Social support in older individuals: The role of the BDNF Val66Met polymorphism†
Please cite this article as follows: Taylor WD, Züchner S, McQuoid DR, Steffens DC, Blazer DG, Krishnan KRR. 2008. Social Support in Older Individuals: The Role of the BDNF Val66Met Polymorphism. Am J Med Genet Part B 147B:1205–1212.
Abstract
Although often viewed as a purely environmental construct, perception of social support may be influenced by genetic factors. This study examined the relationship between the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and social support measures in older subjects. The sample consisted of 243 depressed and 115 nondepressed older subjects, age 60 years or older; 233 were Val66 allele homozygotes, while 125 were Met66 allele carriers. All subjects completed clinical assessments, including a self-report questionnaire assessing four social support domains, and provided blood for genotyping. Statistical models examined the relationship between scale scores of social support and BDNF Val66Met genotype, while controlling for presence or absence of major depressive disorder and other demographic factors significantly associated with social support. As social support measures were not normally distributed, log-transformed scores were examined. After controlling for diagnosis and education level, the Met66 allele was associated with lower levels of subjective social support (F1,357 = 5.33, P = 0.0216) and a trend for fewer social interactions (F1,357 = 3.66, P = 0.0567). To our knowledge, this is the first report associating a measure of social support with a genetic polymorphism. This supports previous work that genetic factors may influence social support perception. Further work is needed to determine the generalizability of this finding to the broader population, as well as its significance for clinical outcomes. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Impairment of social support is a critical factor influencing the development and course of mental and physical illnesses. Lower levels of social support predict poorer health, a greater risk of developing medical and psychiatric illnesses [Wade and Kendler, 2000; Lett et al., 2005; Vanderhorst and McLaren, 2005], poorer response to medical treatment [Lett et al., 2005; Hybels et al., 2006], and higher levels of mortality [Blazer, 1982; House et al., 1988]. Three general domains of social support have been proposed [Barrera, 1986]: social embeddedness, or the frequency of contacts and integration into social networks; received support, or measures of help received; and perceived support, the subjective evaluation of and satisfaction with support. Of these, social support perception and social network integration are most closely linked to emotional adjustment and overall wellbeing [Cohen and Wills, 1985; Kessler et al., 1992].
Lower levels of social support have consistently been associated with depression in older populations [Oxman et al., 1992; Wilson et al., 1999; Vanderhorst and McLaren, 2005], but what is the nature of this relationship? Depressive symptoms may have a direct, negative effect on social relationships through characteristic symptoms of irritability, self-imposed isolation and social withdrawal; it could also be secondary to the negative perceptual bias seen in depression, which may negatively color an individual's perception of social support. These theories imply that depression results in changes in social support or its perception. It is also possible that other factors which negatively influence social interactions or perception of social support may increase the risk of depression.
Although social support was initially conceptualized as a purely environmental variable, recent progress in social neuroscience has broadened our understanding of how biological factors influence social behavior [Cacioppo et al., 2007]. It is plausible that genetic factors influence how individuals perceive the quality of social interactions, which in turn affects the frequency and intensity of their social interactions [Kendler, 1997]. Longitudinal twin studies [Bergeman et al., 1990; Kessler et al., 1992; Herndon et al., 2005] support this hypothesis, as they found that genetic differences account for 24–70% of the temporally stable variance in measured domains of social support [Furukawa and Shibayama, 1997; Kendler, 1997; Bergeman et al., 2001]. Social support domains are generally stable over time [Sarason et al., 1986; Kendler, 1997; Bergeman et al., 2001] while in contrast, environmental influence on social support tends to be less stable across different assessments [Bergeman et al., 2001].
What could mediate a relationship between genetics and social support? One possibility is the influence of genetic differences on personality traits [Kendler, 1997], which are themselves correlated with dimensions of social support [Monroe and Steiner, 1986; Sarason et al., 1986; Windle, 1992]. It is also possible that genetic polymorphisms could affect brain structure and function, which in turn affects how we perceive and evaluate our social environment [Herndon et al., 2005; Cunningham and Zelazo, 2007].
Possible genetic candidates which could be related to social perception or behavior include polymorphisms in genes associated with neurotrophins, which are nerve growth factors that play key roles in neuronal proliferation, differentiation, and synaptogenesis. Brain-Derived Neurotrophic Factor (BDNF) may be particularly important, as it potentiates the release of dopamine in the nucleus accumbens and is a key regulator of the mesolimbic dopamine pathway, which regulates the identification of and response to emotionally salient environmental stimuli [Wise, 2004; Schultz, 2006]. Studies examining animal models have demonstrated that BDNF is a critical mediator of changes in social motivation, particularly in the development of experience-dependent social aversion [Berton et al., 2006], and is affected by psychosocial stress [Branchi et al., 2004]. Further, manipulation of early social environment exerts both short- and long-term effects on BDNF expression in the brain [Cirulli et al., 2003; Roceri et al., 2004; Branchi et al., 2006], which in turn may affect brain development [Liu et al., 2000]. Thus the relationship may be bidirectional, wherein genotype differences may be related to the perception of social support or measures of social interaction, but psychosocial factors may also influence gene expression.
Recent research into the gene coding for BDNF has focused on a single nucleotide polymorphism at nucleotide 196 (G/A), which results in an amino acid substitution at codon 66 of valine to methionine (Val66Met). This polymorphism is located in the 5′ pro-region of the protein, and the Met66 allele is thought to attenuate intracellular trafficking and secretion of BDNF [Egan et al., 2003]. The Met66 allele is associated with smaller hippocampus and prefrontal cortex volumes [Pezawas et al., 2004; Szesko et al., 2005], as well as differences in several cognitive measures [Egan et al., 2003; Hariri et al., 2003; Dempster et al., 2005]. These relationships may be particularly relevant as the prefrontal cortex and temporal lobe regions are associated with assigning meaning to social stimuli [Insel and Fernald, 2004] and stimulus evaluation [Cunningham and Zelazo, 2007].
Interestingly, the Met66 allele is also associated with a weaker sense of coherence [Surtees et al., 2007]. “Sense of coherence” is a validated theoretical construct [Eriksson and Linstrom, 2005] describing a flexible and adaptive orientation, with high levels indicating an ability to successfully cope with adverse experiences [Antonovsky, 1987]. Lower levels of coherence are associated with lower satisfaction with social relationships [Volanen et al., 2004], with greater difficulty in receiving social support [Volanen et al., 2004], and with longer times needed to adapt to adverse social stressors [Surtees et al., 2006].
Based on the observations that BDNF is associated with both a sense of coherence and with social behavior [Berton et al., 2006], we explored the relationship between the BDNF Val66Met polymorphism and social support domains. We elected to pursue this study in an older cohort given the clinically important relationship between social support, health, and mortality [Blazer, 1982; Lett et al., 2005], although low social support may serve as a risk factor for psychopathology in both younger and older adults [Acierno et al., 2006]. This was an exploratory analysis, but based on previous work associating the Met66 allele with weaker sense of coherence [Surtees et al., 2007], which in turn is associated with poorer measures of social support or social adaptation [Volanen et al., 2004; Surtees et al., 2006], we hypothesized that the Met66 allele would be associated with lower levels of social support, particularly perceived social support.
METHODS
Sample
This cross-sectional study was a secondary analysis of data gathered through NIMH Conte Center for the Neuroscience of Depression in Late Life, located at Duke University Medical Center. It examined the relationship between genotype and four social support parameters in a cohort of older depressed and nondepressed individuals. Eligibility for enrollment into the Center was limited to patients aged 60 years or older. Depressed subjects had to meet criteria for Major Depressive Disorder (MDD) on the NIMH Diagnostic Interview Schedule (DIS) [Robins et al., 1981] and were additionally assessed to assure they met DSM-IV diagnostic criteria through an interview with a geriatric psychiatrist. Exclusion criteria included (1) another major psychiatric illness, although coexisting anxiety symptoms considered to be secondary to MDD were allowed; (2) history of alcohol or drug dependence; and (3) primary neurologic illness, including dementia. Subjects were recruited for the study primarily through referrals to the study from primary care physicians at Duke, but also through limited advertising at Duke University Medical Center and through word-of-mouth.
Comparison nondepressed subjects were community-dwelling individuals recruited through advertisements and from the Aging Center Subject Registry at Duke University. Eligible subjects had a non-focal neurological examination, no self-report of neurologic or psychiatric illness, and no evidence of a current or past psychiatric disorder based on the Diagnostic Interview Schedule.
Subjects were excluded if they had a diagnosis of dementia or if the study geriatric psychiatrist suspected dementia at baseline. The majority of subjects had Mini Mental State Examination (MMSE) [Folstein et al., 1975] scores above 24; some severely depressed individuals had scores below 25. These subjects were followed through an acute 3-month treatment phase; if the scores remained below 25, they were not included in this study.
The study protocol was approved by the Duke University Medical Center Institutional Review Board. All subjects provided written informed consent before beginning study procedures.
This study included Caucasian subjects previously included in a study examining BDNF Val66Met allele frequency in geriatric depression [Taylor et al., 2007]. Two of the depressed subjects from that study were not included in this study as they did not provide complete responses to the social support measures. The current study also includes additional nondepressed subjects recruited since the previous study.
Clinical Evaluation
A trained interviewer administered the Duke Depression Evaluation Schedule (DDES) to each subject. The DDES, a composite diagnostic interview instrument, includes sections of the DIS assessing depression, enriched with items assessing sleep problems and the clinical features of melancholia and psychosis, dysthymia, mania, and alcohol abuse or dependence. The DDES also includes questions on age of depression onset, depression history, and family history of psychiatric illness. All depressed subjects were additionally evaluated by a study geriatric psychiatrist, who reviewed entry criteria, current psychiatric symptoms, their psychiatric history, and completed the Montgomery-Asberg Depression Rating Scale (MADRS) [Montgomery and Asberg, 1979].
Social support was measured using the Duke Social Support Index (DSSI) [Landerman et al., 1989]. This 35-item self-report questionnaire was designed to evaluate several domains of a subject's social environment and perception of that environment. It is divided into four subscales previously derived by factor analysis [George et al., 1989; Landerman et al., 1989]. The Social Network Size scale assesses the number of people with whom the individual has contact, including household members, family, coworkers, and friends. The Social Interaction Scale assesses the frequency of contact with family and friends, including both in-person contacts and telephone contacts. The Instrumental Social Support scale assesses assistance a subject receives with day-to-day activities, such as errands, chores, finances. Finally, the Subjective Social Support scale includes items referring to how the individual feels understood, useful, and listened to by family and friends, and whether or not they have a close confidant; this is the only scale that is qualitative and assesses perception of support, while the others are quantitative and assess received assistance or frequency of contacts. Higher scores on all scales indicate greater levels of social support, and the scales have been validated [George et al., 1989]. These scales cannot be combined into an aggregate measure as different metrics are used in different scales.
Genotyping
Fresh blood samples were obtained from all participants and DNA was extracted and stored according to methods and quality checks previously reported [Rimmler et al., 1998; Taylor et al., 2007]. An aliquot of DNA was used for genotyping of the BDNF Val66Met polymorphism. DNA samples were placed in 96-well plates together with no-template controls and four sample duplicates in an asymmetric pattern to avoid unintended plate-switching. DNA was PCR amplified applying a Taqman by-design assay (Applied Biosystems, Foster City, CA) that recognized the single nucleotide polymorphism (SNP) which defines the Val66Met polymorphism (rs6265). The samples were examined with an ABI7900 DNA analyzer (Applied Biosystems) and the genotypes determined with the SDS software package (Applied Biosystems). Greater than 95% genotyping efficiency was required before data were submitted for further analysis.
Analytic Plan
Tests for deviations from Hardy-Weinberg equilibrium (HWE) were conducted in unrelated cases and controls using the exact test from Genetic Data Analysis (GDA) software [Lewis and Zaykin, 2000]. All other statistical analyses were conducted in SAS, version 9.1 (Cary, NC). Following our previous strategy [Taylor et al., 2007], which was based on the observation that there were few subjects homozygous for the Met66 allele, we dichotomized subjects into those who were homozygous for the Val66 allele, and those who were Met66 allele carriers. Analyses of differences in demographic variables between subject groups used chi-square analyses for categorical variables and two-tailed t tests for continuous variables, or the Satterthwaite t-test if variances were unequal.
After examining the distribution of the social support scale scores, we expected that they would not be normally distributed. We confirmed this assumption using the Shapiro-Wilk test, and then proceeded with a log transformation of the four composite social support scale scores to create a normal distribution. We first examined for univariate differences in transformed social support scores between Val66Met polymorphism genotypes. To reduce the number of statistical comparisons, social support domains that did not achieve a level of statistical significance in univariate analyses were not further analyzed. For those that were significantly different between genotype groups, we examined the relationship between those measures and demographic variables, using t tests for dichotomous demographic variables and Spearman correlation coefficients for continuous variables. The demographic variables that were associated with transformed social support scores at a P < 0.10 were incorporated into general linear models, wherein the transformed social support score was the dependent variable, and MDD diagnosis, Val66Met genotype, and demographic measures were independent variables. All models initially included a genotype by MDD diagnosis interaction term, which was removed if it did not reach statistical significance.
RESULTS
Overall Sample
The current sample consisted of 358 older Caucasian individuals; 243 were depressed met criteria for MDD, and 115 were nondepressed comparison subjects. In this sample, 65.1% (N = 233) were homozygous for the Val allele (G/G genotype), 32.1% (N = 115) were heterozygotes (A/G genotype), and 2.8% (N = 10) were homozygotes for the Met allele (A/A genotype).
Differences Between Diagnostic Groups
As previously reported, there were significantly more Met66 allele carriers in the cohort with MDD (Table I) [Taylor et al., 2007]. There was no deviation from HWE for rs6265 (Val66Met) in either diagnostic group. There were no significant differences between depressed and nondepressed subjects in age or sex representation, although the depressed cohort had significantly fewer years of education.
| MDD (N = 243) | Nondepressed (N = 115) | df | Test statistic | P-value | |
|---|---|---|---|---|---|
| Met66 allele carriers (%) | 38.7% (94/243) | 27.0% (31/115) | 1 | χ2 = 4.72 | 0.0298 |
| Age | 69.7 (7.5) | 70.0 (5.60) | 293 | t = 0.34 | 0.7375 |
| Sex (% Female) | 63.8% (155/243) | 72.2% (83/115) | 1 | χ2 = 2.46 | 0.1164 |
| Education | 13.8 (2.8) | 15.6 (1.6) | 340 | t = 7.85 | <0.0001 |
| MADRS score | 26.8 (7.9) | — |
| Val/Val (N = 233) | Met carrier (N = 125) | df | Test statistic | P-value | |
|---|---|---|---|---|---|
| BDNF genotype | |||||
| Age | 70.3 (7.1) | 68.9 (6.6) | 356 | t = 1.80 | 0.0722 |
| Sex (% Female) | 64.8% (151/233) | 69.6% (87/125) | 1 | χ2 = 0.84 | 0.3598 |
| Education | 14.4 (2.6) | 14.3 (2.7) | 356 | t = 0.56 | 0.5779 |
| (N = 150) | (N = 93) | ||||
| MADRS score | 26.8 (8.1) | 26.7 (7.2) | 241 | t = 0.09 | 0.9279 |
- Continuous variables presented as mean (standard deviation); mean age and education reported in years. All analyses used chi-squared tests for categorical variables and two-tailed t tests for continuous variables. For unequal variances, the Satterthwaite t-test was used. Only MDD subjects had MADRS data.
Subjects with MDD reported significantly lower levels of social support than did the nondepressed comparison subjects (Table II) on the subjective, instrumental, and social interaction scales. There was no significant difference between diagnostic groups on the social network scale.
| Social support scale | MDD (N = 243) | Nondepressed (N = 115) | df | Test statistic | P-value |
|---|---|---|---|---|---|
| Subjective | |||||
| Log | 3.12 (0.19) | 3.30 (0.04) | 275 | t = 13.32 | <0.0001 |
| Unadjusted | 23.15 (4.0) | 27.25 (1.14) | |||
| Instrumental | |||||
| Log | 2.19 (0.28) | 2.35 (0.10) | 328 | t = 8.20 | <0.0001 |
| Unadjusted | 9.22 (2.02) | 10.53 (0.96) | |||
| Social interaction | |||||
| Log | 1.70 (0.50) | 2.06 (0.34) | 313 | t = 7.73 | <0.0001 |
| Unadjusted | 5.98 (2.64) | 8.24 (2.44) | |||
| Social network | |||||
| Log | 0.69 (0.69) | 0.55 (0.61) | 252 | t = 1.59 | 0.1125 |
| Unadjusted | 1.98 (2.09) | 1.29 (1.60) | |||
- Social support scale scores, both log-transformed and unadjusted, presented as mean (standard deviation). Group comparisons were only performed on the log-transformed data as the unadjusted data were not normally distributed; these comparisons used two-tailed, pooled t tests, or with the Satterthwaite t-test for unequal variances.
Differences Between Genotype Groups and Relationship With Social Support Domains
There were no significant demographic differences between subjects who did and did not carry a Met66 allele (Table I), although there was a trend for Met66 allele carriers to be younger. In the depressed cohort, we found no difference in MADRS scores between those who did and did not carry the Met66 alele. Univariate analyses of the Val66Met polymorphism groups and measures of social support domains (Table III) showed a significant relationship between genotype and both the Subjective Social Support scale and Social Interaction Scale. The distribution of responses by genotype is shown in Figure 1; in general, subjects reporting higher scores were more frequently Val66 homozygous. To reduce the number of comparisons, no further analyses were performed for the Social Network Size scale or the Instrumental Social Support scale as they were not significantly associated with BDNF genotype.
| Social support scale | Val/Val genotype (N = 233) | Met allele carriers (N = 125) | df | Test statistic | P-value |
|---|---|---|---|---|---|
| Subjective | |||||
| Log | 3.21 (0.15) | 3.14 (0.23) | 176 | t = 2.70 | 0.0076 |
| Unadjusted | 24.92 (2.61) | 23.72 (4.53) | |||
| Instrumental | |||||
| Log | 2.26 (0.23) | 2.21 (0.28) | 216 | t = 1.51 | 0.1335 |
| Unadjusted | 9.76 (1.74) | 9.43 (2.02) | |||
| Social interactions | |||||
| Log | 1.87 (0.47) | 1.73 (0.50) | 346 | t = 2.53 | 0.0119 |
| Unadjusted | 7.02 (2.80) | 6.16 (2.69) | |||
| Social network | |||||
| Log | 0.68 (0.68) | 0.61 (0.65) | 252 | t = 0.81 | 0.4212 |
| Unadjusted | 1.81 (2.01) | 1.66 (1.88) | |||
- Social support scale scores, both log-transformed and unadjusted, presented as mean (standard deviation). Group comparisons were only performed on the log-transformed data as the unadjusted data were not normally distributed; these comparisons used two-tailed, pooled t tests, or with the Satterthwaite t-test for unequal variances.
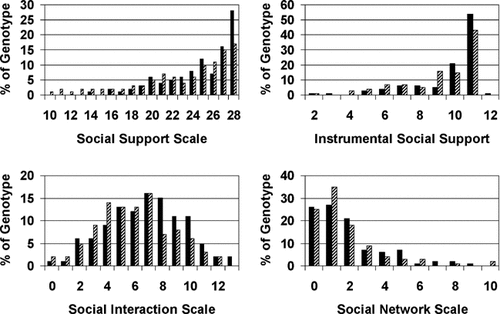
Genotype differences in social support measures. Figures show the percent of subjects with each genotype responding at a given score for each measure. The solid black bars = Val66 homozygotes, striped bars = Met66 allele carriers. In general, subjects endorsing higher scores on these domains were more often Val66 homozygotes.
To determine if the differences observed between the genotype groups in these two scales were purely driven by the depressed population, where the Met66 allele is more frequent, we examined for genotype differences in social support domains separately for the depressed and nondepressed cohorts. Although not consistently reaching a level of statistical significance, depressed Met66 allele carriers exhibited lower log-transformed scores in both subjective support (Met66 carrier = 3.08, SD = 0.25; Val66 homozygotes = 3.15, SD = 0.16; 136 df, Satterthwaite t = 2.11, P = 0.037) and social interaction domains (Met66 carrier = 1.63, SD = 0.52; Val66 homozygotes = 1.75, SD = 0.50; 241 df, pooled t = 1.69, P = 0.093). Similarly, nondepressed Met66 allele carriers also exhibited lower transformed scores than Val66 homozygous nondepressed subjects, although this did not reach statistical significance in either subjective support (Met66 carrier = 3.29, SD = 0.04; Val66 homozygotes = 3.31, SD = 0.05; 113 df, pooled t = 0.94, P = 0.349) or social interaction domains (Met66 carrier = 1.99, SD = 0.35; Val66 homozygotes = 2.08, SD = 0.34; 113 df, pooled t = 1.24, P = 0.217).
Next we examined the univariate relationship between demographic variables and the log transformed measures of the two significantly different scales. Neither sex nor age was significantly associated with scores on either the Subjective Social Support scale or the Social Interaction Scale. Education was positively associated with both the Social Interaction Scale (Spearman Correlation Coefficient, SCC = 0.19, P = 0.0004) and the Subjective Social Support scale (SCC = 0.12, P = 0.0233).
Finally we proceeded to develop statistical models examining the Subjective Social Support scale and Social Interaction Scale as dependent variables which incorporated as independent variables the demographic factors that were statistically significant in univariate analyses (Table IV). Initial models included a MDD diagnosis by BDNF genotype interaction term; this variable did not reach a level of statistical significance in either model, so was removed. After controlling for presence of MDD and education level, carrying the Met66 allele was associated with significantly lower transformed scores on the Subjective Social Support scale, with a trend not reaching statistical significance in models examining the Social Interaction Scale.
| Subjective social support | Social interaction | |||
|---|---|---|---|---|
| F-value | P-value | F-value | P-value | |
| Genotype | 5.33 | 0.0216 | 3.66 | 0.0567 |
| Depression | 81.62 | <0.0001 | 28.96 | <0.0001 |
| Education | 0.15 | 0.6967 | 3.16 | 0.0762 |
- Social support measures were log-transformed. Genotype (Val66 homozygote or Met66 allele carrier) and depression (MDD or nondepressed) were dichotomous variables. Age and sex were not included in models as they were not significantly different between BDNF genotype groups, and not significantly related to social support measures. Education (in years) was a continuous variable. Each variable had 1 degree of freedom while the overall model had 357 degrees of freedom. Initial models included a depression by genotype interaction term, however these were not statistically significant so were removed.
DISCUSSION
To our knowledge, this is the first report to associate a specific single nucleotide polymorphism with measures of a social support domain in older individuals. As a whole, individuals homozygous for the Val66 allele report higher scores on all four parameters of social support than do carriers of the Met66 allele, but after controlling for covariates only the Subjective Social Support domain score was consistently associated with BDNF genotype. This measure assesses perception of support through questions of feeling listened to, of feeling useful, of having close confidants, and the perceived ability to go to family or friends with problems. The relationship between genotype and the Social Interaction Scale, which assesses the number of friends and frequency of contact, approached but did not reach a level of statistical significance on the log-transformed scale score. These relationships are independent of MDD, and there does not appear to be an interaction between MDD and BDNF genotype. The Val66Met polymorphism was not significantly related to the more objective measures of social support, specifically instrumental social support or social network size.
Despite past conceptualization of social support as a purely environmental variable, epidemiological population-based or twin studies have found a genetic basis for both perception of social support [Bergeman et al., 1990; Kessler et al., 1992; Herndon et al., 2005] and measures of social interaction [Kessler et al., 1992; Kendler, 1997]. One of the most common rationales presented that might explain this relationship is that there is a genetic influence on personality. Such hypotheses have been difficult to consistently demonstrate for isolated single nucleotide polymorphisms, given how personality is likely determined by both differences in multiple genes as well as environmental effects. However, a genetic predisposition to specific personality traits may affect one's social network or perception of support, as extraversion is positively associated but neuroticism negatively associated with perceived social support [Monroe and Steiner, 1986; Sarason et al., 1986; Windle, 1992]. Although appealing, this explanation may not be applicable for the current findings. Although serum BDNF concentrations are associated with neuroticism [Lang et al., 2004] and an initial report found that the Val66 allele was associated with higher levels of neuroticism [Sen et al., 2003], others have not found similar relationships [Tsai et al., 2004; Lang et al., 2005; Willis-Owen et al., 2005; Tochigi et al., 2006].
Our data demonstrate a genetic influence on perception of social support, but this does not negate the role of environmental factors. Maltreated children with the BDNF Met66 allele and the short/short (s/s) serotonin transporter linked promoter region polymorphism (5-HTTLPR) exhibit high levels of depressive symptomatology [Kaufman et al., 2006]. The authors identified a four-way interaction between these factors and a protective role of social support, wherein maltreated children with the Met66 allele and s/s genotype who reported higher levels of social support had lower depression scores. Our current study found no relationship between the BDNF Val66Met allele and depressive symptomatology in older depressed subjects, but we did not examine the 5-HTTLPR polymorphism. However, the Kaufman study emphasizes the need to include environmental variables in future studies, as older individuals often experience their own environmental adversity, such as financial difficulties, moving away from independent living, and bereavement. Recent research indicates that gene-environment interactions may contribute to cognitive deficits [Reynolds et al., 2007] and perception of stressors in older populations [Charles and Almeida, 2007], and some environmental influences may have a greater affect on older populations than younger [Charles and Almeida, 2007]. Thus there may be gene–environment interactions that may not be observable except in older populations.
BDNF affects systems involved in stimulus assessment and in turn is affected by stress in ways that may provide insights into the mechanism behind the association we describe in this report. BDNF has a regulatory effect on the mesolimbic dopamine pathway and nucleus accumbens, which is achieved through BDNF's activation of tyrosine kinase B (TrkB) receptors [Horger et al., 1999; Guillin et al., 2001; Grimm et al., 2003]. The mesolimbic dopamine pathway consists of dopaminergic neurons projecting from the ventral tegmental area to the nucleus accumbens and is involved with the identification of and response to emotional environmental stimuli [Wise, 2004; Schultz, 2006]. Components of this pathway are activated by positive social actions such as cooperation [Rilling et al., 2002] or during attachment processes [Insel and Fernald, 2004], but also by aversive social stimuli [Louilot et al., 1986; Tidey and Miczek, 1996; Cabib et al., 2000]. Presumably by its effect on this pathway, BDNF plays a critical role in the recognition of threatening stimuli and is the development of experience-dependent social aversion [Berton et al., 2006]. In addition, acute social stress reduces BDNF expression in the brain [Pizarro et al., 2004], although recurrent social stress or social confrontations may result in increased BDNF expression [Fiore et al., 2003; Pardon et al., 2005]. Individuals who carry the Met66 allele may be less able to mount this response to recurrent stress, which in turn may affect perception of social interactions or the drive to seek out social attachments.
One potential study limitation is that all social support measures are from subject self report. Although we describe one scale as being “subjective social support” because it is asking questions about how subjects feel in relation to their social system, even the “objective” questions (such as do family members help with problems, how often do you see people, etc.) are still susceptible to recall bias. Therefore our study cannot determine if the BDNF Val66Met polymorphism is related to how people truly interact with their social environment or if it is only associated with the perception of one's social environment. Nevertheless, perception of health and well-being, including the well-being of one's social support system, has been consistently associated with health outcomes such as depression.
Another limitation is the sample used in this study. It is exclusively an elderly cohort, which limits the generalizability of our findings to younger populations. This polymorphism may also affect social support domains in younger populations, but its effect may be clinically apparent only in the context of medical illness or environmental changes that occur with aging, such as loss of independence or death of peers.
Further limitations include using a Social Support Index with different metrics for different scales. Similar metrics would have allowed creation of an aggregate measure, which could have provided a broader, single glimpse of the relationship between this polymorphism and social support. Also, had we obtained MADRS data on the controls, we could have utilized a continuous measure of depressive symptomatology in our primary analyses, rather than a categorical definition of MDD.
Despite examining a population of only Caucasian subjects, this is a mixed sample of subjects with and without MDD. Depressed individuals generally report lower levels of social support [Blazer, 1983; George et al., 1989; Lin and Dean, 1984], however in multivariate models the Val66Met polymorphism remains significantly related to our measure of subjective social support even when controlling for the presence of MDD. Moreover, there was no significant interaction between MDD and the Val66Met polymorphism on subjective social support. Although not demonstrating a statistically significant difference, separate univariate analyses of social support domain scores by genotype within each cohort demonstrated that Met66 allele carriers tend to have lower mean scores than do Val66 homozygotes. Thus, the relationship between this polymorphism and social support appears to be independent of the presence of MDD.
This study is important as it not only improves our understanding of differences in how individuals perceive their social environment, but also because poor social support is a risk factor for many psychiatric illnesses. Using depression as an example, lower levels of subjective social support are associated with illness severity [George et al., 1989] and with poorer treatment outcomes [Blazer, 1983; Lin and Dean, 1984; George et al., 1989; Wade and Kendler, 2000; Bruce, 2002]. Our finding raises the theory that while some genetic polymorphisms may increase the risk of or severity of psychiatric illness by modulating the severity of one's response to stress [Caspi et al., 2003], others may affect how we perceive our social environment, which in turn increases the risk of illness. This hypothesis does not imply that depression itself does not result in impaired social relationships or impaired perception of support. The relationship between depression and social support is likely bidirectional. Importantly, the hypothesis that genotype influences social support, which in turn increases the risk of depression, cannot be answered in a cross-sectional study, but rather requires a longitudinal study design.
This study identifies a single polymorphism that is associated with lower levels of perceived, subjective social support, but not more objective measures of social support. However, perceived social support is such a complex construct that perception of it is likely influenced by multiple genes as well as current and past environmental influences. Future studies should consider pursuing this hypothesis using truly objective as well as subjective measures of social interactions, as well as detailed measures of environmental factors, a measure not included in the current study. Additionally, neuroimaging methods could examine how the Val66Met polymorphism is associated with differential brain activation to socially salient stimuli, and how depressive symptoms may affect that relationship. Such work could improve our understanding of how the brain processes social stimuli and what factors contribute to perception of the social environment, which will help us better understand risk factors for psychiatric illness.