Insomnia improvement during antidepressant treatment and CLOCK gene polymorphism
Abstract
Sleep disturbances are commonly observed in mood disorders, and sleep manipulations can influence the clinical status. In the present study, we investigated the possible effect of the 3111 T/C circadian locomotor output cycles kaput (CLOCK) gene polymorphism on insomnia symptomatology during antidepressant treatment. One hundred seventy-eight inpatients were treated with fluvoxamine 300 mg/day (n = 147) or paroxetine 20–40 mg/day (n = 31), and either placebo or pindolol in a double blind design for 6 weeks. The severity of depressive symptoms was weekly assessed with the Hamilton Rating Scale for Depression (HAM-D). We observed a significantly higher presence of insomnia throughout the trial in homozygotes for the C variant (P = 0.026). Other demographic and clinical features were found not to be related with CLOCK polymorphisms. Overall, our findings may suggest that CLOCK genotype influences the time course of insomnia during antidepressant treatment. This, together with previous findings on this polymorphism could lead to a further dissection of the complexity of mood disorders. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Alterations in biological rhythms have been repeatedly observed in subjects affected by mood disorders; for example, circadian mood fluctuations with typical morning worsening and improvement in the evening time have been considered a distinctive trait of those diseases, and they have been also associated with a positive outcome [Reinink et al., 1993]. Further, sleep abnormalities such as failure to fall asleep (initial insomnia), to maintain sleep (middle insomnia), and early wakening in the morning (terminal insomnia) are commonly observed. In the manic phase, a general reduction of need of sleep is almost the rule. Besides, in clinical practice, rhythm manipulations have a proven clinical efficacy: in fact, sleep deprivation is an effective treatment for depressed bipolar patients [Smeraldi et al., 1999]; conversely, a prevention of sleep loss could inhibit an early manic phase in patients affected by bipolar disorder [Wehr et al., 1987].
The endogenous control of circadian rhythms is under the control of a central pacemaker localized in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. Several genes are thought to interact in rhythmic control (see review in Reppert and Weaver, 2001) and they are called “clock” for their function of regulation of timing in biological functions [Bunney and Bunney, 2000]. In particular, the circadian locomotor output cycles kaput (CLOCK) gene was identified in mice [King et al., 1997] and in humans [Steeves et al., 1999].
In the mouse and human orthologs of clock, the high level of sequence conservation suggests a conserved function for the CLOCK protein in the circadian system of mammals. The mRNA of human CLOCK gene (4q12-GDB: 9785615) has been found in the SCN, hippocampus, piriform cortex, and cerebellum [Steeves et al., 1999], all areas involved in biological rhythms.
One polymorphism named 3111 T/C located in the 3′ flanking region has been shown to affect mRNA stability and half-life [Mignone et al., 2002]. The C allele has been associated with significantly higher “eveningness” in healthy subjects and with a delay in preferred timing for activity or sleep episodes, with no changes in sleep architecture [Katzenberg et al., 1998], though this result was not confirmed in a subsequent study [Robilliard et al., 2002]. In patients affected by mood disorders, two studies performed by our research group reported no association between CLOCK 3111 T/C polymorphism and diagnosis, and no association with circadian mood fluctuations, but found a significantly higher recurrence rate of illness episodes in bipolar patients homozygotes for the C variant [Benedetti et al., 2003] and a strong association between the C variant and an increased lifetime sleep disturbances (in particular, initial and middle insomnia) [Serretti et al., 2003].
Those findings confirmed the clinical relevance of CLOCK 3111 T/C polymorphism in mood disorders, raising the possibility of a genetic dissection of psychopathological symptoms associated with depression: the moderate and clinically not relevant phase preference associated with CLOCK C/C genotype in healthy subjects could become symptomatic insomnia in patients affected by mood disorders. We then hypothesized that the efficacy of antidepressant treatments on insomnia symptomatology may vary depending on 3111 T/C CLOCK gene variants.
METHODS
Sample
One hundred seventy-eight subjects consecutively admitted to the Mood Disorder Center, Department of Psychiatry at the Institute H. San Raffaele, Milan were included in this study (age = 52.6 ± 12.6 years; onset = 37.3 ± 12.6 years; female/male = 113/65; bipolars/major depressives = 88/90; delusional/non-delusional = 70/108). Assessment methods were previously reported [Smeraldi et al., 1998; Serretti et al., 2001]. All patients were evaluated at baseline and weekly thereafter until the sixth week using the 21-item Hamilton Rating Scale for Depression (HAM-D-21) [Hamilton, 1967] administered by trained senior psychiatrists, blind to genetic data and to treatment. Subjects for the present study are part of a larger sample where we studied the effect of CLOCK variants on lifetime insomnia and periodicity [Serretti et al., 2003]. They have been treated in the context of previous trials under double blind conditions [Smeraldi et al., 1998; Serretti et al., 2001] where 5-HTTLPR and TPH variants were studied. The procedure was the same in all trials. Briefly, after a 7-day washout period, fluvoxamine or paroxetine was administered to reach respectively, 300 mg and 20–40 mg daily from day 8 until the end of the trial. Pindolol was blindly added to approximately one-third of subjects (n = 68). Concomitant psychotropic drugs were not allowed except flurazepam at bedtime (up to 45 mg) or lithium maintenance (n = 45). Detailed data about hypnotics were not available, however, an analysis performed in a subsample of 59 individuals showed that flurazepam was administered to 80% of patients. The distribution of flurazepam was not significantly different depending on CLOCK variants χ2 = 4.29; d.f. = 2; P = n.s.) with a trend of higher use in C/C genotype. We also analyzed in this subsample, if the dose was reduced, maintained, or increased during hospitalization, and it was not different across genotypes (χ2 = 0.97; d.f. = 4; P = n.s.). Therefore, hypnotics were not considered for the calculations. HAM-D scores during treatment are the main outcome measure; insomnia symptomatology was scored based on the actual symptoms of the current week, independent from treatment. A decrease in HAM-D scores to eight or less, with delusion factor equal to zero (items 2, 15, 20) [Bellini et al., 1992; Bech et al., 1993; Sobin and Sackeim, 1997], was used to define responders. After the procedure had been fully explained to all subjects, written informed consent was obtained. Two subjects with extreme (± 1.96 SD) plasma fluvoxamine and paroxetine levels were excluded [Lucca et al., 1994].
DNA Analysis
Genomic DNA was extracted from leukocytes by NaCl precipitation [Lahiri and Nurnberger, 1991]. PCR was performed according to the methods reported by Katzenberg et al. [1998]. Amplified fragments were digested by use of Bsp 1286I restriction enzyme (New England Biolabs, England, UK). The incubation is performed at 37°C overnight and fragments were separated in agarose gels. The unrestricted PCR product (TT genotype) had a size of 221bp; complete restriction (CC genotype) produced bands of 125 and 96 bp. The sample was in Hardy–Weinberg equilibrium (χ2 = 0.33; P = 0.56).
Statistical Analysis
Seven HAM-D insomnia (items 4-5-6) scores measurements (baseline and 6 weeks) were analyzed. Repeated measures analysis of variance (MANOVA) was used to examine the differences between gene variants on HAM-D scores. Analysis of covariance (MANCOVA) was used when including covariants. An “intent-to-treat” analysis was carried out for all patients who had a baseline assessment and at least one assessment after randomization, with the last observation carried forward on the HAM-D. Variation from baseline was used for all calculations. A Student's t-test and χ2 were used to compare demographic data and baseline ratings. All P values are 2-tailed, and statistical significance was set at the 5% level (P < 0.05).
With these parameters, our sample had a high power (0.80) to detect a medium-large effect size (d = 0.74), which corresponded to a difference of approximately 0.9 points on the final HAM-D insomnia between the two genotypes [Cohen, 1988]. The analysis was performed, pooling fluvoxamine and paroxetine, given their similarity of action, previous reports suggesting similar genetic influences [Smeraldi et al., 1998; Serretti et al., 2001], and comparing C/C variants versus T/T and T/C following previous evidences [Katzenberg et al., 1998; Benedetti et al., 2003; Serretti et al., 2003].
RESULTS
The clinical outcome of the sample has been separately reported in previous studies [Smeraldi et al., 1998; Zanardi et al., 2000; Serretti et al., 2001]. A brief description of demographic and clinical variables is summarized in Table I. Baseline characteristics of subjects grouped according to CLOCK variants did not show any significant difference except for a significant association of the C/C genotype with females χ2 = 10.42; d.f. = 2; P = 0.0055).
| TT number (%) | TC number (%) | CC number (%) | Total* | χ2 | P | |
|---|---|---|---|---|---|---|
| Sex | 10.42 | 0.0055 | ||||
| Female | 59 (52.22%) | 39 (34.51%) | 15 (13.27%) | 113 | ||
| Male | 32 (49.23%) | 32 (49.23%) | 1 (1.54%) | 65 | ||
| 178 | ||||||
| Marital status | 2.86 | 0.8267 | ||||
| Single | 17 (53.13%) | 13 (40.63%) | 2 (6.24%) | 32 | ||
| Married | 45 (47.87%) | 40 (42.55%) | 9 (9.58%) | 94 | ||
| Separated/divorced | 13 (61.90%) | 6 (28.57) | 2 (9.53%) | 21 | ||
| Widowed | 3 (60.00) | 2 (40.00) | 0 (0.00%) | 5 | ||
| 152 | ||||||
| Diagnosis | 2.26 | 0.3224 | ||||
| UP | 50 (55.56%) | 31 (34.44%) | 9 (10.00%) | 90 | ||
| BP | 41 (46.59%) | 40 (45.46%) | 7 (7.95%) | 88 | ||
| 178 | ||||||
| Psychotic features | 2.14 | 0.3435 | ||||
| Absent | 39 (55.71%) | 23 (32.89%) | 8 (11.40%) | 70 | ||
| Present | 52 (48.15%) | 48 (44.44%) | 8 (7.41%) | 108 | ||
| 178 | ||||||
| Personality disorders | 0.12 | 0.9401 | ||||
| Absent | 17 (48.57%) | 14 (40.00%) | 4 (11.43%) | 35 | ||
| Present | 36 (49.32%) | 31 (42.46%) | 6 (8.22%) | 73 | ||
| 108 | ||||||
| Pindolol augmentation | 0.76 | 0.6832 | ||||
| Yes | 32 (46.38%) | 30 (43.48%) | 7 (10.14%) | 69 | ||
| No | 59 (54.13%) | 41 (37.61%) | 9 (8.26%) | 109 | ||
| 178 | ||||||
| Responders | 1.75 | 0.4168 | ||||
| Yes | 56 (53.33%) | 42 (40.00%) | 7 (6.67%) | 105 | ||
| No | 35 (47.95%) | 29 (39.72%) | 9 (12.33%) | 73 | ||
| 178 | ||||||
| Initial insomnia at baseline | 1.24 | 0.5362 | ||||
| Absent | 3 (50.00%) | 3 (50.00%) | 0 (0.00%) | 6 | ||
| Present | 88 (51.16%) | 68 (39.53%) | 16 (9.30%) | 172 | ||
| 178 | ||||||
| Middle insomnia at baseline | 0.53 | 0.7659 | ||||
| Absent | 9 (60.00%) | 5 (33.33%) | 1 (6.67%) | 15 | ||
| Present | 82 (50.31%) | 66 (40.49%) | 15 (9.20%) | 163 | ||
| 178 | ||||||
| Early morning waking insomnia at baseline | 4.32 | 0.1155 | ||||
| Absent | 9 (75.00%) | 3 (25.00%) | 0 (0.00%) | 12 | ||
| Present | 82 (49.40%) | 68 (40.96%) | 16 (9.64%) | 166 | ||
| 178 | ||||||
| Mean ± SD | Mean ± SD | Mean ± SD | F | P | ||
| Age | 52.6 ± 13.16 | 52.04 ± 12.67 | 54.81 ± 9.65 | 161 | 0.31 | 0.7347 |
| Education (years) | 9.08 ± 4.05 | 9.23 ± 3.99 | 7.61 ± 3.64 | 155 | 0.90 | 0.4076 |
| Onset | 35.87 ± 11.74 | 38.78 ± 13.42 | 39.13 ± 13.61 | 161 | 1.13 | 0.3256 |
| Number of episodes | 4.45 ± 3.71 | 4.33 ± 3.23 | 4.44 ± 3.36 | 110 | 0.02 | 0.9842 |
| Age at first lifetime treatment | 38.15 ± 12.64 | 40.20 ± 13.77 | 40.33 ± 14.01 | 142 | 0.44 | 0.6466 |
| Number of hospitalizations | 3.66 ± 4.15 | 3.83 ± 3.50 | 2.50 ± 2.11 | 138 | 0.62 | 0.5416 |
| Duration current episode (weeks) | 19.04 ± 21.14 | 16.25 ± 20.69 | 13.10 ± 12.40 | 113 | 0.48 | 0.6224 |
| Baseline HAM-D score | 28.67 ± 6.18 | 30.53 ± 7.11 | 27.94 ± 6.43 | 178 | 2.00 | 0.1383 |
| HAM-D score at T6 | 8.27 ± 9.40 | 9.15 ± 10.50 | 11.94 ± 12.74 | 178 | 0.90 | 0.4065 |
| Fluvoxamine mean blood level | 370.98 ± 201.57 | 300.81 ± 175.55 | 344.00 ± 168.03 | 147 | 1.37 | 0.2583 |
| Paroxetine mean blood level | 150.00 ± 92.97 | 104.50 ± 89.99 | — | 31 | 0.43 | 0.5424 |
- * Some data are available for subset of patients.
We then analyzed insomnia score (sum of the HAM-D items 4-5-6) during antidepressant treatment with baseline level as a covariant. CLOCK genotypes were not associated with insomnia at baseline, there was a non-significant trend in the direction of higher terminal insomnia in CLOCK C/C genotype. The CLOCK C/C genotype resulted associated with a more severe insomnia symptomatology during SSRIs treatment (see Fig. 1; MANCOVA: F = 5.04; d.f. = 1,175; P = 0.026; baseline covariant not significant). More in detail, the effect of genotype was greater for middle insomnia (P = 0.02) compared to both early and late insomnia respectively, (P = 0.07 and P = 0.05).
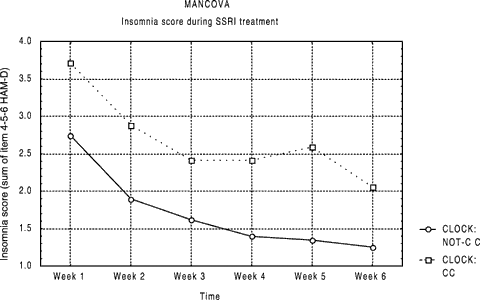
Shows the time course of response divided by CLOCK variants: subjects with the C/C variant showed a constantly higher insomnia score (F = 5.04; d.f. = 1,175; P = 0.026).
Figure 1 shows that the decrease of insomnia score followed a parallel slope of time course in the C/T and T/T subjects, but with C/C patients maintaining a higher severity throughout the treatment. The whole HAM-D score decrease comparing genotypes C/C versus others did not show any significant difference (Table I).
Following, we compared mean insomnia score at each observation time and we observed that the difference between genotypes was present as a trend since week 1, reaching significance only in week 5 (Student's t-test: week 1: P = 0.08; week 2: P = 0.08; week 3: P = 0.2; week 4: P = 0.05; week 5: P = 0.02; week 6: P = 0.06), but not at baseline (P = 0.9). Consideration of sex, presence of delusional features, and drug did not affect the genotype effect, while considering diagnosis, we observed a slightly more marked effect in bipolars subject compared to MDD. Female sex, which was associated with CLOCK*C/C genotype, was associated with higher baseline scores for middle insomnia (t = 2.64; d.f. = 169; P = 0.0092).
DISCUSSION
We observed a significant association between 3111 T/C CLOCK polymorphism and sleep disturbances during SSRI treatment in patients affected by a major depressive episode. We previously reported that the same C/C variant was associated with lifetime insomnia [Serretti et al., 2003] and mood disorders time course [Benedetti et al., 2003]. In particular, inpatients affected by recurrent major depression, the presence of the C variant was linked to a higher occurrence of initial insomnia, while inpatients affected by bipolar disorder, the same variant was associated with insomnia throughout the whole night and reduced need for sleep.
Interestingly, the present results suggest that part of the clinical picture of acute depressive episodes could be linked to stable trait features, genetically controlled, and relatively resistant to antidepressant treatment. We observed this phenomenon for insomnia, which is a stable lifetime feature in subjects with 3111 T/C CLOCK variants and it is significantly less influenced by antidepressant treatment. An early recognition of those subjects could help the clinicians to individualize the treatments, and to avoid the erroneous interpretation of an incomplete remission due to persistence of sleep disturbances. However, we should consider that the present finding was obtained in a relatively small number of subjects (16 with CLOCK C/C) and with a low significance value.
The use of hypnotic drugs may bias studies on sleep. Unfortunately, in the present sample we do not have reliable data for all subjects. However, we should consider that our analysis in a subgroup of patients did not show any significant difference between CLOCK variants. Moreover, a trend toward a heavier use of sleep inducing drugs in C/C subjects could bias toward an overtreatment of insomnia in those subjects leading to an effect opposite compared to the one we observed.
We previously investigated the influence of genes of the serotonergic pathway in response to SSRIs, however, we did not observe any association of CLOCK with total HAM-D time course but only with insomnia symptomatology, thus evidencing how polymorphisms may have a different impact on the drug response phenotype. Further, a case-control association study in subjects affected by mood disorders, showed no positive result for CLOCK and major depression [Desan et al., 2000].
The functional significance of the CLOCK polymorphism has not yet been clearly defined at a molecular level. However, it is important to underline that this polymorphism is in a well conserved region between mice and humans, and that in mouse it contains several functional polyadenylation signals [King et al., 1997]. Alternatively, this polymorphism could be in linkage disequilibrium with others, as yet unidentified, but more functionally significant [Savov et al., 1995].
Differences in allele frequency for different populations have been reported [Desan et al., 2000]. However, our sample was genetically homogenous [Gasparini et al., 1997].
Overall, our findings suggest that CLOCK genotype influences the severity of insomnia during antidepressant treatment of a major depressive episode; this together with previous findings on this polymorphism lead to further dissect the complexity of mood disorders.




