Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity†
Please cite this article as follows: Congdon E, Lesch KP, Canli T. 2007. Analysis of DRD4 and DAT Polymorphisms and Behavioral Inhibition in Healthy Adults: Implications for Impulsivity. Am J Med Genet Part B 147B:27–32.
Abstract
Impulsivity, a highly prevalent symptom in multiple psychiatric disorders, is a partially heritable trait influenced by specific biological mechanisms. In particular, dopamine is proposed to play a role in impulsive behaviors and recent studies have implicated functional polymorphisms of dopamine-related genes in impulsive behaviors across different clinical and behavioral classifications. However, most have not isolated the impulsivity construct per se as a biologically based and measurable endophenotype. The present study was therefore undertaken in a sample of healthy adults to investigate the influence of two candidate dopaminergic gene polymorphisms (DRD4 and DAT) on the endophenotype of impulsivity, which we operationalized as behavioral inhibition during the Stop-signal task. We recruited an ethnically diverse sample of 119 healthy adults to complete a self-report questionnaire of impulsivity and to perform a Stop-signal task. We report significant differences in inhibitory control between individuals with at least one 7-repeat allele of the DRD4 polymorphism, as well as an interaction between DRD4 and DAT genotypes, on inhibitory control. Results of the present study support the influence of dopaminergic variation on impulsive-related measures, as well as the advantage of using measures which are likely more sensitive to the effects of such genetic variation. © 2007 Wiley-Liss, Inc.
Impulsivity is the predisposition to respond to internal or external stimuli without regard to the negative consequences [Moeller et al., 2001]. There are multiple psychiatric disorders that are characterized by elevated impulsivity, including personality disorders, substance abuse and addiction, mood disorders, impulse control disorders (ICDs), and attention deficit hyperactivity disorder (ADHD) [American Psychiatric Association (APA, 2000)]. The high prevalence of ICDs [Kessler et al., 2006] and the high comorbidity between ICDs and other psychiatric disorders also supports the idea that impulsivity is a predisposing factor to psychiatric illnesses. Understanding the biological mechanisms influencing both normal and pathological levels of impulsivity, therefore, may have implications for our understanding of psychiatric illnesses.
Research supporting the contribution of impulsivity to a wide range of psychopathologies, however, has meant that impulsivity is typically studied in the context of discrete diagnostic categories of patient populations [Moeller et al., 2001]. Diagnostic categories are heterogeneous and non-biologically based, which limits our ability to detect small gene effects. Furthermore, impulsivity is multidimensional, encompassing inhibitory control, response to punishment, response to novelty, and delay discounting aversion [Nigg, 2000]. An alternative way to understand the biological mechanisms influencing psychiatric disorders is to identify intervening variables along the pathway between genes and a behavior that are likely to be more sensitive to the effects of genetic variation [Gottesman and Gould, 2003; Hariri and Weinberger, 2003]. This approach, known as an endophenotype approach, has the advantage that it may yield more precise and sensitive measures than self-report alone, which can be vague and subjective [De Geus and Boomsma, 2001; Hariri and Weinberger, 2003; Goldberg and Weinberger, 2004].
Central to the definition of impulsivity is the inability to inhibit thoughts or actions. This aspect of impulsivity can be directly assessed using tasks in which inhibition of a response is required, such as the Stop-signal task [Logan, 1994]. In the tracking Stop-signal task, participants are told to respond to a certain set of stimuli but to inhibit their response when a stop-signal is presented. By tracking participants' responses on inhibition trials and modifying the timing parameters on subsequent inhibition trials, this procedure ensures that participants successfully inhibit on 50% of inhibition trials, thereby ensuring a comparable level of experienced difficulty, behavioral performance, and number of trials across participants.
There is some evidence suggesting that serotonin is involved in impulsivity [for review, see Evenden, 1999; Carver and Miller, 2006] and, in particular, recent evidence relates variants of serotonin-related genes to behavioral inhibition [Nomura et al., 2006; Stoltenberg et al., 2006]. However, there is also widespread evidence supporting the role of dopamine in impulsivity, specifically behavioral inhibition. The present study will focus on the role of dopamine because there is substantial evidence relating behavioral inhibition, our proxy of impulsivity, to the dopaminergic system.
Dopamine plays a role in a number of neural processes related to cognition, emotion, motivation, motor functioning, and hormone release [Robbins, 2000; Tzschentke, 2001]. Support for the role of dopamine in impulsive behaviors comes from pharmacological studies in humans [de Wit et al., 2002; Friedel, 2004], and from pharmacological, metabolite, lesion, and knockout studies in animals [Rubinstein et al., 1997; Puumala, 1998; Dulawa et al., 1999; Cardinal et al., 2001; Winstanley et al., 2004, 2006]. The identification of variation in genes of the dopaminergic signaling pathway that are hypothesized to be functionally relevant to impulsive behaviors allows us to examine the association between dopaminergic polymorphisms and our measure of behavioral inhibition.
In particular, there is support for two polymorphisms in genes of the dopaminergic system: the dopamine D4 receptor (DRD4) and the dopamine transporter (DAT). The gene coding for the D4 receptor, which participates in the mediation of dopaminergic transmission, contains a variable number of tandem repeats (VNTR) polymorphism that varies from 2 to 11 repeats across individuals [Asghari et al., 1995; Cravchik and Goldman, 2000]. Presence of the 7-repeat allele has been associated with ADHD [Li et al., 2006], and with performance on behavioral measures of impulsivity in ADHD patients [Langley et al., 2004]. The gene coding for the protein that removes dopamine from the extracellular space, the DAT, also contains a VNTR polymorphic region that varies between individuals. The 10/10 DAT genotype has been associated with ADHD [Waldman et al., 1998; Faraone et al., 2005], and more specifically, with a measure of behavioral inhibition [Cornish et al., 2005].
Based on evidence supporting the relationship between gene variants of the dopaminergic system and psychiatric disorders characterized by impulsivity, we conducted the present study to test the association between dopamine-related gene polymorphisms and a measure of behavioral inhibition in an attempt (1) to overcome some of the limitations associated with self-reported impulsivity or diagnostic categories and (2) to elucidate the role of two dopaminergic gene polymorphisms in this major component of impulsivity. We expected that the risk-variants of DRD4 (7-allele) and DAT (10/10 genotype) would be associated with increased impulsive responding on measures of impulsivity. We further expected that there would be an interaction between these risk variants on impulsive responding, such that participants with both risk variants/genotype would show the greatest impairment in behavioral inhibition.
MATERIALS AND METHODS
One hundred nineteen participants (78 females), with a mean age of 20.87 (SD = 3.25) were recruited among undergraduates through a college subject pool and received course credit for their participation. All participants gave written informed consent and the study was approved by the Institutional Review Board at Stony Brook University. All participants were screened for history of psychiatric illness in order to ensure that the sample included only healthy individuals. The K10-Psychological Distress Scale (K-10) [Kessler et al., 2002] was used to assess general mental health status, the Iowa Personality Disorder Screen, Version 1.2 (IPDS) [Langbehn et al., 1999] was used to screen for the presence of personality disorders, and the Adult ADHD Self-Report Scale (ASRS-v1.1) Symptom Checklist [Kessler et al., 2005] was used to screen for symptoms of ADHD.
A subset of participants was excluded from analyses due to incomplete data sets: 2 participants due to high scores on the Adult ADHD scale; 4 participants due to an inability to obtain complete genotype results; 1 participant due to an incomplete Barratt Impulsiveness Scale, Version 11 (BIS-11) questionnaire; 26 participants due to incomplete task performance. Of the 86 participants (56 females) with complete data sets, the mean age was 20.57 (SD = 3.14). The distribution of ethnic groups in the final sample was as follows: 44 were Caucasian, 26 were Asian, 7 were Latino, 3 were African-American, 1 was Native-American, and 5 were self-identified as Other.
Participants in the present study were not matched for age, sex, ethnicity, or education. However, all participants were drawn from a college subject pool population and were therefore of comparable age and educational level. There were no significant differences on any of our dependent variables between sexes, ethnic groups, or college levels, and age did not correlate with self-reported impulsivity (BIS-11 total scores) or our measure of behavioral inhibition. Means and standard deviations for demographic variables are provided (Table I).
| Measure | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age | 20.57 | 3.14 | 18 | 37 |
| College year | 2.65 | 1.24 | 1 | 4 |
| BIS-11 Total Score | 63.97 | 9.54 | 45 | 86 |
| BIS-11 Non-planning | 24.55 | 4.91 | 12 | 35 |
| BIS-11 Motor | 22.05 | 4.61 | 14 | 43 |
| BIS-11 Cognitive | 18.04 | 3.78 | 11 | 26 |
| SSRT | 239.09 | 68.94 | 119 | 472 |
- BIS-11 Non-planning, Motor, and Cognitive scores represent subscales of the BIS-11; SSRT = Stop-signal reaction time.
Self-reported impulsivity was assessed with the BIS-11 [Patton et al., 1995]. The BIS-11 is a widely used questionnaire that measures self-reported levels of impulsivity and has been validated in impulsive and normal populations [Patton et al., 1995]. Each of the 30 questions is answered on a 4-point scale, ranging from Rarely/Never to Almost Always/Always. The scale has three major components: a Motor subscale addressing a tendency to act without thinking and lack of perseverance; a Non-planning subscale addressing a lack of self-control and “present orientation;” and a Cognitive subscale addressing inattention and a tendency to make quick decisions [Patton et al., 1995].
In this study, our behavioral measure of impulsivity was a tracking Stop-signal task, which is a well-characterized measure of behavioral inhibition [Logan, 1994; Logan et al., 1997]. Participants saw a right- or leftwards pointing arrow and were instructed to press one key in response to a right arrow and a separate key for a left arrow [Rubia et al., 2003]. On a subset of trials (20%), a right- or leftwards pointing arrow was replaced by an upwards-pointing arrow (the stop-signal), after a variable interval. Participants were instructed to inhibit responses on trials in which the stop-signal appears, and to respond as quickly and accurately throughout the task. There was a total of 160 trials with right- and leftwards pointing arrows only (equal number of each), and there was a total of 40 trials in which an upwards pointing arrow (the stop-signal) appeared, making for a total number of 200 trials lasting for approximately 6 min.
Each trial consisted of a 500 msec stimulus presentation (right- or leftwards-pointing arrow) and a 1,800 msec inter-stimulus interval (ISI) (blank screen). The onset of the stop-signal on the first stop trial was 250 msec, but the delay between onset of a right- or leftwards pointing arrow and onset of a stop-signal (stop-signal delay, SSD) increased or decreased by 50 msec on each successive trial, depending on the participant's performance on the previous inhibition trial. The SSD became 50 msec longer after the participant was able to inhibit successfully on the previous inhibition trial, and the SSD became 50 msec shorter after the participant failed to inhibit on the previous inhibition trial. This tracking procedure in the Stop-signal task allows for the computation of an individual's inhibitory function [Logan, 1994]. The main dependent variable of the task is the Stop-signal reaction time (SSRT), or duration of the stopping process; longer SSRT values reflect poorer inhibitory control.
SSRT is estimated using the race model, which assumes that the processes underlying the Go response and the Stop response are independent, thereby allowing for the estimation of the Stop distribution (which cannot be directly observed) from the Go distribution (which is directly measured) [Logan, 1994]. By adjusting the SSD to obtain 50% inhibition on stop trials, we were able to subtract the SSD (where participants were able to successfully inhibit 50% of the time) from median reaction time on Go (non-inhibition) trials [Band et al., 2003].
DNA was collected with cheek cell swabs and extracted using the Epicentre BuccalAmp DNA Extraction Kit (EpiCentre, Madison, WI). The DRD4 polymorphism was genotyped as previously reported [Ebstein et al., 1996]. For the DAT polymorphism, the 40-base pair VNTR located in the 3′-untranslated region of the DAT cDNA was amplified using 5 U of Taq DNA polymerase (following Hunnerkopf et al., 2007). After an initial denaturation for 3 min at 95°C, 35 cycles of denaturing at 95°C for 45 sec, annealing at 67.5°C for 45 sec and extension at 72°C for 45 sec were performed in the presence of primers 5′-TGT GGT GTA GGG AAC GGC CTG AG-3′ and 5′-CTT CCT GGA GGT CAC GGC TCA AGG-3′, followed by a final extension at 72°C for 3 min. PCR amplification was carried out in a final volume of 25 µl consisting of 80 ng of DNA, 250 µM of each deoxyribonucleotide, 10 pmol of sense and antisense primers, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 1.5 mM MgCl2. Amplification products were analyzed using 3% agarose gels containing ethidium bromide, and product sizes were determined by comparison with molecular weight standards.
RESULTS
Inspection of the data reveals adequate allele frequencies. In the final sample, 24 participants fell in the DRD4 7-allele present group and 62 participants fell in the DRD4 7-allele absent group; 41 participants fell in the DAT 10/10 group, and 45 fell in the DAT non-10/10 group. These distributions did not depart from the Hardy–Weinberg equilibrium (for DRD4: χ2 = 0.07, df = 2, P = 0.77; for DAT: χ2 = 0.03, df = 2, P = 0.70). The specific allele frequencies for both genes are listed in Table II. Non-parametric tests (Mann–Whitney) revealed no significant differences in the frequency distribution for sex, ethnicity, or college level for either DRD4 or DAT. While independent t-tests did not reveal any significant differences in age for DRD4, there were significant differences in age between DAT genotype groups (t (84) = 2.15, P < 0.05). Therefore, age was added as a covariate in the remaining analyses.
| Allele | N | % |
|---|---|---|
| DRD4 | ||
| 2 | 16 | 9.30 |
| 3 | 7 | 4.07 |
| 4 | 115 | 66.86 |
| 5 | 4 | 2.33 |
| 6 | 3 | 1.74 |
| 7 | 26 | 15.12 |
| 8 | 1 | 0.58 |
| Total | 172 | 100.0 |
| DAT | ||
| 6 | 1 | 0.58 |
| 8 | 1 | 0.58 |
| 9 | 43 | 25.00 |
| 10 | 124 | 72.09 |
| 11 | 3 | 1.74 |
| Total | 172 | 100.0 |
Inspection of the behavioral data reveals that the tracking design of the task worked well and ensured comparable performance across participants. The mean percent commission errors (or failure to inhibit) on stop trials across the final sample were 50.90 (SD = 9.70). That is, participants were, on average, able to successfully inhibit a response on 50% of stop trials.
We conducted a two-way analysis of variance to test the effect of each gene and the interaction between them on the two major dependent variables, BIS-11 total scores and SSRT. Genotypes were entered as independent variables and grouped as follows: for the DRD4 genotype, presence versus absence of at least one 7-repeat allele; and for the DAT genotype, individuals homozygous for the 10-repeat variants were compared to all others. Age was included as a covariate because there was a significant age difference between DAT genotype groups. In addition, a Pearson's correlation was conducted on SSRT, BIS-11 total score, and BIS-11 subscales. Data are summarized to show means and standard deviations for each dependent variable (Table I) and for each dependent variable as a function of genotype (Table III).
| Measure | Polymorphism | Genotype Group | |
|---|---|---|---|
| 7-repeat allele present | 7-repeat allele absent | ||
| BIS-11 | DRD4 | 63.38 (10.40) (N = 24) | 64.19 (9.26) (N = 62) |
| SSRT | DRD4 | 262.74 (89.71) (N = 24) | 229.93 (57.30) (N = 62) |
| 10/10 genotype | Non-10/10 genotype | ||
| BIS-11 | DAT | 65.00 (9.99) (N = 45) | 62.83 (8.99) (N = 41) |
| SSRT | DAT | 243.53 (75.99) (N = 45) | 234.22 (60.84) (N = 41) |
In terms of the analysis of variance to test the effect of each gene and the interaction between them on our dependent variables, there were three main findings. There was a significant interaction between DRD4 and DAT genotypes on SSRT, F (1, 81) = 6.61, P < 0.05, with genotype accounting for 7.5% of the variance. Inspection of the data revealed that those individuals with both risk variants/genotype had the highest SSRT, as compared to those with only one, or without either, risk variant/genotype (see Fig. 1).
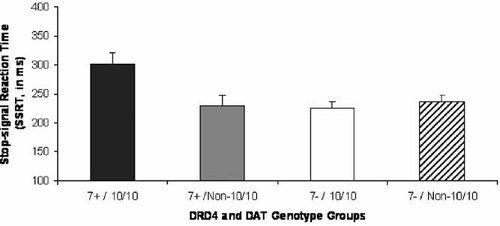
Stop-signal reaction time (SSRT) as a function of DRD4 and DAT genotypes. DRD4 7-allele present (7+) versus absent (7−) and DAT 10/10 genotype (10/10) versus non-10/10 genotype (Non-10/10) were combined to form four groups. There was a significant difference between groups on SSRT. Longer SSRT (msec) reflects greater difficulty inhibiting a behavioral response to a stop-signal.
While interpretation of main effects is less clear in light of a significant interaction, there was a significant effect of DRD4 and a trend towards an effect of DAT on SSRT. For DRD4, there was a significant difference between genotype groups on SSRT, F (1, 81) = 4.95, P < 0.05, with DRD4 genotype accounting for 5.8% of the variance. Figure 2 shows that for the DRD4 groups, subjects with the risk variant (the 7-repeat allele, N = 24) had a higher mean SSRT than subjects without the risk variant (N = 62). For DAT, there was a trend for a difference between genotype groups in SSRT, F (1, 81) = 3.59, P = 0.062.
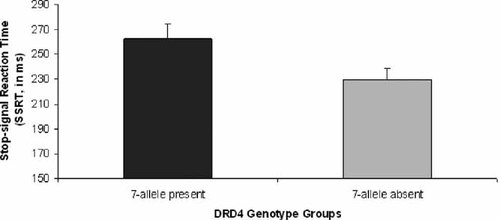
Stop-signal reaction time (SSRT) across DRD4 genotype groups. There was a significant difference in SSRT between those with the 7-allele of the DRD4 polymorphism as compared to those without the 7-allele. Greater values for SSRT indicate poorer inhibitory control.
There were no significant differences between DRD4 or DAT genotype groups, or the interaction between them, on BIS-11 total scores. The amount of variance in BIS-11 scores accounted for by DRD4 genotype was 0.2%, by DAT genotype was 0.7%, and by the interaction between DRD4 and DAT was 0.2%. Post-hoc analyses revealed that there were no significant differences between allele or genotype groups on any of the subscales of the BIS-11. In addition, although the BIS-11 total score significantly correlated with each of the subscales (all P < 0.01), SSRT did not correlate with BIS-11 total score or any subscale.
DISCUSSION
The present study was designed to address an intervening variable, or endophenotype, of impulsivity, by using a performance measure on a well-characterized behavioral task to quantify impulsivity. Specifically, we tested the relationship between dopaminergic genetic polymorphisms (DRD4 and DAT) and behavioral inhibition (as assessed with a Stop-signal task) in a sample of healthy adults. We found that there was a significant interaction between DRD4 and DAT genotypes. Specifically, those individuals with both the DRD4 7-repeat allele and DAT 10/10 genotype had the longest SSRTs, as compared to those with only one, or without either, risk variant/genotype. While the difference in SSRT between DAT genotype groups alone did not reach significance, there was a significant difference in SSRT between DRD4 genotype groups, with those individuals carrying at least one 7-repeat allele having significantly longer SSRTs, reflecting poorer inhibitory control.
The finding of an interaction between DRD4 and DAT genotype groups on SSRT in a cohort of healthy volunteers is novel, although there have been two previous reports of an epistatic effect of DRD4 and DAT on impulsive behavior in ADHD samples. ADHD children diagnosed with the hyperactive-impulsive subtype who carried at least one 7-repeat variant of the DRD4 and were homozygous for the DAT 10-repeat allele were reported to have significantly higher hyperactive-impulsive scores than all other groups [Roman et al., 2001]. Subsequently, an increased rate of having at least one 7-allele of the DRD4 and both 10-alleles for the DAT was reported in ADHD children as compared to their unaffected siblings [Carrasco et al., 2006]. Furthermore, an interaction between DRD4 and DAT genotype groups was reported in an imaging study [Szobot et al., 2005]; these authors reported significantly higher perfusion (as measured by single photon emission-computed tomography (SPECT)) in the right middle temporal gyrus of ADHD boys with the 7-repeat allele of the DRD4 and the 10/10 genotype of the DAT compared to ADHD boys without the risk variant/genotype. These studies further support the argument that diagnostic categories may be, in some cases, too heterogeneous for association studies as two of these studies looked not at presence versus absence of clinical diagnosis, but rather at more specific measures of ADHD symptomology within clinically defined samples. In addition, these studies suggest that DRD4 and DAT polymorphisms may be interacting to influence impulsive-related behaviors.
The results of the present study support the relationship between variants of dopaminergic genetic polymorphisms and behavioral inhibition, a major component of impulsivity. These results highlight the advantage of using quantitative measures of endophenotypes (i.e., phenotypes that are hypothesized to be closer to the effects of genetic variation) over self-report measures. Indeed, we found a significant effect of genetic variation on a quantitative measure of an endophenotype of impulsivity (SSRT), with DRD4 genotype accounting for 5.8% of the variance and DAT genotype accounting for 4.2% of the variance. In contrast, we did not find a significant effect of genetic variation on a self-report measure of impulsivity (BIS-11), in which only 0.2% and 0.7% of the variance was accounted for by the DRD4 and DAT genotypes, respectively. Thus, our study illustrates that quantitative measures of an endophenotype elicited by a neuropsychological paradigm are more sensitive to the effects of genetic variation than self report measures by more than an order of magnitude times five.
In the present study, there were no significant differences on the BIS-11 self-report measure of impulsivity, or on any of its subscales, between DRD4 or DAT genotype groups, but there were significant differences in SSRT as a function of genotype. Impulsivity itself is a multidimensional construct and self-report measures of trait impulsivity assess multiple aspects of impulsivity. Indeed, a strength of the BIS-11 scale is that it contains three subscales assessing Motor, Non-planning, and Cognitive aspects of impulsivity. Yet we still failed to find significant differences on any subscale of the BIS-11 between allele or genotype groups.
Based on our results, we conclude that use of the Stop-signal task is more sensitive to the effects of dopaminergic genetic variation than are self-report measures of trait impulsivity. That is not to say that we think SSRT and BIS-11 scores are meant to address the same components of impulsivity, but rather that behavioral inhibition (as indexed by SSRT) is a more suitable endophenotype of impulsivity when testing for the effects of dopaminergic genetic variation than are multifaceted, high-order traits (assessed with self-report measures). The implication of this conclusion is that the BIS-11 and its subscales may be less suitable for assessing behavioral inhibition, but may be more suited for assessing other components of impulsivity, which are potentially influenced by other neurotransmitter systems (such as serotonin).
Our findings demonstrate that behavioral inhibition, a major component of impulsivity, is associated with dopaminergic genotype in healthy individuals. We speculate that the underlying mechanism involves differential dopaminergic regulation, through the interactions of DRD4 and DAT, of a right-lateralized frontostriatal circuit that has been shown to mediate behavioral inhibition (reviewed in Congdon and Canli, 2005). One possibility is that the effects of DRD4 and DAT variation lead to individual differences in neural morphometry within this circuit [Durston et al., 2005]. Another possibility is that the effects of DRD4 and DAT variation lead to individual differences in neural activation within this circuit: individual differences in dopamine reuptake (conferred through the DAT) in conjunction with individual differences in dopamine binding (conferred through DRD4) may alter signal-to-noise ratio in dopaminergic signaling within the frontostriatal circuit, affecting behavioral inhibition [Arnsten, 1998]. These hypotheses remain to be tested in future work.
The strengths and limitations of the current study should be considered when interpreting the findings. The study sample size was modest, though this sample size provided enough power to detect a significant interaction and main effect of gene variants, of small effect size, on our measure of behavioral inhibition. Participants completed self-report measures of mental health in order to exclude the presence of psychiatric illness in the sample; we therefore ensured that our sample included only healthy individuals. Participants were not matched for age, sex, ethnicity, or education. However, there were no significant differences on any dependent variable as a function of sex, ethnicity, or college level, and age did not correlate with our two main dependent variables (BIS-11 score or SSRT). There were no differences in the frequency distribution of genotypes for sex, ethnicity, or college level, and no differences in age between DRD4 genotype groups. As the DAT genotype groups did, however, differ in age, we added age as a covariate in our analyses. Nonetheless, the possibility of population stratification cannot be excluded and, as such, caution must be used when interpreting our results, which were drawn from an ethnically diverse sample in which participants were not matched for demographic variables.
Our results suggest that by moving beyond self-report measures, which may not only be vague and subjective, but which also are multidimensional and likely influenced by many genes of small effect size, we are better equipped to detect differences between genotype groups in a relatively small sample of healthy adults. In essence, our results support the use of behavioral inhibition as a proxy for impulsivity in association studies testing for the influence of dopaminergic genetic variation. Additional research is warranted to test whether these effects hold in samples characterized by extreme levels of impulsivity, and whether these variants in dopamine-system related genes interact with other genes known to influence impulsive behavior.
Acknowledgements
The authors would like to thank J.-F. Sisante, N. Tokunaga, and C. Sandiford for their help in data collection and analysis, and K. Omura for his excellent help in task design. This work was supported by the National Science Foundation grant BCS-0224221.