An Update on 3M Syndrome: Review of Clinical and Molecular Aspects and Report of Additional Families
Funding: The authors received no specific funding for this work.
ABSTRACT
3M syndrome is a rare autosomal recessive disorder characterized by short stature and recognizable facial and musculoskeletal features. Pathogenic variants in the CUL7, OBSL1, and CCDC8 genes are implicated in the pathogenesis of 3M syndrome. In this review, we discuss the history, epidemiology, molecular basis, clinical features, and management strategies for 3M syndrome. Moreover, we report on 11 new patients (from 9 unrelated families) with short stature and dysmorphic features consistent with 3M syndrome, in whom we identified five novel pathogenic variants expanding the genetic landscape of the syndrome. Finally, we have reviewed the molecularly confirmed cases of 3M published to date.
1 Introduction and Historical Context
3M syndrome (OMIM #273750) is a rare autosomal recessive disorder characterized by pre- and postnatal growth retardation, proportionate short stature, recognizable facial features, and distinctive skeletal malformations, with intelligence typically remaining unaffected (Winter et al. 1984). The name “3M” refers to the last initials of three researchers (Miller, McKusick, and Malvaux) who first described the condition in 1975 (Miller et al. 1975). Thirty years later, using homozygosity (autozygosity) mapping in 7 consanguineous families with 3M syndrome, Huber et al. reported linkage of the underlying gene to a 3.84-Mb interval on chromosome 6p21.1, and subsequent analyses revealed pathogenic variants within CUL7 (Huber et al. 2005). In 3M syndrome patients who did not carry CUL7 variants, pathogenic variants were subsequently identified in the OBSL1 gene and later in CCDC8 (Geisler et al. 2007; Hanson, Murray, O'Sullivan, et al. 2011).
1.1 Epidemiology of 3M Syndrome
3M syndrome is an extremely rare disorder, with fewer than 300 cases reported in medical literature worldwide (Orphanet Report Series 2024). Notably, the prevalence of 3M syndrome may be underestimated due to the normal mental development and nonspecific presentation of growth retardation. Therefore, the exact prevalence is unknown, and the actual number of cases could be higher than that previously reported. Advances in molecular diagnostics, such as next-generation sequencing (NGS), have led to increased detection of 3M syndrome in recent years. The majority of molecularly confirmed cases have been reported in isolated populations or communities with higher rates of consanguinity, such as the Middle East and South Asia, reflecting the autosomal recessive inheritance pattern (Huber et al. 2005; Maksimova et al. 2007; Simsek-Kiper et al. 2019; Tuysuz et al. 2021; Temtamy et al. 2006; Lee et al. 2020; Al-Dosari et al. 2012; Khachnaoui-Zaafrane et al. 2022; Akalin et al. 2023; Ceylan et al. 2023; Karacan Kucukali et al. 2023; Jacob and Girisha 2021; Alkhawaldeh et al. 2024). However, sporadic cases have been reported worldwide, highlighting the global nature of the disorder (Huber et al. 2005; Maksimova et al. 2007; Ceylan et al. 2023; Huber et al. 2009; Hanson et al. 2009; Sasaki et al. 2011; Meazza et al. 2013; Takatani et al. 2018; Huber et al. 2010; Hu et al. 2017; Takizaki et al. 2020; Shaikh et al. 2019; Wang et al. 2024).
1.2 Genetics and Etiology of 3M Syndrome
3M syndrome is a genetically heterogeneous disorder. To date, the disease has been associated with biallelic pathogenic variants in three separate genes: Cullin-7 (CUL7-609577), Obscurin-like 1 (OBSL1-610991) and Coiled coil domain containing protein 8 (CCDC8-614145) (Huber et al. 2005; Hanson et al. 2009; Hanson, Murray, Sud, et al. 2011; Hanson et al. 2012; Demir et al. 2013; Keskin et al. 2017; Hu et al. 2020; Luo et al. 2024; Yang and Patni 2020; Xu et al. 2023).
It is still unclear how gene variants are linked to the clinical manifestations of 3M syndrome. Nonetheless, these three genes were found to encode proteins that are essential components of the ubiquitin-proteasome system, which regulates protein degradation and cellular growth (Yan et al. 2014). The majority of genetically confirmed 3M syndrome patients have been identified with CUL7 variants (~70%) with OBSL1 accounting for 25% of cases and pathogenic variants in CCDC8 identified in the remaining 5% (Huber et al. 2005; Geisler et al. 2007; Hanson, Murray, Sud, et al. 2011; Luo et al. 2024; Deeb et al. 2015; Liao et al. 2017; Dauber et al. 2013).
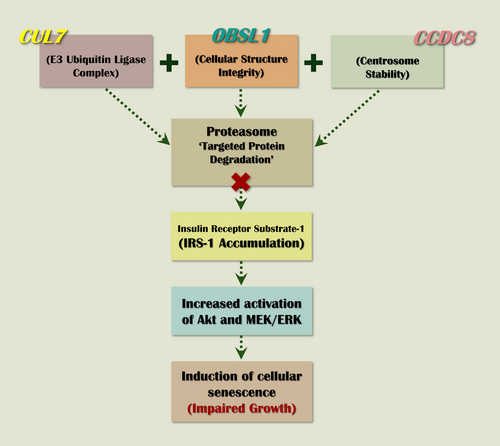
The primary pathogenic mechanism in 3M syndrome involves the dysregulation of protein degradation within the ubiquitin-proteasome system. CUL7, a cullin scaffold protein, assembles an E3 ubiquitin ligase complex, which tags target proteins for degradation by the 26S proteasome (Willems et al. 2004; Dias et al. 2002). OBSL1 and CCDC8 work in conjunction with CUL7 to stabilize the E3 ubiquitin ligase complex. OBSL1 is thought to mediate protein–protein interactions within the complex, while CCDC8 supports structural integrity. The CUL7-OBSL1-CCDC8 complex is crucial for cellular processes such as cell cycle control, apoptosis, and signal transduction, and it is required to regulate microtubule dynamics and genome integrity (Yan et al. 2014). Pathogenic variants in any of these genes impair proteasomal activity, leading to the diverse clinical manifestations observed in 3M syndrome. However, loss of ubiquitination may be the main pathological mechanism underlying 3M syndrome (Hanson, Murray, Sud, et al. 2011; Hanson et al. 2014), and according to functional experiments, insulin receptor substrate 1 (IRS-1), a critical mediator of the insulin/insulin-like growth factor 1 (IGF-1) signaling, has been identified as a proteolytic target of the CUL7 E3 ubiquitin-dependent degradation (Xu et al. 2008). In support of these observations, embryonic fibroblasts of Cul7 −/− mice were found to accumulate IRS-1 and exhibit increased activation of IRS-1's downstream Akt and MEK/ERK pathways (Xu et al. 2008). It is postulated that the overstimulation of these pathways ultimately leads to induction of cellular senescence (Litterman et al. 2011), which is a contributing factor for the pathogenesis of growth restriction observed in patients with 3M (Figure 1).
1.3 Clinical Features of 3M Syndrome
Patients with 3M syndrome exhibit a unique set of clinical manifestations, including growth retardation, craniofacial features, and skeletal abnormalities. However, the range and severity of symptoms and physical features are very variable. Individuals with 3M syndrome generally retain normal cognitive abilities, although some cases have reported slight developmental delay (Huber et al. 2005; Irving and Holder-Espinasse 1993; Hennekam et al. 1987).
1.3.1 Growth Retardation
The primary manifestation is severe pre- and postnatal growth failure, resulting in proportionate short stature. There is no catch-up growth, and the final adult height is often 4–6 standard deviations below the mean (Hennekam et al. 1987; Huber et al. 2011; van der Wal et al. 2001). From an endocrine point of view, serum growth hormone (GH) levels are usually normal and IGF-1 is normal or low, while the growth response to recombinant human GH therapy is variable but usually poor (Clayton et al. 2012).
1.3.2 Craniofacial Features
The characteristic craniofacial features typically include relative macrocephaly, dolichocephaly, frontal bossing, triangular-shaped face with broad forehead and pointed chin, flat midface, fleshy tipped nose, long philtrum, and large ears. In addition, some affected children may display overcrowding of teeth (Miller et al. 1975; Temtamy et al. 2006; Dauber et al. 2013; van der Wal et al. 2001).
1.3.3 Musculoskeletal Abnormalities
The main skeletal anomalies are long, slender tubular bones, reduced anteroposterior diameter of the vertebral bodies, small pelvic bones, and delayed bone age. Other manifestations include joint hypermobility and dislocation, short neck, square shoulders, short thorax, transverse chest groove, hyperlordosis, winged scapulae, pes planus, clinodactyly of fifth fingers, and developmental hip dysplasia. In younger patients, prominent fleshy heels are an almost universal feature (Maksimova et al. 2007; Hanson et al. 2009; Hennekam et al. 1987; van der Wal et al. 2001).
1.3.4 Genitourinary Anomalies
Males with 3M syndrome may manifest gonadal dysfunction and subfertility or infertility as documented by high follicle-stimulating hormone (FSH) levels, low testicular volume, and abnormal semen analysis (Irving and Holder-Espinasse 1993; van der Wal et al. 2001). Hypospadias has been seen in a few males with 3M syndrome. Female gonadal function appears normal (Irving and Holder-Espinasse 1993).
1.4 Differential Diagnosis
3M syndrome was reported to share a high degree of similarity with two other short stature conditions: gloomy face syndrome (Merrer and Maroteaux 1991) and Yakut short stature syndrome (Maksimova et al. 2007). Both were confirmed to have the same genetic cause as 3M syndrome and are therefore considered to be part of a wider 3M syndrome spectrum.
Nevertheless, 3M syndrome shares overlapping features with other syndromic growth restriction disorders, requiring careful differentiation. The main differential diagnosis is Silver-Russel syndrome (OMIM #180860), which is characterized by proportionate short stature, normal head circumference, limb length asymmetry, and feeding difficulties (Price et al. 1999). Other differential diagnoses include Mulibrey nanism (OMIM #605073) characterized by prenatal and post-natal growth deficiency (often less severe than in infants with 3M), yellowish dots on the fundus, pericardial constriction, hepatomegaly, J-shaped sella turcica, and fibrous dysplastic lesions of the tibiae (Avela et al. 2000).
Both syndromes are characterized by relative macrocephaly, a triangular face with frontal bossing and a pointed chin, which resembles 3M syndrome. However, the other conditions lack the characteristic radiologic features of 3M (Irving and Holder-Espinasse 1993).
1.5 Investigation and Management Considerations
1.5.1 Diagnostic Approaches
The diagnosis of 3M syndrome is primarily based on clinical and radiological findings, further confirmed by genetic testing.
1.5.1.1 Clinical Diagnosis
The diagnosis of 3M syndrome begins with the recognition of the characteristic clinical features described in the previous section.
1.5.1.2 Radiological Evaluation
The clinical diagnosis of 3M syndrome is supported by specific radiographic findings, which include slender diaphyses of the long bones with normal epiphyses, delayed bone age, foreshortened vertebral bodies, small pelvic bones, and a broad thorax with slender and horizontal ribs. These radiographic features are highly variable and most often present after age 2 years (Huber et al. 2005; Irving and Holder-Espinasse 1993).
1.5.1.3 Molecular Diagnosis
Definitive diagnosis is established via genetic testing, which would identify biallelic pathogenic variants in the CUL7, OBSL1, or CCDC8 genes. Current molecular diagnostic approaches include Sanger sequencing, targeted gene panels, exome sequencing, and genome sequencing.
1.5.1.4 Endocrinologic Assessment
It is crucial that individuals with 3M syndrome see an endocrinologist at the time of diagnosis to assess for GH deficiency and to evaluate gonadal function in pubertal males (Irving and Holder-Espinasse 1993).
1.5.2 Management Strategies
There is no specific treatment for 3M syndrome. Management focuses on addressing growth failure and associated complications.
1.5.2.1 Supportive Care
Supportive care is the cornerstone of managing 3M syndrome, focusing on optimizing growth potential and addressing skeletal complications. Early nutritional support is essential to mitigate secondary effects of growth retardation.
1.5.2.2 Growth Hormone Therapy
The use of GH treatment is controversial, and its efficacy remains doubtful. Although most early research found GH ineffective and did not recommend GH treatment (Meazza et al. 2013; Huber et al. 2011), some recent studies claim that GH treatment may be effective in 3M syndrome (Lee et al. 2020; Isik et al. 2021), particularly in high doses (van der Wal et al. 2001). A recent study (Karacan Kucukali et al. 2023) reported a good response during the early stages of the long-term GH treatment, which decreased in the following years, and the desired improvement in height could not be achieved.
1.5.2.3 Orthopedic Interventions
Significant joint laxity or hypermobility should prompt orthopedic interventions to control the development of arthritis. Orthopedic assessment and physiotherapy may also be required in cases of severe back pain due to excessive lordosis (Clayton et al. 2012). Corrective procedures such as spinal fusion or osteotomies can significantly improve functional mobility and alleviate pain. Surgical bone lengthening may be an option (Irving and Holder-Espinasse 1993).
1.5.2.4 Long-Term Management and Surveillance
Given the chronic nature of the condition, a multidisciplinary team approach is essential. Endocrinologists, geneticists, orthopedists, and rehabilitation specialists should collaborate to optimize patient outcomes. Regular follow-up visits are vital to monitor growth progression and to ensure early detection of musculoskeletal complications. Monitoring of growth every 6–12 months on standard growth charts should be performed, with special attention to growth velocity (Irving and Holder-Espinasse 1993).
2 Case Reports
Herein, we are reporting 11 patients, from nine unrelated families, who all had short stature and fulfilled the diagnostic criteria for 3M syndrome. A detailed medical history was taken, and a thorough clinical examination, including careful recording of dysmorphic features, was performed. Anthropometric measurements were obtained and plotted on the Egyptian standard growth charts. In addition, a complete skeletal survey was requested. After the initial diagnosis of 3M syndrome, based on the clinical and radiological findings, molecular confirmation was pursued.
High molecular weight genomic DNA was extracted from a peripheral blood sample. Exome sequencing (ES) was performed following the CAP/CLIA validated standard operating protocol by 3billion (Seoul, Republic of Korea). Briefly, exome capture was performed using the xGen Exome Research Panel v2 (Integrated DNA Technologies, Coralville, Iowa, USA) and sequencing was performed using the NovaSeq platform (Illumina, San Diego, CA, USA) as 150 bp paired-end reads. Sequencing data were aligned to the GRCh37/hg19 human reference genome using BWA-MEM and processed for variant calling by GATK v.3 (van der Auwera et al. 2013; Li and Durbin 2011). Variants were then annotated by the Ensembl Variant Effect Predictor (VEP) and filtered and classified by EVIDENCE (Seo et al. 2020), following the American College of Medical Genetics and Genomics (ACMG) guidelines (Jacob and Girisha 2021; Richards et al. 2015). The filtered and classified variant list was manually reviewed by medical geneticists and physicians. The most likely variants that could explain the patient's phenotype were selected for reporting.
2.1 Clinical Phenotype
All enrolled 3M patients—eight males and three females—were born to healthy, consanguineous parents, with no significant family history, except for one family with three affected cousins. The studied cohort included 2 adults, 7 children, and 2 infants, with a mean age of 9.75 years at the time of diagnosis (range 8 months to 21 years). The demographic and history data, clinical findings, and molecular results of the entire cohort are summarized in Table 1.
| Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | Family 7 | Family 8 | Family 9 | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |||
| Personal history | |||||||||||||
| Sex | M | M | M | M | F | F | F | M | M | M | M | 8M 3F | |
| Age | 7.5 y | 11.5 y | 1 y | 8.5 y | 13 y | 7 y | 21 y | 21 y | 8 m | 5 y | 11 y | 8m–21y | |
| Consanguinity | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive | 9/9 | |
| Family history | Negative | Negative | Negative | Negative | Negative | Positive | Positive | Positive | Negative | Negative | Negative | 1/9 | |
| Pregnancy and delivery history | |||||||||||||
| Gestational age | |||||||||||||
| Complications | − | − | − | − | − | − | − | − | − | − | − | 0/11 | |
| Low BW | − | + | + | − | − | + | + | + | + | + | + | 8/11 | |
| Anomalies noted at birth | − | − |
+ Short stature |
+ Club foot |
+ Cong. hip dislocation |
+ Cong. hip dislocation |
− | − |
+ Short limbs |
+ Short limbs & ASD |
+ Club foot |
7/11 | |
| Development | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | NA | Normal | Normal | 10/10 | |
| General examination | |||||||||||||
| Cognition | Normal | Normal | NA | Normal | Normal | Normal | Normal | Normal | NA | Normal | Normal | 9/9 | |
| Abnormal voice | + | − | − | − | − | − | − | − | NA | + | + | 3/10 | |
| Weight | Below P3 | Below P3 | Below P3 | Below P3 | P10 | Below P3 | P25 | P10 | P10 | P50 | Below P3 | ||
| Height | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | Below P3 | ||
| HC | P25 | P25 | P25 | P10 | P50 | P25 | Above P97 | P50 | P10 | P10 | Just below P3 | ||
| Short stature | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Disproportionate short stature | − | − | + | − | − | − | − | + | + | − | − | 3/11 | |
| Craniofacial features | |||||||||||||
| Large head | + R | + R | + R | + R | + | + R | + | + | + R | + R | + R | 11/11 | |
| Dolichocephaly | + | − | + | − | + | + | + | + | − | + | + | 8/11 | |
| Triangular face | + | + | + | + | + | + | + | − | + | + | + | 10/11 | |
| Frontal bossing | + | − | + | + | + | + | + | + | + | − | + | 9/11 | |
| Midface retrusion | + | − | + | + | + | + | + | + | + | − | + | 9/11 | |
| Thick eyebrows | − | + | + | + | + | + | + | + | − | + | − | 8/11 | |
| Fleshy nasal tip | + | + | + | − | + | + | + | + | + | + | + | 10/11 | |
| Long philtrum | + | + | + | + | − | + | + | + | + | + | + | 10/11 | |
| Full lips | + | + | − | − | + | − | + | + | + | + | + | 8/11 | |
| Prominent ears | + | + | + | + | + | + | + | − | − | + | + | 9/11 | |
| Pointed chin | + | + | + | + | + | + | + | − | + | + | + | 10/11 | |
| Musculoskeletal features | |||||||||||||
| Loose joints | + | − | + | + | + | + | + | + | + | + | + | 10/11 | |
| Short broad neck | + | + | + | − | + | + | + | + | + | + | − | 9/11 | |
| Short thorax | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Square shoulders | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Pectus deformity | + | + | + | + | − | − | − | − | + | + | + | 7/11 | |
| Hyperlordosis | + | + | − | + | + | + | + | + | + | + | + | 10/11 | |
| Prominent fleshy heels | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Pes planus | + | + | + | + | + | + | + | + | + | + | + | 11/11 | |
| Short 5th finger | + | − | − | + | + | + | + | + | + | + | + | 9/11 | |
| DHD | − | − | − | − | + | + | − | − | − | − | − | 2/11 | |
| Genitourinary anomalies | |||||||||||||
| Hypogonadism | NA | − | − | NA | − | NA | − | − | NA | NA | − | 0/6 | |
| Hypospadias | − | − | − | − | NA | NA | NA | − | − | − | − | 0/8 | |
| Radiographic features | |||||||||||||
| Slender long bone | − | ND | + | ND | ND | + | + | ND | + | − | + | 5/7 | |
| Tall vertebral bodies | − | ND | − | ND | ND | − | − | ND | − | − | + | 1/7 | |
| Growth hormone status | |||||||||||||
| GH deficiency | + | − | ND | + | + | − | − | ND | − | − | + | 4/9 | |
| Molecular results | |||||||||||||
| 3M syndrome | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 1 | 3-M 2 | 3-M 2 | 3-M 2 | ||
| Gene | CUL7 | CUL7 | CUL7 | CUL7 | CUL7 | CUL7 | CUL7 | CUL7 | OBSL1 | OBSL1 | OBSL1 | ||
| Genome position | 6-43,014,674-T-A | 6-43,015,905-GACCGCAGGC-TG (GRCh37) | 6-43,015,905-GACCGCAGGC-TG (GRCh37) | 6-43,013,324-C-T (GRCh37) | 6-43,013,724-CGAGTT-C (GRCh37) | 6-43,006,723-G-A | 6-43,006,723-G-A | 6-43,006,723-G-A | 2-220430050-A-ATCAGACGGTGTTTGCGCCCATCCATCTTC (GRCh37) | 2-220,435,919-C-CG (GRCh37) | 2-220,435,919-C-CG (GRCh37) | ||
| cDNA | NM_014780.5:c.2341A>T | NM_014780.5:c.2141_2150delinsCA | NM_014780.5:c.2141_2150delinsCA | NM_014780.5:c.2862+1G>A | NM_014780.5:c.2761_2765del | NM_014780.5:c.4297C>T | NM_014780.5:c.4297C>T | NM_014780.5:c.4297C>T | NM_015311.3:c.2292_2320dup | NM_015311.3:c.35dup | NM_015311.3:c.35dup | ||
| Protein | NP_055595.2:p.Lys781Ter | NP_055595.2:p.Cys714Serfs Ter5 | NP_055595.2:p.Cys714SerfsTer5 | NP_055595.2:p.? | NP_055595.2:p.Asn921GlyfsTer36 | NP_055595.2:p.Gln1433Ter | NP_055595.2:p.Gln1433Ter | NP_055595.2:p.Gln1433Ter | NP_056126.1:p.Ile774ArgfsTer10 | NP_056126.1:p.Cys13ValfsTer241 | NP_056126.1:p.Cys13ValfsTer241 | ||
| Molecular consequence | Nonsense | Frameshift | Frameshift | Splice donor site | Frameshift | Nonsense | Nonsense | Nonsense | Nonsense | Frameshift | Frameshift | ||
| Allele frequency in population databases | GnomAD (Exome) | 0 | 0 | 0 | 0 | 0 | 0.000008 | 0.000008 | 0.000008 | 0 | 0.00008 | 0.00008 | |
| GnomAD (Genome) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00007 | 0.00007 | ||
| ExAC | 0 | 0 | 0 | 0 | 0 | 0.000008 | 0.000008 | 0.000008 | 0 | 0.0002 | 0.0002 | ||
| Novel (N)/Reported (R) | N |
N |
N |
N |
N |
R | R | R |
N |
R | R | ||
| Zygosity | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | ||
| ACMG/AMP evidences | PVS1_very strong PM2_moderate | PVS1_very strong PM2_moderate |
PVS1_very strong PM2_moderate |
PVS1_ strong PM2_moderate | PVS1_very strong PM2_moderate | PVS1_very strong PM3_strong PM2_moderate | PVS1_very strong PM3_strong PM2_moderate) | PVS1_very strong PM3_strong PM2_moderate |
PVS1_very strong PM2_moderate |
PVS1_very strong PM3_very strong PM2_moderate |
PVS1_very strong PM3_very strong PM2_moderate |
||
| ACMG Classification | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Pathogenic | Pathogenic | ||
All the patients were born uneventfully at term. Their intellectual and motor development was normal. Although all the patients manifested postnatal growth retardation and short stature, only 72.7% (8/11) had prenatal growth retardation. At birth, disproportionate short stature (short limbs) was recognized in only three patients. Additionally, congenital hip dislocation and talipes equinovarus were each reported in two patients.
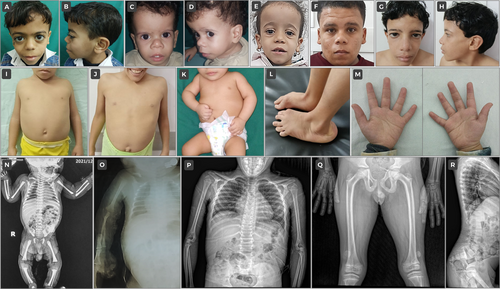
On examination, growth retardation/short stature, relative macrocephaly, square shoulders, short broad thorax, pes planus, and prominent fleshy heels were consistent features recorded in 100% of the cases. Triangular face was also noted in all cases, while other features like frontal bossing, flat midface, fleshy nasal tip, long philtrum, prominent ears, and pointed chin were observed in over 80%. Moreover, of the classic musculoskeletal features of the syndrome, joint hypermobility and hyperlordosis were noted in 90.9% (10/11) of cases. A short and broad neck and short fifth fingers were each evident in 81.8% (9/11) of the patients. Of the available skeletal radiographs, 71.4% (5/7) showed slender long tubular bones, but tall vertebral bodies could be detected in only one patient (1/7; 14.3%). Figure 2 shows some of the phenotypic features displayed by the current cohort.
2.2 The Genetic Profile of 3M Syndrome in Egyptian Patients
Seven distinct pathogenic/likely pathogenic (P/LP) homozygous variants were detected in two genes: CUL7 and OBSL1, while no variants were identified in CCDC8. All variants were predicted to be null variants, including three nonsense, three frameshift, and one splicing variant. The CUL7 gene was most frequently involved, occurring in six different families.
One variant, OBSL1 c.35dup (p.Cys13ValfsTer241), was reported in an Indian patient (Jacob and Girisha 2021). Another variant, CUL7 c.4297C>T (p.Gln1433Ter), was found in an extremely low allele frequency in gnomAD population databases < 0.001%. The other five variants are novel, including OBSL1 c.2292_2320dup (p.Ile774ArgfsTer10) and CUL7 c.2341A>T (p.Lys781Ter), c.2141_2150delinsCA (p.Cys714SerfsTer5), c.2862+1G>A (p.?), and c.2761_2765del (p.Asn921GlyfsTer36). One novel variant, CUL7 c.2141_2150delinsCA, was identified in two unrelated families, and the previously reported OBSL1 variant (c.35dup) was also harbored by two unrelated families.
2.3 Review of Literature of 3M Syndrome (Molecularly Confirmed Cases)
On comprehensive research of literature, 281 genetically confirmed 3M syndrome patients, from 221 families, were found. More than 30 (34) articles and case reports have been published in the last 20 years since Huber et al. first described CUL7 variants in families with 3M syndrome (Huber et al. 2005). In Table S1, we summarize the molecular findings and the phenotypic features of all the genetically confirmed cases of 3M syndrome, which were reported prior to December 2024 (Huber et al. 2005; Hanson, Murray, O'Sullivan, et al. 2011; Maksimova et al. 2007; Simsek-Kiper et al. 2019; Tuysuz et al. 2021; Lee et al. 2020; Al-Dosari et al. 2012; Khachnaoui-Zaafrane et al. 2022; Akalin et al. 2023; Ceylan et al. 2023; Karacan Kucukali et al. 2023; Jacob and Girisha 2021; Alkhawaldeh et al. 2024; Huber et al. 2009; Hanson et al. 2009; Sasaki et al. 2011; Meazza et al. 2013; Takatani et al. 2018; Huber et al. 2010; Hu et al. 2017; Takizaki et al. 2020; Shaikh et al. 2019; Wang et al. 2024; Hanson et al. 2012; Demir et al. 2013; Keskin et al. 2017; Hu et al. 2020; Luo et al. 2024; Yang and Patni 2020; Xu et al. 2023; Deeb et al. 2015; Liao et al. 2017; Dauber et al. 2013; Isik et al. 2021).
In relation to the etiology of 3M syndrome, out of the 221 reported families, CUL7 variants were responsible for the disease etiology in 153 families (69.2%), 27.2% (60/221) had OBSL1 variants, and only 8 families (3.6%) had CCDC8 variants. The addition of our cohort to the genetic pool of 3M syndrome brings the genetically confirmed cases up to 292 cases, from 230 families, and minimally changes the genetic contribution of the three genes to 69.1% for CUL7 variants, 27.4% for OBSL1 variants, and 3.5% for CCDC8 variants.
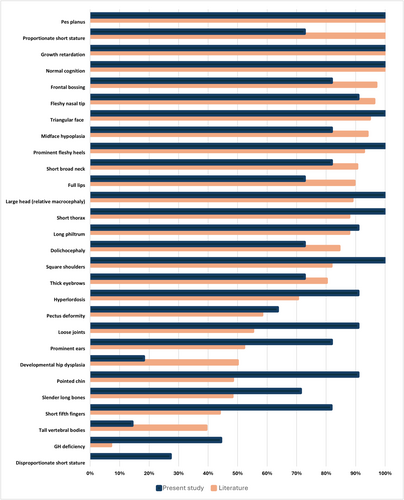
For the sake of clinical comparison, we excluded the case reports of single patients (or families) as well as the cohorts for which detailed clinical data were not available. Therefore, we reviewed 12 remaining studies (Hanson, Murray, O'Sullivan, et al. 2011; Maksimova et al. 2007; Simsek-Kiper et al. 2019; Tuysuz et al. 2021; Al-Dosari et al. 2012; Khachnaoui-Zaafrane et al. 2022; Karacan Kucukali et al. 2023; Hanson et al. 2009; Hanson et al. 2012; Hu et al. 2020; Xu et al. 2023; Isik et al. 2021). Using the clinical data of 157 molecularly confirmed patients described in those 12 published studies, we calculated the frequency of the main clinical manifestations of 3M syndrome, as shown in Table 2.
| Clinical feature | Frequency in literature (%) |
|---|---|
| Normal cognition | 93/93 (100) |
| Growth retardation | 141/141 (100) |
| Proportionate short stature | 141/141 (100) |
| Pes planus | 23/23 (100) |
| Fleshy nasal tip | 129/134 (96.3) |
| Frontal bossing | 130/134 (97) |
| Triangular face | 127/134 (94.8) |
| Midface hypoplasia | 126/134 (94) |
| Prominent fleshy heels | 103/111 (92.8) |
| Short broad neck | 95/109 (90.5) |
| Full lips | 120/134 (89.6) |
| Large head (relative macrocephaly) | 112/126 (88.9) |
| Long philtrum | 101/115 (87.8) |
| Short thorax | 101/115 (87.8) |
| Dolichocephaly | 71/84 (84.5) |
| Square shoulders | 45/55 (81.8) |
| Thick eyebrows | 69/86 (80.2) |
| Hyperlordosis | 81/115 (70.4) |
| Pectus deformity | 42/72 (58.3) |
| Loose joints | 37/67 (55.2) |
| Prominent ears | 48/92 (52.2) |
| Developmental hip dysplasia | 12/24 (50) |
| Pointed chin | 44/91 (48.4) |
| Slender long bones | 54/112 (48.2) |
| Short fifth fingers | 25/57 (43.9) |
| Tall vertebral bodies | 43/109 (39.4) |
| GH deficiency | 2/28 (7.1) |
| Disproportionate short stature | 0 |
- Abbreviation: GH: growth hormone.
Furthermore, in Figure 3, we compare the frequency of the main clinical features of 3M syndrome, observed in our cohort, to the corresponding literature-based frequency (arranged by descending order of frequency).
3 Discussion
3M syndrome is an underdiagnosed and underreported autosomal recessive disorder, characterized by severe pre- and postnatal growth retardation with subtle yet noticeable facial dysmorphism in addition to distinctive radiological findings (Tuysuz et al. 2021; Al-Dosari et al. 2012; Hanson et al. 2009). In this study, we describe 11 Egyptian patients from 9 unrelated families who were found to carry P/LP variants in two of the genes known to cause 3M syndrome (CUL7, OBSL1). As part of our ongoing attempts to determine the genetic etiology of short stature in Egypt, ES was conducted on a cohort of patients with short stature, and 11 cases of 3M syndrome were diagnosed. Throughout their long journey to seek medical help, most of the studied patients had been misdiagnosed with another growth retardation syndrome, and some of them even had undergone extensive evaluation for skeletal dysplasias.
A total of seven P/LP variants in CUL7 and OBSL1 were identified in our cohort. After classifying the detected variants using Franklin Genoox program (franklin.genoox.com) in accordance with the AMP/ACMG guidelines (Richards et al. 2015), and considering their molecular implications, it was found that the five CUL7 variants were all null variants, including one splice site, two frameshift and two nonsense variants resulting in a loss or disruption of normal protein function. This holds particular importance because loss of function (LOF) is a well-established disease mechanism, as other cohort studies have demonstrated (Huber et al. 2005; Tuysuz et al. 2021; Huber et al. 2009; Hanson et al. 2012). Similarly, the two OBSL1 variants, that were identified in our cohort, were null variants (one nonsense and one frameshift), emphasizing LOF as the pathogenetic mechanism of 3M syndrome 2 in agreement with earlier studies (Tuysuz et al. 2021; Huber et al. 2010; Hanson et al. 2012).
Thus far, three variants; CUL7:c.1398delC, CUL7:c.4108_4111delGGAG and OBSL1:c.1534+2T>C, have been identified in Egyptian patients exhibiting 3M syndrome manifestations (Hanson et al. 2012). Herein, we additionally describe four CUL7 variants (c.2341A>T, c.2141_2150delinsCA, c.2862+1G>A, c.2761_2765del) and one additional OBSL1 variant (c.2292_2320dup) that have not yet been reported in the literature or added to any population databases, thus expanding the mutational spectrum of 3M syndrome and demonstrating the high allelic heterogeneity of 3M syndrome in Egypt.
Despite the small number of molecularly diagnosed cases, it is remarkable that two unrelated cases, originating from different regions of Egypt, both displayed the same novel variant in CUL7 (c.2141_2150delinsCA) potentially implying a founder effect for this variant in Egypt. Moreover, the previously reported OBSL1 variant (c.35dup) was also detected in two unrelated cases. It should be noted that all variants are homozygous, which may be explained by Egypt's high percentage of consanguineous marriages, estimated at 35% (Shawky et al. 2011).
Clinically, the current cohort showed a strikingly uniform clinical phenotype in spite of the considerable allelic and locus variation among the families, which is consistent with other research studies (Al-Dosari et al. 2012; Clayton et al. 2012). All patients exhibited the distinctive craniofacial dysmorphism, musculoskeletal features, and radiological findings of 3M syndrome. However, it was notable that three out of the 11 patients studied displayed limb shortening. 3M syndrome is a recognizable cause of proportionate short stature. To the best of our knowledge, disproportionate short stature had never been associated with 3M syndrome, apart from a single patient (with homozygous CUL7:c3041T>G variant) reported by Karatsiolis et al. (2021).
Upon reviewing the literature (Table S1), it was evident that the bulk of reports of 3M syndrome were from the Middle East; particularly from Turkish, Arab, Iranian, and Pakistani ancestry, with East Asian cases, including Chinese, Japanese, Korean, and Indian ancestry, also common. Conversely, just a small percentage of the published cases were from Western countries. As an autosomal recessive condition, the higher incidence of 3M syndrome could be attributed to the higher rates of consanguinity in the Arab and Muslim communities. Larger populations in Eastern Asian countries may theoretically lead to a higher overall number of cases, which could explain the increased reporting from these regions. Finally, differences in genetic makeup between populations could influence the prevalence of specific genetic disorders, including 3M syndrome.
4 Conclusion
3M syndrome is a genetically heterogeneous disorder with distinct clinical and radiological features. Despite its unique clinical manifestations, 3M syndrome is often overlooked in pediatric endocrine clinics. Based on our experience with our first 1000 ES (unpublished data), we suggest that the actual prevalence of 3M syndrome may be higher than currently believed.
The review of molecularly confirmed cases underscores the critical role of genetic testing in diagnosing 3M syndrome. Finally, further research into the molecular mechanisms underlying 3M syndrome may pave the way for targeted therapies.
Author Contributions
Shaymaa Elsayed: conceptualization, writing – review and editing. Gehad A. Elmakkawy: data curation, methodology, writing – review and editing. Ibrahim M. Abdelrazek: data curation, methodology, writing – review and editing. Dina A. Fawzy: data curation, original draft preparation. JiHye Kim: data curation, methodology, writing – review and editing. YongJun Song: data curation, methodology, writing – review and editing. Omneya M. Omar: data curation, final editing of the manuscript. Ebtesam M. Abdalla: conceptualization, final editing of the manuscript.
Acknowledgments
The authors thank the families who participated in this study for their cooperation.
Ethics Statement
The research was reviewed and approved by the Ethics Committees of the Faculty of Medicine and the Medical Research Institute (Alexandria, Egypt). All study participants/legal guardians provided written informed consent. Specific written parental permission was obtained for the publication of photographs presented in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




