Severe myoclonus-dystonia syndrome associated with a novel epsilon-sarcoglycan gene truncating mutation
Abstract
Myoclonus-dystonia syndrome (MDS) is an autosomal dominant disorder characterized by myoclonic and dystonic muscle contractions, associated with psychiatric manifestations. MDS is usually considered as a benign disease. In most of the families, MDS is linked to chromosome 7q21 and mutations within epsilon-sarcoglycan (SGCE) gene have been recently described. We report a MDS family with a severe and heterogeneous phenotype, including myoclonus with important functional impact and several psychiatric features, characterized by obsessive-compulsive disorder, depression, and anxiety. This phenotype was shown to be associated with a novel truncating mutation located within exon 4 of SGCE. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Inherited myoclonus-dystonia syndrome (MDS, DYT 11; MIM: 159900) is a rare movement disorder that is characterized by myoclonic jerks and dystonia. Myoclonic jerks affect mainly proximal muscles of the extremities. Mild dystonia is common but rarely the sole symptom of the disease. Myoclonic dystonia may be highly responsive to alcohol ingestion. In addition, patients often show psychiatric symptoms, including panic attacks, anxiety, depression, and obsessive-compulsive disorder (OCD) [Nygaard et al., 1999]. Age of onset is usually in the first or second decade. Evolution is usually benign and compatible with normal life [Gasser, 1998]. The mode of inheritance is autosomal dominant with reduced penetrance. A missense mutation in a conserved region of the D2 dopamine receptor (DRD2) on chromosome 11q23 was shown to cosegregate with MDS in one large family [Klein et al., 1999]. Nevertheless, in most of the MDS families, the disease is linked to a second locus on chromosome 7q21-31 [Nygaard et al., 1999]. Five different heterozygous truncating mutations within the epsilon-sarcoglycan (SGCE) gene were recently reported in six MDS families [Zimprich et al., 2001]. SGCE is a component of the dystrophin–sarcoglycan complex, which links the extracellular matrix to the cytoskeleton. SGCE is probably involved in cell adhesion and tissue integrity. In this study, we report the detailed phenotype, including neuropsychological and psychiatric assessment, of a large French MDS family in which a novel mutation of the SGCE gene segregates. This family demonstrates that SGCE mutation can result into a severe phenotype not compatible with normal life.
PATIENTS AND METHODS
Subjects
This family included 14 individuals (Fig. 1). Clinical and molecular investigations were performed with the informed consent of participating family members. Clinical diagnosis of MDS was established according to published criteria [Gasser, 1998]. Age of onset, neurological signs, and psychiatric features were established by consulting medical records, by individual examination in 12 cases, and by interview of other family members for two deceased cases (I.1, III.3). Myoclonic jerks were assessed by the Unified Myoclonus Rating Scale [UMRS, Frucht et al., 2002] and by videotaping. Algorithms for the DSM-IV [American Psychiatric Association, 1994] diagnosis of OCD, generalized anxiety disorder, major affective disorder (recurrent depression), alcohol abuse without alcohol dependence and alcohol dependence were used. Relatives were investigated with a battery of tests assessing memory [Grober and Buschke Verbal Learning Test, Grober and Buschke, 1987], visuospatial abilities [Rey Figure Copy, Rey, 1970], non-verbal (Standard Progressive Matrices) and verbal (Mill Hill) intellectual functions [Raven, 1998]. Scores on the tests were considered abnormal if they were more than two standard deviations below the age and sex related means from the normative data. Psychiatric assessment, included the Montgomery–Asberg Depression Rating Scale [MADRS, Montgomery and Asberg, 1979], the Yale–Brown Obsessive Compulsive Scale [Y-BOCS, Goodman et al., 1989], and the Goldberg Anxiety Rating Scale [Goldberg et al., 1988]. Subjects were considered impaired if their score was above the cut-off point, either determined by the instruction of the scale or used in previous studies [Goldberg et al., 1988; Zitterl et al., 2001; Marangell et al., 2002]: 12/60, 4/9, 16/40 for MADRS, Anxiety Rating Scale, and Y-BOCS, respectively.
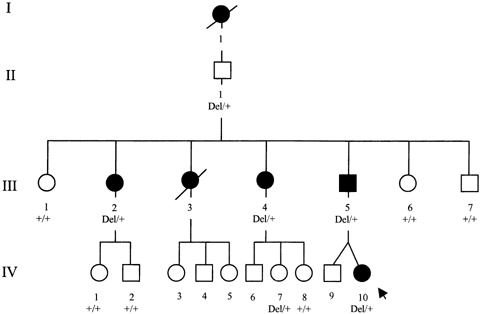
Partial pedigree of the family. The proband was indicated by an arrow.
Molecular Studies
DNA was extracted from blood samples using the QIAGEN blood DNA kit (Qiagen). The 11 exons of the SGCE gene were PCR-amplified using intronic primers (sequences available upon request). Sequencing reactions were performed using the DYEnamic ET Terminator Cycle Sequencing kit (Amersham Pharmacia Biotech, Piscataway, NJ) and sequences were analyzed on an ABI 377 automated DNA sequencer (Applied Biosystems, Perkin Elmer-Cetus, Foster City, CA). Screening for the 4 bp deletion of exon 4 in relatives was performed on genomic DNA by restriction fragments analysis since this mutation abolishes an Ase I restriction site. A 529 bp fragment was PCR-amplified using exon 4 primers and one fifth of the PCR reaction was digested with 1 U of Ase I restriction enzyme (New England Biolabs, Beverly, MA) in a final volume of 20 μl for 2 h. Fragments were then resolved on a 3% GTG agarose (FMC) gel. Wild type allele leads to two fragments of 201 and 328 bp.
RESULTS
The proband (IV.10) was a 4-year-old girl who belonged to a French four-generation pedigree (Fig. 1). Since she was 2 years old, she had severe myoclonus with important functional impact. She had difficulty in walking because of dystonia of the right leg. She was examined at age of 4 years: action myoclonus was so severe that she could not eat or drink alone and global disability score of UMRS was the most severe. Her learning for drawing and writing was impaired. She had normal psychomotor development, comparable to that of her twin brother. Clinical, neuropsychological, psychiatric findings observed among the affected and non affected relatives are presented in Table I. Three relatives were also affected. For two other relatives who were deceased (Fig. 1: I.1 and III.3), probable MDS diagnosis was based on interview and medical records. I.1 had myoclonic jerks with important functional impact and died at 73. III.3 had myoclonic jerks and was suffering of OCD and severe depression with major consequences in her family and social life. Several admissions were required in psychiatric hospital. She died by suicide at 33. In the six affected patients, the predominant clinical feature was myoclonus. Only subject III.5 reported less severe myoclonic symptoms after ingestion of alcohol but did not use alcohol.
| Relatives (age, years) | Genetic status | UMRS global disability (/4) | Localization of dystonia | Age of onset (years) | Alcohol sensitivity | Memorya TR3/DTR (/16) | Verbal efficiencyb (/88) | Non-verbal efficiencyc (/60) | Visuospatial functiond (/36) | Depressione (/60) | Anxietyf (/9) | Obsessive-compulsive disorderg (/40) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II.1 (74) | Del/+ | 0 | — | — | 9**/10*** | 27** | 15* | 19*** | 0 | 0 | 0 | |
| III.2 (48) | Del/+ | 1 | Arm | 20 | No | 16/16 | 53* | 35* | 31*** | +:12# | +:7 | 3 |
| III.4 (43) | Del/+ | 2 | — | 30 | No | 16/16 | 40*** | 29* | 22*** | 7 | +:9 | +:18 |
| III.5 (38) | Del/+ | 2 | Writer's cramp leg | 5 | Yes | 7***/4*** | 34** | 15*** | 26*** | 10 | +:7 | +:19 |
| IV.10 (4) | Del/+ | 4 | 2 | ? | ? | ? | ? | ? | ? | ? | ? | |
| IV.7 (17) | Del/+ | 0 | — | — | 15***/15*** | 32** | 41 | 32 | 0 | 0 | 0 | |
| III.1 (51) | +/+ | 0 | 16/16 | 73 | 41 | 34 | 5 | 0 | 0 | |||
| III.6 (36) | +/+ | 0 | 14***/14*** | 6*** | 26** | 30** | 0 | 1 | 2 | |||
| III.7 (34) | +/+ | 0 | 16/15*** | 19*** | 40 | 34 | 4 | 0 | 0 | |||
| IV.1 (30) | +/+ | 0 | 16/16 | 73 | 50 | 36 | 0 | 0 | 0 | |||
| IV.2 (24) | +/+ | 0 | 16/16 | 55 | 42 | 36 | 9 | +:9 | 0 | |||
| IV.8 (15) | +/+ | 0 | 16/16 | 27*** | 39 | 30 | 0 | 0 | 0 |
- ?, Not applicable because of age; UMRS, unified myoclonic rating scale; −, absence of sign; +, above the cut-off; *, score ≤ −1 standard deviation (SD); **, ≤ −2 SD; ***, ≤ −3 SD; #, with antidepressant.
- a Grober and Buschke verbal learning test (TR3: third total recall/DTR: delayed total recall).
- b Mill Hill test.
- c Standard Progressive Matrices Test.
- d Rey figure copy.
- e Montgomery–Asberg Depression Rating Scale.
- f Goldberg Anxiety Rating Scale.
- g Yale–Brown Obsessive Compulsive Scale.
Sequencing analysis of the SGCE gene revealed in the index case a 4 bp deletion within exon 4 (484–487 del) resulting into a premature stop codon, one codon downstream. Segregation analysis (Fig. 1) confirmed the presence of the mutation in the affected relatives.
DISCUSSION
We studied a French family with autosomal dominant myoclonus-dystonia and identified a novel truncating mutation within exon 4 of the SGCE gene. Like previously reported SGCE alterations, this mutation obviously results into a loss of function [Zimprich et al., 2001]. In mice, SGCE is an imprinted gene mainly expressed from the paternal allele in all adult tissues, with a residual weak expression of the maternal allele in brain [Piras et al., 2000]. Segregation analysis of MDS families linked to the SGCE locus had previously suggested that the penetrance of the pathological genotype is highly dependent on the parental origin of the disease allele. In agreement with this observation, asymptomatic mutation carriers II.1 and IV.7 (Fig. 1) carried the mutation on their maternal chromosome.
While MDS is usually considered as benign genetic disease, the observation of the index case, who was incapacitated and dependent for daily life since the childhood, demonstrates that SGCE mutation can result into a severe phenotype.
In some families with MDS, OCD, generalized anxiety disorder, major affective disorder, and alcohol dependence had been previously observed [Klein et al., 1999; Nygaard et al., 1999; Asmus et al., 2001]. These families were not systematically assessed for psychiatric disorders and before the 7q21 locus identification, it was impossible to determine whether these features segregate with the mutant allele or not. Recently, Saunders-Pullman et al. [2002], reported a possible association between OCD and MDS. In agreement with this observation, in our family, OCD was detected in three MDS affected subjects (Table I) but in none relative without SGCE mutation. Depression, leading to a suicide in one case, was also observed only in SGCE mutation carriers. Although one cannot systematically exclude a random cosegregation, description of this family suggests that OCD and depression could be linked to the SGCE mutation. Assessment of additional families with SGCE mutation is necessary to compare the presence of alcohol dependence and generalized anxiety disorder between carriers and non-carriers.
Within this family, several subjects had a poor scholar performance. Neuropsychological assessment confirmed that at least one cognitive function (memory, verbal, non-verbal, and visuospatial intellectual abilities) was impaired in eight relatives (Table I). Nevertheless, these cognitive difficulties were not linked to the SGCE mutation since they did not cosegregate with the mutation. Therefore, in agreement with previous descriptions, cognitive impairment cannot be considered as part of the MDS phenotype.
Acknowledgements
We thank the subjects and their families for their cooperation. LM was supported by la Fondation pour la Recherche Médicale.




