes at 3q25-27 for association with autistic disorder.
The families were recruited via Finnish university and central hospitals. Detailed clinical and medical examinations were performed by experienced child neurologists as described elsewhere [Auranen et al., 2002]. Diagnoses were based on ICD-10 [World Health Organization, 1993] and DSM-IV [American Psychiatric Association, 1994] diagnostic nomenclatures. Families with known associated medical conditions or chromosomal abnormalities were excluded from the study. A total of 97 families, with one to three individuals affected with autistic disorder were included in this study. The total number of genotyped subjects is 356, and 118 of these are affected with autistic disorder. Families were used as they provide greater power for association analysis compared with case-control design [Goring and Terwilliger, 2000; Thornton and McPeek, 2007]. The families in this study include 32 of the 38 families included in the Finnish genome-wide scan for ASDs where linkage to 3q25-27 was first reported [Auranen et al., 2002]. Only individuals diagnosed with autistic disorder were assigned affected status. Informed written consent was obtained from participating individuals or their parents. The study has been approved by the Ethical Committee of the Hospital of Children and Adolescents, Helsinki University Hospital.
For candidate gene selection, all known genes at the linkage peak at 3q25-27 were identified, and their function was explored in silico through data-base searches (UCSC http://genome.ucsc.edu, PubMed http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=PubMed, OMIM http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM&itool=toolbar). Candidate genes are presented in Table I.
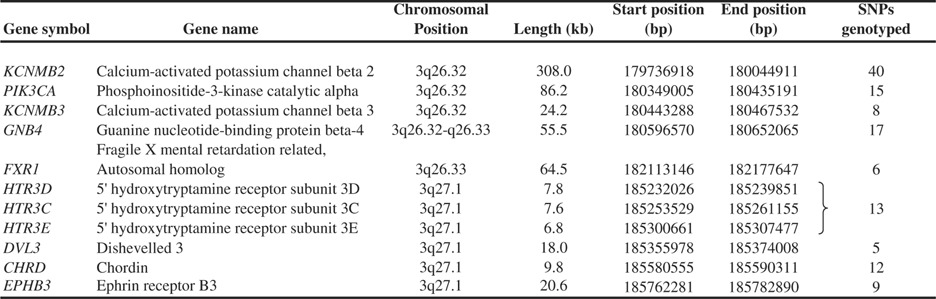
- kb, kilobase pairs.
- Positions correspond to NCBI build 36.1.
SNPs for genotyping were selected from the HapMap web-site (www.hapmap.org/index.html.en) and dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/). The Tagger algorithm implemented in HaploView was used to select SNPs tagging the majority of common variation in the candidate genes [Barrett et al., 2005]. In addition, all non-synonymous SNPs with a verified allele frequency in dbSNP at the time of assay design were included. A total of 125 SNPs were polymorphic and successfully genotyped (see Supplementary Table I). Genomic sequences for SNP primer design were obtained from dbSNP. SNP genotyping was performed using allele-specific primer extension on microarrays performed as described elsewhere [Pastinen et al., 2001; Ylisaukko-oja et al., 2005] and using the Sequenom iPLEX platform (Sequenom, San Diego, CA) according to manufacturers' instructions. Mendelian inheritance of genotypes was checked using PEDCHECK [O'Connell and Weeks, 1998], and Hardy–Weinberg equilibrium (HWE) was tested for all SNPs using a two-tailed χ2 test. Coding regions and exon–intron junctions of FXR1 and HTR3C were sequenced to identify possible rare variants in linkage disequilibrium with the genotyped SNPs. PCR and sequencing were performed as described elsewhere [Ylisaukko-oja et al., 2005]. Primers for the genotyping as well as sequencing are available from the authors at request.
We used PSEUDOMARKER and HBAT for family-based association analyses [Goring and Terwilliger, 2000; Horvath et al., 2001]. In PSEUDOMARKER analysis, the test of LD conditional on linkage was used, as linkage in the tested region has been observed using a subset of the families included in this study. Likelihood ratio tests implemented in PSEUDOMARKER have been shown to be stochastically equivalent to the commonly used haplotype relative risk and transmission/disequilibrium tests when performed on trios, but are generalized for analysis of extended pedigrees. Both recessive and dominant PSEUDOMARKER analyses were conducted. We used HBAT for haplotype association tests, as haplotype analysis is not implemented in PSEUDOMARKER. The empirical variance option of HBAT was used to correct for linkage in multiplex families. Haploview was used to determine LD between markers; haplotype blocks were constructed using the solid spine of LD method using a D′ threshold of 0.8 [Barrett et al., 2005]. In all analyses an affected only approach was employed.
To test post hoc if the most significantly associated SNPs could partly account for the linkage at 3p25-27 identified in the original genome-wide screen, we used the genotype-IBD Sharing Test (GIST) implemented in the GIST software package [Li et al., 2004]. GIST is used to test if affected individuals with a certain genotype contribute more than expected to the linkage evidence at a locus. Analyses were performed for all families from the original genome-wide linkage scan which were also included in this study (32 of 38 families). Analyses were performed using results for multipoint NPL scores from the original genome-wide linkage scan [Auranen et al., 2002].
We analyzed a total of 125 SNPs in 11 positional candidate genes at 3q25-27 in 97 Finnish families (see Supplementary Table I). These SNPs were chosen to efficiently capture the allelic diversity of the genes among Finns based on the haplotype block structure from the HapMap Caucasian sample. None of the SNPs was found to deviate from HWE (P > 0.01). Results from PSEUDOMARKER analysis using a dominant model are shown in Table II and Supplementary Table I. Analysis using a recessive model resulted in general in less significant P-values (data not shown). The most significant association was observed with two non-synonymous SNPs in HTR3C, rs6766410 (N163K) and rs6807362 (G405A), both resulting in P = 0.0012 in dominant PSEUDOMARKER analysis. The non-synonymous variants are common SNPs, with minor allele frequencies (MAF) ranging from 0.42 to 0.49. The effects of the substitutions on the protein structure was modeled using Polyphen (http://www.bork.embl-heidelberg.de/PolyPhen/), but were not predicted to result in significant structural of functional changes. However, the replacement of a neutral asparagine with a basic lysine could confer functional changes. GIST analysis for the two non-synonymous SNPs in HTR3C resulted in non-significant P-values (P = 0.15 for rs6766410 and P = 0.30 for rs6807362 using a dominant model). In addition, the association analysis revealed several SNPs in FXR1, two SNPs in KCMNB2 and one SNP in HTR3E and GNB4 respectively, showing suggestive association to autistic disorder (P < 0.05).
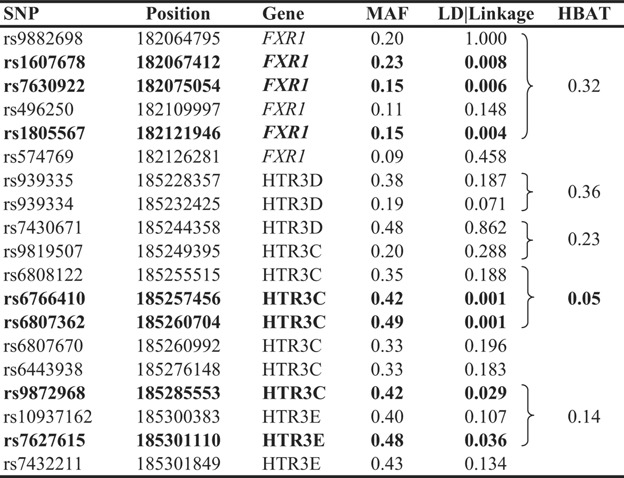
- For single marker analysis, a family-based, combined test for linkage and association (LD|linkage) was performed using PSEUDOMARKER software. Family-based haplotype association analysis was performed using HBAT. All results are given as P-values, which are not corrected for multiple tests and P-values <0.05 are indicated in bold text.
To extract maximum possible information from the genotype data, we constructed haplotype blocks for the genes using HaploView. Haplotype association was tested within the blocks using HBAT (Table II and Supplementary Table II). The haplotype block containing the two non-synonymous SNPs showing the most promising results in single-marker tests, composed of rs6808122, rs6766410 and rs6807362, yielded suggestive association with autistic disorder (P = 0.050). The two-marker haplotype consisting of the two non-synonymous SNPs in this block (rs6766410 and rs6807362) also showed suggestive association with autistic disorder (P = 0.020). The haplotype C-C, corresponding to amino acids N163 and A405 was overtransmitted to individuals with autistic disorder (P = 0.006), whereas the haplotype A-G, corresponding to amino acids K163 and G405 showed suggestive undertransmission to individuals with autistic disorder (P = 0.014). No other haplotypes resulted in evidence for association with autistic disorder.
To identify possible rare variants located in FXR1 and HTR3C contributing to autism susceptibility tagged by SNPs identified in the association analysis, we sequenced coding regions and splice sites in seven individuals with autistic disorder, from families contributing to the original linkage finding [Auranen et al., 2002]. No novel variants were identified in the sequence analysis.
In this study we have investigated 11 positional and functional candidate genes for association with autistic disorder in 97 Finnish families. Family-based association analyses of single SNPs and haplotypes resulted in suggestive evidence for association between two non-synonymous SNPs in HTR3C and autistic disorder. These two SNPs are in high LD (D′ = 0.96 in the HapMap CEU data) and are located within the same haplotype block. The two-marker haplotype constructed from these markers as well as the haplotype comprised of the three tag-SNPs within this block (rs6808122, rs6766410, and rs6807362) resulted in suggestive evidence of association with autistic disorder when tested with HBAT. These two SNPs are located approximately 6 Mb from D3S3037, the best microsatellite markers in the Finnish genome-wide scan for ASDs. Interestingly, GIST analysis indicated that these SNPs by themselves do not entirely account for the linkage observed in the original study, suggesting that more than one susceptibility gene for ASDs is located in the 3q25-27 region. This is also supported by the fact that several SNPs in FXR1 and KCMNB2 showed suggestive association with autistic disorder, and replications in larger study samples are needed to confirm the role of HTR3C in autism.
HTR3C has been shown to have a wide expression pattern, including both adult and fetal brain [Niesler et al., 2003], making it a plausible candidate gene for ASDs. The function of HTR3C is still unknown, but data from the two well-characterized subunits of this receptor, HTR3A and HTR3B, show that the heteromeric HTR3A/HTR3B receptor displays different functional properties compared to the homomeric HTR3A receptor [Davies et al., 1999]. It has recently been shown, that HTR3C, HTR3D and HTR3E subunits are able to form heteromeric receptors with HTR3A, and modulate receptor function and properties [Niesler et al., 2007]. A specific expression pattern for HTR3C in the CNS has not been established, and it will be interesting to see whether HTR3C is co-expressed with HTR3A or HTR3B in certain parts of the CNS where it could contribute to development by modifying channel function.
Evidence for the role of the serotonin system in ASDs is provided by reports of reduced obsessive-compulsive routines, anxiety, and aggression in individuals with autistic disorder after administration of serotonin reuptake inhibitors [Hollander et al., 2003]. The finding of elevated whole blood serotonin in individuals with autism and their close relatives has been well replicated [McDougle et al., 2005], whereas the genetic studies of the serotonin system in autism present a more controversial picture [Devlin et al., 2005]. Although the HTR3 receptors cannot be directly linked to the levels of blood serotonin, interestingly variants in HTR3A have been shown to modulate neural activation of the amygdala during a face recognition task [Iidaka et al., 2005]. Abnormalities in several aspects of face processing have been repeatedly reported in ASDs, and in combination with the results obtained here warrant further study of the HTR3 receptors in ASDs.
Replication of linkage to 3q25-27 has recently been reported in one multiple incidence ASD family with Northern European descent [Coon et al., 2005], the most significant linkage was observed at rs1402229 (P = 0.0003), located 2.6 Mb from the best marker (D3S3037) in the Finnish genome-wide scan. Few, if any, autism linkage regions have been replicated with a comparably high precision. However, it should be noted that the most genome-wide screens for ASDs have been performed using microsatellites, whereas the study by Coon et al. was performed using a dense set of SNP markers, enabling a higher resolution of the linkage peak. Further support for linkage between autism and 3q was obtained in a study of language QTLs in autism, with the strongest evidence for “age at first word” (P < 0.001) at 147cM, around 40cM from the best findings in the Finnish genome-wide scan [Alarcon et al., 2005]. In the same study suggestive evidence for linkage of “age of first phrase” (P = 0.04) was obtained at 180 cM, only 10 cM proximal to our peak at 190cM.
In their study, Coon et al. 2005 also analyzed FXR1, located at the linkage peak, by direct sequencing. They found no variants likely to contribute to autism, and our results support this conclusion. We sequenced the complete coding region and exon–intron junctions of FXR1, and identified only one variant in the whole region. This variant corresponds to SNP rs1805599, which is a common SNP, with a MAF of 0.16 in the HapMap CEU population. However, the family-based association analysis of SNPs in the promoter and introns of FXR1 revealed suggestive evidence of association using three SNPs in FXR1, making it impossible to totally exclude the role of FXR1 in ASDs.
As for all candidate gene studies in complex disorders, these results should be interpreted with care until replication in independent study samples is reported, especially since the association analysis results do not stay significant after correction for 125 tests. It should be noted that the association studies cannot determine whether the associated variants are true susceptibility variants or in LD with other unknown or non-genotyped variants in the region. The non-synonymous SNPs showing most significant association in this study are both common SNPs with near equal allele frequencies for both alleles, and amino acid changes introduced by these SNPs were not predicted to affect protein structure. However, non-synonymous SNPs may affect protein structure in ways that can only be detected in functional studies, and not identified by computational methods. In addition, sequence analysis of HTR3C did not reveal any other variants in the coding region or splice sites.
In conclusion, linkage to 3q has been observed in independent ASD datasets, and the role of 3q in ASDs warrants further investigation. After previously excluding the role of known autism susceptibility genes NLGN1 and ATP13A4 at this locus, here we have analyzed 11 functional candidate genes. The results revealed two non-synonymous SNPs in HTR3C showing association to autistic disorder, and we propose that the role of HTR3C in autism needs further investigation in other datasets.
Acknowledgements
We are grateful to the families who have made this study possible. Reija Alen, Mari Auranen, Ismo Makkonen, and Raili Riikonen are warmly thanked for their contribution in collecting and characterizing the sample. Minna Suvela and Anna-Maija Sulonen are thanked for technical assistance with SNP microarrays. Tero Hiekkalinna is thanked for help with statistical analysis and related issues. This study was supported by the Center of Excellence of Complex Disease Genetics of the Academy of Finland, Biocentrum Helsinki Foundation, Academy of Finland, Helsinki University Hospital Research Funding, Päivikki and Sakari Sohlberg Foundation, The Medical Society of Finland (KR), Helsinki Biomedical Graduate School (KR) and Cure Autism Now (TY).




