Human Phenotype Ontology Annotations for Rare Congenital Conditions: Application to Arthrogryposis Multiplex Congenita
Funding: Dr. Dahan-Oliel is supported by a clinical research scholar award from the Fonds de recherche du Québec – Santé (FRQS; Junior 1: 2020–2023; Junior 2: 2024–2027) and a Rising Star Award from the American Society for Bone and Mineral Research. Dr. Peter N Robinson, Dr. Melissa Haendel, and Dr. Monica Munoz-Torres are funded by theNIH National Human Genome Research Institute grant # U24HG011449-03. Shriners' Children funded the North American AMC registry for children through a multisite clinical grant (#79150, 2019–2022; #79137, 2023–2026).
ABSTRACT
Arthrogryposis multiplex congenita (AMC) represents a large, rare group of congenital conditions. This study addressed major challenges in AMC research posed by the lack of systematic frameworks for data collection and the use of inconsistent terminologies and text descriptions. We aimed to systematically review the Human Phenotype Ontology (HPO) terms, encode AMC phenotypic traits as HPO terms, and pilot test the encoding process in a cohort of children with AMC. An international consensus-based dataset for AMC was used to extract phenotypic traits from the fetal period to adulthood. The encoding process was developed by an international expert panel to expand and revise HPO ontology for joint contractures, as the main characterizing traits in AMC. Using a pre-tested mapping algorithm, the HPO mapping process resulted in a 62% complete match, a 12% incomplete match, and a 26% no match. The encoding process included 37 new terms and annotations and 13 re-structures across 10 different joints. The implemented annotations significantly increased the number of available HPO terms for joint contractures in a cohort of children with AMC (p-value = 0.04). Our encoding and annotation approach may be used as a blueprint for systematic HPO (re)annotations for musculoskeletal and non-musculoskeletal phenotypic traits of AMC.
1 Introduction
Arthrogryposis multiplex congenita (AMC) is a term to describe a group of rare musculoskeletal (MSK) conditions, characterized by congenital joint contractures in two or more body areas (Dahan-Oliel, Cachecho, et al. 2019). AMC conditions are rare, occurring in every 3000–5200 live births (Lowry et al. 2010). Over 400 genes and numerous environmental factors are attributed to the development of non-progressive contractures in AMC as a consequence of diminished fetal movement in the prenatal period (Dieterich et al. 2019; Hunter et al. 2015). Individuals with AMC present with a broad spectrum of phenotypic traits in the MSK system, as well as in other body systems (e.g., neurological, gastrointestinal, etc.). While similar phenotypes due to different etiologies are common (Dieterich et al. 2019; Hall 2014), most individuals with AMC report pain, activity limitations, and impaired quality of life (Altiok et al. 2019; Nouraei et al. 2017). To address the challenges to AMC research caused by diverse etiologies, and heterogeneity and rarity of clinical presentations, an international AMC consortium was established in 2020. The consortium comprises 16 AMC experts in complementary clinical disciplines (i.e., orthopedics, rehabilitation, pediatric neurology, obstetrics, genetics, epidemiology, kinesiology), bioinformatics, and individuals with lived experience across North America and Europe. Similar to other rare diseases (Jia and Shi 2017; The European Union Committee of Experts on Rare Diseases (EUCERD) n.d.), the consortium acknowledged that advancing AMC research across countries requires concerted datasets and systematic data frameworks using a common language for data collection and sharing (Dahan-Oliel, van Bosse, et al. 2019). This prompted us to identify a set of common data elements (CDEs) for AMC conditions using the AMC-annotated definition of the presence of congenital contractures in two or more body areas (Nematollahi et al. 2024). In line with a North American AMC registry for children (funded by Shriners' Children in 2017 (Dahan-Oliel, van Bosse, et al. 2019; Dahan-Oliel et al. 2018) including eight sites in Canada, US, and Mexico (Dahan-Oliel et al. 2022)) and a consensus-based annotated definition for AMC, the CDEs have facilitated large-scale studies and multi-institutional patient registries (Dahan-Oliel, Cachecho, et al. 2019; Cachecho et al. 2019) to broaden the knowledge on etiology, epidemiology, and phenotypic diagnoses in AMC (Dahan-Oliel et al. 2022, 2021). The importance of international data collection and sharing to advance AMC knowledge has been unanimously acknowledged by the AMC community (Dahan-Oliel et al. 2022; Dahan-Oliel, Hall, et al. 2019; Hall et al. 2015). However, in the absence of a standardized vocabulary, data exchange and integration into larger datasets are challenging. This is an important concern in AMC research, as inconsistent terminologies due to limited utilization of disease coding systems and challenges due to text descriptions across and within jurisdictions are very common (Bedard and Lowry 2019).
Standardized vocabularies for phenotypic abnormalities, such as Human Phenotype Ontology (HPO), are necessary tools to ensure a common language is maintained in health information systems to effectively contribute to research, program planning, and development (Bedard and Lowry 2019). In rare diseases, HPO is a widely used resource to support differential diagnosis (Köhler et al. 2021; Gargano et al. 2024). HPO does not presuppose that a phenotype must only belong to a single syndrome or disease diagnosis. This feature is of particular interest for AMC. With a very heterogeneous group of more than 400 conditions, AMC is a sign rather than a diagnosis (Dahan-Oliel, Cachecho, et al. 2019; Hall et al. 2019). Therefore, this study aimed to (i) Systematically review HPO terms for phenotypic traits observed in AMC, especially contractures and restricted joint mobility; (ii) Encode AMC phenotypic traits as HPO terms by extending and, where necessary, revisiting the structure of the HPO, and pilot test the encoding process in a cohort of children with AMC. Our goal was to standardize the phenotypic description of AMC conditions and provide consensus definitions for joint contractures in AMC. This will facilitate deep phenotyping, data harmonization, and knowledge sharing for AMC and other rare diseases characterized by joint contractures.
2 Materials and Methods
2.1 Ethical Consideration
This study is part of a research project to identify international common data elements in AMC for which research ethics approval was obtained from the McGill University Faculty of Medicine Institutional Review Board (IRB) (A09-B66-21B). Administrative approval was obtained from the Department of Medical Research at Shriners' Children (CAN2110). Data to conduct the pilot testing was provided from a North American AMC registry with McGill University Faculty of Medicine IRB (A08-M30-19B) and administrative approval (CAN1903). Photographs used for the annotations were also obtained from the registry, with signed informed consent for use of the photos for scientific and educational purposes.
2.2 Methodology
This cross-sectional study was a collaborative effort between members of the AMC consortium and HPO team (Figure 1).
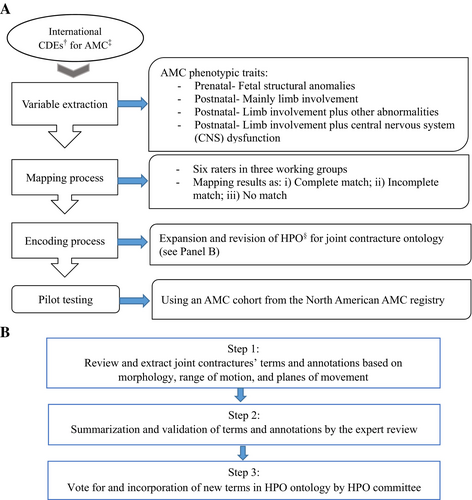
2.2.1 Systematic Mapping of AMC Phenotypic Traits to HPO
The international CDEs for AMC were used as a proof of concept (Nematollahi et al. 2024). The CDEs contain 321 data elements that were identified through a mixed-methods study among 45 experts across 11 countries in North America, Europe, and Australia. To reflect the life course perspective from the fetal development to adulthood, the CDEs address various aspects of AMC including general/demographic characteristics, family history of AMC, pregnancy (e.g., tests/assessments, maternal lifestyle), birth (e.g., complications at birth, Apgar score), current health status of the individual with AMC (e.g., phenotypic traits, anthropometric measurements), rehabilitation utilization (e.g., use of mobility aids), and genetic findings (e.g., chromosomal array, targeted gene panel, Whole Exome Sequencing, Whole Genome Sequencing (WGS)) (Nematollahi et al. 2024). For this study, a primary list of data elements describing phenotypic traits in AMC, variables hereafter, were extracted from the CDEs. To ensure comprehensiveness, the variables were revised using the North American AMC registry dataset and available published materials (Hall 2014; Nouraei et al. 2017; Dahan-Oliel, van Bosse, et al. 2019; Staheli et al. 1998). The variables were then characterized to “Fetal structural anomalies” (phenotypic abnormalities that were detected during pregnancy tests/assessments, e.g., clubfeet); “Postnatal-mainly limb involvement” (presence of joint contractures as examined/recorded by a trained healthcare professional, e.g., knee flexion contracture); “Postnatal-limb involvement plus other abnormalities” (presence of phenotypic abnormalities in other body systems/organs e.g., cardiovascular, soft tissues, etc.); and “Postnatal-limb involvement plus central nervous system (CNS) dysfunction” (presence of neurological abnormalities e.g., seizure) (Staheli et al. 1998) (Table 1).
| # | Data domain | No. of data elements (%) | Extraction of phenotypic traits for systematic mapping to HPO b |
|---|---|---|---|
| 1 | General/Identification | 32 (10) | Not applicable. |
| 2 | Family history of AMC | 11 (3) | Not applicable. |
| 3 | Pregnancy with individual with AMC | 85 (26) | |
| 3.1 Tests/Assessments | 20 (23) | Fetal structural anomalies | |
| 3.2 Parental health/Lifestyle | 23 (27) | Not applicable. | |
| 3.3 Complications/Plurality | 32 (38) | Not applicable. | |
| 3.4 Deceased fetus with AMC | 10 (12) | Not applicable. | |
| 4 | Birth | 23 (7) | Postnatal-Mainly limb involvement |
| 5 | Health status | 110 (35) | |
| 5.1 Anthropometry | 12 (11) | Postnatal-Mainly limb involvement | |
| 5.2 Musculoskeletal involvement | 74 (67) | Postnatal-Mainly limb involvement | |
| 5.3 Non-musculoskeletal involvement | 24 (22) |
Postnatal-Limb involvement plus other abnormalities Postnatal-Limb involvement plus central nervous system (CNS) dysfunction |
|
| 6 | Rehabilitation utilization | 33 (10) | Not applicable. |
| 7 | Genetics | 27 (9) | Not applicable. |
| Total | 321 (100) | ||
- a Arthrogryposis multiplex congenita.
- b Human phenotype ontology.
2.2.1.1 Organization of Working Groups
Within the AMC consortium, three working groups were established, each with two members (for a total of six raters). A coordinator from the Shriners' Children-Canada accompanied the groups to facilitate communication and organization through a general agenda, group-specific tasks, meetings, and results communications via teleconferencing and email.
2.2.1.2 Mapping Process
We defined a mapping algorithm describing linking rules to systematically review the existing HPO terms using similar literature (Haimel et al. 2022; Shi et al. 2014; Dhombres et al. 2022; Le and Dao 2018), three workshops with HPO team, and numerous teleconferences with the AMC consortium (Table 2). The reliability of the algorithm was tested on a random subset of variables from the North American AMC registry (n = 30), yielding a satisfactory agreement of 75% (Intra Class Correlation Coefficient (ICC): 0.75, 95%CIs: 0.50, 0.91) among the six raters. For each variable, available HPO terms and the ontology structure were extracted from the v2022-06-12 HPO disease annotation and ontology release (retrieved from https://hpo.jax.org) and summarized in a spreadsheet. For each variable, the spreadsheet contained the currently available HPO term(s), annotation (i.e., definition), semantic description (e.g., synonyms), and HPO hierarchy of ancestors (i.e., upstream/parent) and descendant (i.e., downstream/offspring) terms associated with each variable. The AMC consortium spearheaded the process. The mapping results were presented in three mutual groups of “Complete match,” “Incomplete match,” and “No match.” Complete match referred to when there was an HPO term with exact semantic characteristics, annotation, and/or clinical applicability for a variable. Incomplete match referred to when one/several HPO term(s) were available for a variable with semantic/annotative similarities and distinct discrepancies. No match referred to when there was no HPO term(s) for a variable. For the complete match category, we defined no further action. However, we addressed the incomplete match and no match categories by developing an encoding process to expand and revise the HPO structure to suggest standardized new terms, re-annotations, and/or re-structuring (Figure 1A).
| Domain | Rule |
|---|---|
| Sensitivity |
|
| Comprehensibility |
|
| Reproducibility |
|
| Data linkage |
|
- Abbreviations: HPO, human phenotype ontology; ICD, international statistical classification of diseases and related health problems; MeSH, medical subject headings; SNOMED-CT, systematic nomenclature of medicine-clinical terms; UMLS, unified medical language system.
2.2.2 Expansion and Revision of HPO Structure
2.2.2.1 Expert Panel
A multidisciplinary panel consisting of the HPO team, members of the AMC consortium (orthopedists, obstetrician, medical geneticist, epidemiologist), and a panel of clinical specialists in AMC care and rehabilitation (occupational therapists, pediatricians) participated in this component.
2.2.2.2 Encoding Process
The encoding process addressed phenotypic traits of joint contractures within the categories of incomplete match and no match. To annotate joint contractures in AMC with HPO terms, we provided standardized re-annotations by a series of workshops using a 3-step process (Figure 1B). First, we formulated a literature search strategy to extract terms and annotations for joint contractures based on morphology, range of motion, and planes of movement. Second, the expert panel reviewed the content validity of the extracted terms and annotations during multiple meetings, while agreement of at least 70% among the panelists was considered sufficient to submit the suggestions to the HPO (Miles and Huberman 1998). We used Uberon Ontology (retrieved from: https://www.ebi.ac.uk/ols/ontologies/uberon) (Mungall et al. 2012) to classify the suggestions based on anatomic location, for example, to describe “elbow” vs. “forearm” for “elbow contracture.” The suggestions for annotations did not specify laterality (i.e., bilateral/unilateral). We specified the axes of the limbs as per embryological terminology: proximal-distal from the shoulder/hip to the wrist/ankle; radial/tibial—ulnar/fibular from the medially oriented to the laterally oriented parts; and ventral/dorsal from the knee/the back of the hand to the back of the knee/the palm of the hand (the person under investigation standing in the standard anatomic position [palms facing forward]). We did not use radiographs or other imaging modalities in the terminology, nor did we make any judgment to be normal or abnormal (Biesecker et al. 2009, 2022). Third, we submitted the suggestions via an electronic Artificial Intelligence (AI)-powered web-based platform to the HPO team. The suggestions were then reviewed and voted for implementation during multiple teleconference meetings with the study panel and HPO experts. Subsequently, the working groups and HPO team discussed and resolved any inconsistencies.
2.2.2.3 Pilot Testing in an AMC Cohort
To compare the encoding process performance for systematic mapping of joint contractures to HPO terms, we extracted clinical summaries of 80 individuals with AMC (Dahan-Oliel, Cachecho, et al. 2019; Cachecho et al. 2019) from the North American AMC registry database (Dahan-Oliel et al. 2022). The phenotypic traits were collected prospectively once recruited to the registry and retrospectively with regard to pregnancy and birth outcomes and were mapped to HPO one time before and one time after the encoding process by a member of the study team (SN). Paired t-tests were used to compare the mapping results at those two time points.
3 Results
3.1 Systematic Mapping of AMC Phenotypic Traits to HPO
We extracted two hundred and twenty-two (222) variables and categorized them into fetal structural anomalies (n = 22, 10%), postnatal-mainly limb involvement (n = 126, 57%), postnatal-limb involvement plus other abnormalities (n = 58, 26%), and postnatal-limb involvement plus CNS dysfunction (n = 16, 7%). The mapping process was conducted using two main branches of the HPO structure: (Dahan-Oliel, Cachecho, et al. 2019) Abnormality of the musculoskeletal system (HP:0033127), and (Lowry et al. 2010) Abnormality of limbs (HP:0040064). Complete match was found for 62% of the variables, for example the variable “Overlapping fingers” was matched completely to the HPO term “Overlapping fingers (HP:0010557).” Incomplete match was found for 12% of the variables, for example, the variable “Hip abduction contracture” was incompletely matched to the HPO term “Hip contracture (HP:0003273).” No match was found for 26% of the variables, for example no HPO term was found for the variable “Ankle Plantarflexion contracture.” The proportion of findings with a complete match was highest for limb involvement plus other abnormalities (83%) and lowest for mainly limb involvement (50%). The proportion of incomplete match was highest for fetal structural anomalies (36%) and lowest for mainly limb involvement (5%). No match results were highest for mainly limb involvement (45%) and lowest for limb involvement plus other abnormalities (2%) (Table 3).
| Phenotypic trait | Total, N (%) | Mapping results N (%) | |||||
|---|---|---|---|---|---|---|---|
| Complete match n = 138 (62%) | Incomplete match n = 26 (12%) | No match n = 58 (26%) | |||||
| No. of variables (%) | Example(s) | No. of variables (%) | Example(s) | No. of variables (%) | Example(s) | ||
| Fetal structural anomalies | 22 (10%) | 14 (64%) |
|
8 (36%) |
|
0 | Not available. |
| Postnatal-Mainly limb involvement | 126 (57%) | 63 (50%) |
|
7 (5%) |
|
56 (45%) |
|
| Postnatal-Limb involvement plus other abnormalities | 58 (26%) | 48 (83%) |
|
9 (16%) |
|
1 (2%) |
|
| Postnatal-Limb involvement plus central nervous system (CNS) dysfunction | 16 (7%) | 13 (81%) |
|
2 (16%) |
|
1 (4%) |
|
3.2 Expansion and Revision of HPO Structure
3.2.1 Expert Panel
Fourteen participants, including four members of the HPO team and 12 AMC clinical experts (seven members of the AMC consortium and five non-members) participated in four teleconferences, four in-person focus groups, and multiple email correspondences.
3.2.2 Encoding Process
The 3-step process resulted in the extraction and review of 19 articles and book chapters (range: 2–8 reference per joint contracture) that delineated concepts and morphologies of joint contractures for the encoding process (Dahan-Oliel et al. 2021; Köhler et al. 2021; Biesecker et al. 2009, 2022; Chan and Miller 2014; Hoffman et al. 2005; Mann and Coughlin 1981; Leemrijse and Devos-Bevernage 2020; Stedman 2005; Chadwick and Saxby 2011; Betts et al. 2021; Farlex Partner Medical Dictionary 2012; Merks et al. 2003; Langston and Chu 2020; Skaria et al. 2019; Bahm et al. 2020; Suto et al. 2016; Inman 1976; Hagert and Hagert 2010). Using these references, we provided 50 suggestions, comprising of 37 (74%) new terms and (re)annotations, and 13 (26%) re-structures for joint contractures' ontology in 10 joints including Shoulder (UBERON0016884), Elbow (UBERON0001490), Wrist (UBERON0001491), Hand (Manus: UBERON0002398), Thumb (Manual digit 1: UBERON0001463), Fingers (Manual digit 2:UBERON0003622-Manual digit 3:UBERON0003623-Manual digit 4:UBERON0003624-Manual digit 5:UBERON0003625), Hip (UBERON0001486), Knee (UBERON001485), Ankle (UBERON0001488), and Toe (Pedal digit: UBERON0001466) (File S1; Figure 2). More specifically, the suggested annotations included unspecific (i.e., frequently annotated) HPO terms such as “Shoulder joint contracture” as well as specific (i.e., less frequently annotated) HPO terms such as “Shoulder internal rotation contracture,” holding less and more content sensitivity, respectively.
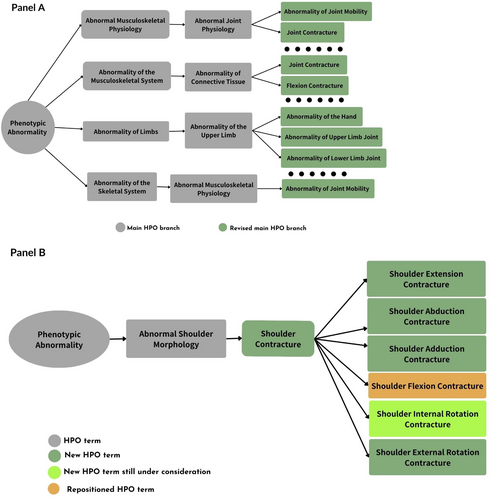
The implementation process took place during 13 months (November 2022–December 2023), resulting in the implementation of 37 (100%) new terms, 31 (84%) (re)annotations, and 13 (100%) re-structures across four main HPO branches: Abnormal musculoskeletal physiology (HP:0011843), Abnormality of the musculoskeletal system (HP:0033127), Abnormality of limbs (HP:0040064), and Abnormal skeletal muscle morphology (HP:0011805). The new annotations for joint contractures that are implemented in HPO ontology as of October 2024 are provided below with accompanying photographs (when available).
3.2.3 Implemented Annotations in HPO for Joint Contractures Ontology
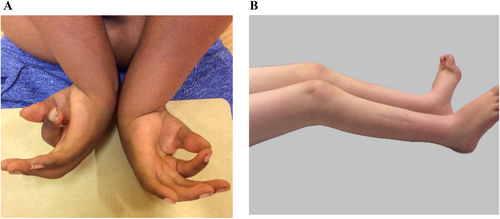
3.2.3.1 Flexion Contracture (HP:0001371)
- Thumb flexion contracture (HP:0009600)
Definition: Lack of full passive range of motion (restrictions in flexion, extension, or other movements) of the thumb joint resulting from structural changes of non-bony tissues, such as muscles, tendons, ligaments, joint capsules, and/or skin.
Comment: The term camptodactyly is used if the distal and/or proximal interphalangeal joints are affected.
Figure 3 depicts flexion contracture in the wrist, thumb, and fingers (A) and bilateral flexion contracture of the knee joints (B).
3.2.3.2 Extension Contracture
- Shoulder extension contracture (HP:0034666)
- Thumb extension contracture (HP:0034667)
- Extension contracture of finger (HP:0034682)
- Extension contracture of hip (HPO code under consideration)
- Knee extension contracture (HP:0034672)
Definition: A type of knee joint contracture in which the knee lacks full expected flexion, such that the upper and lower leg cannot be brought together (Figure 4B).
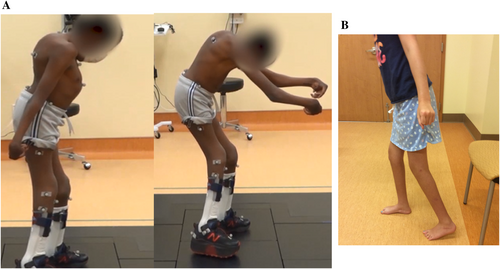
- Extension contracture of toe (HP:0034676)
Definition: A type of toe joint contracture in which the joint lacks full expected flexion, so that the two bony segments on either side of their connecting joint cannot be brought together.
3.2.3.3 Supination Contracture
- Forearm supination contracture (HP:0034394)
- Forefoot supination contracture (HP:0034679)
Definition: Supination contracture of the forefoot joint is characterized by the relative plantar rotation of the lateral border of the foot and/or relative dorsal rotation of the medial border, as viewed with respect to the axial plane of the foot.
3.2.3.4 Pronation Contracture
- Forearm pronation contracture (HP:0034395)
- Forefoot pronation contracture (HP:0034678)
Definition: Pronation contracture of the forefoot joint is characterized by the relative dorsal rotation of the lateral border of the foot and/or relative plantar rotation of the medial border, as viewed with respect to the axial plane of the foot.
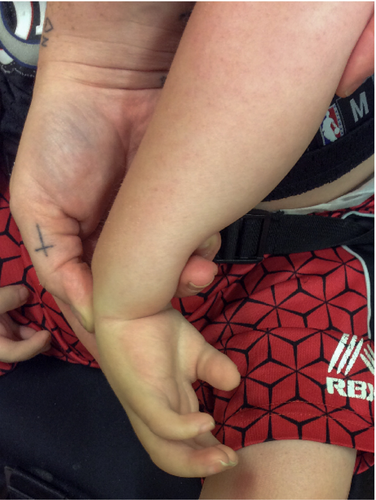
3.2.3.5 Ulnar Deviation of the Hand (HP:0009487)
Definition: A condition generally attributed to a muscular imbalance between radial and ulnar wrist stabilizers. An abnormal position of the hand in which the wrist is bent towards the ulna (i.e., towards the little finger and away from the midline) (Figure 6A,B).
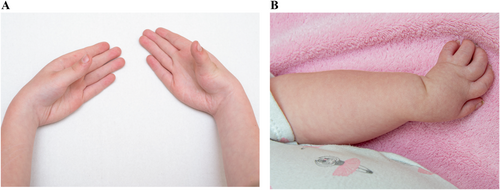
3.2.3.6 Radial Deviation of the Hand (HP:0009486)
Definition: A condition generally attributed to the central axis of the hand is radially angulated relative to the longitudinal axis of the radius. An abnormal position of the hand in which the wrist is bent towards the radius (i.e., towards the thumb) (Figure 7).
3.2.3.7 Internal Rotation Contracture
- Hip internal rotation contracture (HP:0034993)
Definition: Turning the knee from a neutral position to one facing more the midline, where the neutral position for the hip is with the knee pointing directly anteriorly (less than or equal to 45°).
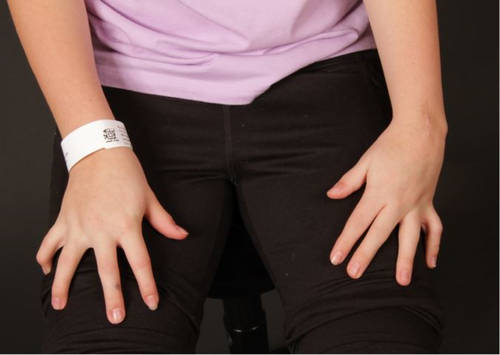
3.2.3.8 External Rotation Contracture
- Shoulder external rotation contracture (HP:0034991)
- Hip external rotation contracture (HPO code under consideration)
Definition: Turning the knee past neutral in the direction moving away from the midline, where the neutral position for the hip is with the knee pointing directly anteriorly (Figure 8A,B).
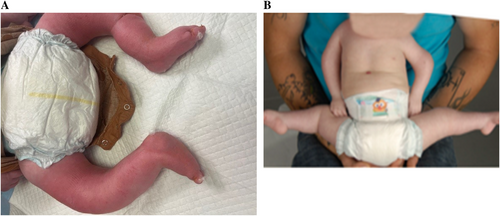
3.2.3.9 Adduction Contracture
- Shoulder adduction contracture (HP:003509)
- Thumb adduction contracture (HP:0034992)
- Forefoot adduction contracture (HP:0034680)
Definition: Adduction contracture of the forefoot is when the axis of the forefoot (usually considered as the axis of the middle ray) is angulated medially in comparison to the axis of the hindfoot (usually considered as the axis of the talus), as viewed in the coronal plane of the foot (Figure 9B).
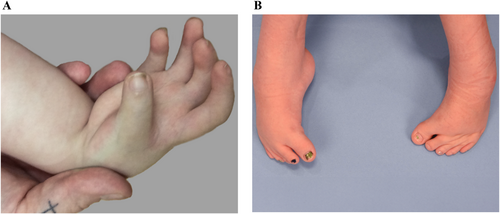
3.2.3.10 Abduction Contracture
- Shoulder abduction contracture (HP:0034990)
- Hip abduction contracture (HP:0003184)
Definition: Abduction contracture of the hip is characterized by the limitation of active and/or passive adduction of the hip joint in the coronal plane; a chronic reduction in the ability to move the proximal segment of the lower extremity actively or passively medially towards the body midline past the body's sagittal plane (Figure 10).
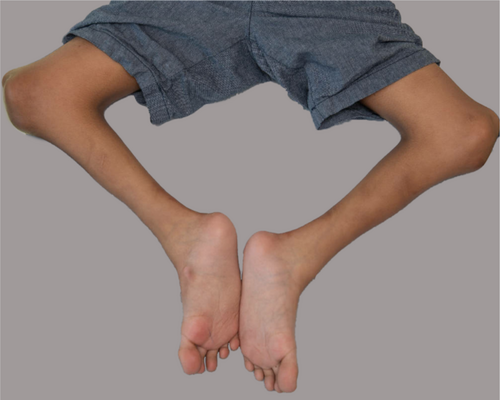
3.2.3.11 Plantar Flexion Contracture (HP:0008112)
Definition: This is the inability to dorsiflex the foot so that the calcaneus cannot be brought into an anatomically neutral position relative to the longitudinal axis of the tibia in the sagittal plane. In other words, this is the movement of the foot at the ankle in which the heel is lifted off the ground (Figure 11).

3.2.3.12 Dorsiflexion Contracture (HP:0033526)
Definition: Limitation of the ankle joint to move the top of the foot from the anterior leg.
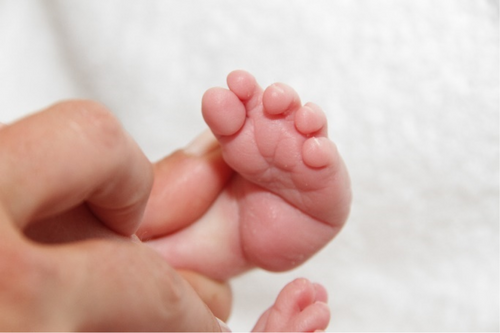
3.2.3.13 Metatarsus Adductus (HP:0001840)
Definition: Metatarsus adductus is characterized by the deviation of the metatarsals medially (tibially) (Figure 12).
3.2.4 Pilot Testing in an AMC Cohort
Among the 80 randomly selected individuals with AMC, we extracted 658 phenotypic traits, of which 426 (65%) were mainly limb involvement (i.e., joint contractures), 210 (32%) were limb involvement plus other abnormalities, and 22 (3%) were limb involvement plus CNS dysfunction. Among the mainly limb involvement group, there was an average of three (range = 2–11) contractures per person in different joints of the shoulder (n = 36, 8%), elbow (n = 45, 11%), wrist (n = 46, 11%), hand (n = 21, 5%), thumb (n = 16, 4%), fingers (n = 45, 11%), hip (n = 56, 13%), knees (n = 65, 15%), ankles (n = 79, 18%), and toes (n = 17, 4%). Other findings of phenotypic traits in this group included clubfoot (n = 59, 9%), spine involvement (n = 56, 8%), joint dislocation (n = 28, 4%), jaw involvement (n = 18, 3%), pterygium (n = 16, 3%), and neck involvement (n = 14, 2%). Among the group of other abnormalities, phenotypic traits included 84 (40%) skin/soft tissue (e.g., hemangioma, dimples, abnormal skin crease), 43 (20%) craniofacial (e.g., micrognathia, high palate, facial asymmetry), 18 (8%) reproductive (e.g., cryptorchidism, ambiguous genitalia), 16 (7%) respiratory (e.g., lung hypoplasia, tracheal cleft/stenosis), 13 (6%) gastrointestinal (e.g., hernia, anterior anus), 13 (6%) cardiac (e.g., heart murmur, ventricular septal defect [VSD]), 7 (3%) sensory (e.g., decreased sensation, hypersensitivity to touch), 7 (3%) metabolic/endocrine (e.g., growth hormone deficiency, hypothyroidism), 6 (2%) circulatory (e.g., blue cold distal limbs, angioma), and 3 (1%) genitourinary (e.g., groin webbing). Hypotonia (n = 8, 36%) was the most common phenotypic trait in the group of CNS dysfunction group.
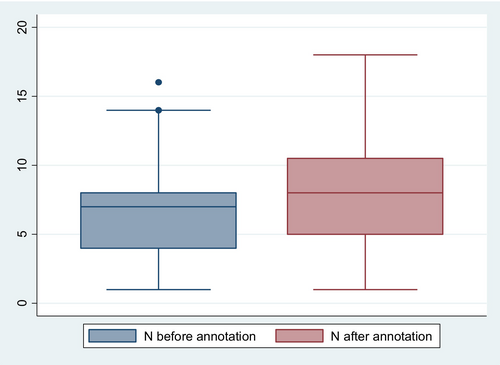
Before the encoding, the mean number of available HPO terms per person was 2.6 (95% CIs: 2.1, 2.9), which was significantly increased to 3.1 (95% CIs: 2.6, 3.6) after the encoding process (p-value = 0.04) (Figure 13).
4 Discussion
Our study aimed to review the HPO ontology to describe AMC phenotypic traits and modify joint contracture annotations to reflect the current knowledge in AMC. The first aim of our study was to perform a systematic mapping of HPO terms to phenotypic traits in AMC. Our mapping exercise showed major gaps in describing AMC phenotypic traits by HPO terms. This was more pronounced for the phenotypic traits with mainly limb involvement, which were largely joint contractures. Phenotypic traits of joint contractures are the main characterizing traits in AMC (Dahan-Oliel, Cachecho, et al. 2019), and their description is an indispensable part of AMC management and care (Dahan-Oliel et al. 2022; Hall et al. 2019; Hall 1984). Nevertheless, the standardized HPO terminology was available for only half of these traits. The lack of standardized terms to describe joint contractures poses crucial barriers toward harmonized data collection and exchange in AMC research. Phenotypic traits of joint contractures in AMC have a broad spectrum of clinical presentations with heterogeneous etiologies and disorders. Limited standardized terms to describe joint contractures challenge correct classification of AMC considering numerous bone abnormalities (e.g., symphalangism, coalition of the carpals, etc.) that are usually confused with AMC (Staheli et al. 1998), and available classification approaches defining various parameters (Hall 2014; Hall et al. 2019; Bamshad et al. 2009).
Therefore, the second aim of the study was to present a comprehensive overview of annotations for joint contractures, which are most seen in AMC. We used a cross-community collaboration to expand and re-annotate four branches of the HPO tree for joint contractures' terminology. To date, there is no standard criterion for performing an expert-based review of HPO; therefore, guidance on annotating rare conditions with HPO terms can vary (Köhler et al. 2021; K¨ohler et al. 2019). Our suggestions and their corresponding HPO terms were scrutinized in-depth by our expert panel to confer validity, resulting in high-quality annotations. We restricted the encoding process to joint contractures, as this helped us maintain the balance between the computational accuracy of HPO terms and the clinical applicability of our encoding process for differential diagnosis of AMC (Gargano et al. 2024). This led to the development of 37 new HPO terms (i.e., 26 terms implemented in HPO, 11 terms under consideration) to describe AMC phenotypic traits. Our systematic expert-driven approach is in line with previous work to improve HPO annotations, phenotype-driven disease classifications, and diagnosis for defined groups of inborn errors of immunity (Haimel et al. 2022), coronary heart diseases (Shi et al. 2014), prenatal phenotypic traits (Dhombres et al. 2022), and novel cancer-associated long non-coding RNAs (lncRNAs) (Le and Dao 2018).
Our comprehensive annotations based on anatomic location, range of motion, and severity of contractures contribute substantially to advance AMC orthopedic and rehabilitation care pathways to improve treatments, natural history, and long-term functional outcomes (Hall 2014; Hall et al. 2019). Moreover, our suggested annotations facilitate multidisciplinary data collection for AMC within multiple jurisdictions. This is because the application of our standardized terminology to describe phenotypic traits of joint contractures offers administrative health systems a parsimonious approach to utilize time, education, and expertise for data collection and sharing (Bedard and Lowry 2019). By implementing all our suggestions in HPO terminology (Gargano et al. 2024), we used the implemented terms to classify AMC phenotypic traits in an independent cohort of children with AMC from a North American AMC registry. Overall, we have achieved a two-fold gain in the number of HPO terms annotated for joint contracture traits. This finding indicates substantial improvement in the qualitative and quantitative content of HPO terminology to enhance phenotypic detection, phenotypic-driven categorization, and differential diagnosis of AMC (Dieterich et al. 2019; Dahan-Oliel, van Bosse, et al. 2019; Hall et al. 2019). Similarly, the adoption of HPO organizes the set of phenotypic traits in AMC in a hierarchical fashion that enables differential diagnostics using computational means in rare diseases (K¨ohler et al. 2019). By defining unified data structures for sophisticated machine learning algorithms, the proposed ontologies will facilitate multidisciplinary collaborations to advance genotype–phenotype investigations, as one of the major research questions in AMC, in diverse clinical and research settings (Dahan-Oliel, van Bosse, et al. 2019; Haendel et al. 2018). Although most phenotype–genotype correlations are currently not yet identified for AMC, it will be intriguing to see how well the results of the HPO annotations aid in genetic diagnoses of AMC in accordance with the Online Mendelian Inheritance in Man (OMIM) catalogue (OMIM 2019). Formation of joint contractures in AMC are influenced by heterogeneous genetic etiologies, and some genes often have more than one function and are expressed slightly differently in various body tissues. The complex and interactive constellation of effects of gene mutations in AMC (over 400 gene mutations) is just beginning to be appreciated (Dieterich et al. 2019; Hall et al. 2019), meaning that the use of the Gene Ontology databases for AMC diagnoses is on the way.
The international common dataset for AMC was used as a proof of concept to extract phenotypic traits. The CDEs for AMC is a recently developed dataset based on international consensus from the AMC expert community (Nematollahi et al. 2024), ensuring the comprehensiveness of the phenotypic traits used in this study. Use of the HPO vocabulary is an efficient strategy to avoid text-associated terminologies datasets, as a major obstacle to advance AMC research (Bedard and Lowry 2019). As an example, AMC is interchangeably referred to as “Arthrogryposis multiplex congenita” (HP:0002804) or “Congenital contracture” (HP:0002803), while specific and recurrent phenotypic traits of AMC maybe known by specific names. This is well illustrated by Freeman-Sheldon syndrome (FSS), which is also known as whistling-face syndrome, while there are several specific underlying diagnoses including distal arthrogryposis type 2A (DA2A), craniocarpotarsal dysplasia, and craniocarpotarsal dystrophy (Bedard and Lowry 2019).
As a group of rare heterogeneous congenital conditions, a standardized set of CDEs according to HPO facilitates knowledge sharing and exchange in AMC in accordance with the recommendations by the Global Rare Diseases Patient Registry Data Repository (Rubinstein and McInnes 2015; Rubinstein et al. 2010), the European Union Committee of Experts on Rare Diseases (The European Union Committee of Experts on Rare Diseases (EUCERD) n.d.), the European Rare Disease Registration Infrastructure (Kölker et al. 2022), and the US National Institute of Neurological Disorders and Stroke (Grinnon et al. 2012). The HPO-standardized data elements of phenotypic traits are designed to be incorporated into an international AMC registry and future multisite studies. As the HPO vocabulary continuously evolves, future routine updates of the standardized terms according to the scientific advancements and knowledge in rare diseases are warranted. This is the first study to describe methodology to improve the standardized organization of the current knowledge about phenotypic traits in a large, yet rare group of congenital rare diseases. Despite the existence of ontologies for rare diseases (Pavan et al. 2017; Schriml et al. 2019), by leveraging clinical features and anatomical and functional presentations, HPO enhances patient matching, diagnosis efficiency, and quality and specificity of global data sharing in rare diseases (Gargano et al. 2024). The Human Phenotype Ontology's efforts and their ongoing collaboration and integration with the Mondo Disease Ontology facilitate these advances (Vasilevsky et al. 2022). Our study used the classical approach to categorize AMC phenotypic traits based on limb involvement. Despite the usefulness of this approach, as there are over 400 genes associated with AMC (Kiefer and Hall 2019) and individuals with the same familial mutation may present heterogeneous phenotypes, including other approaches, such as the one recommended by Bamshad et al. based on fetal neurological assessments, might be helpful (Bamshad et al. 2009). The comprehensive overview of joint contractures' annotation we provided here will never be complete since the expression of AMC with its heterogeneous etiology varies. However, the systematic approach of the annotation will enable medical caregivers to extend their descriptions in the same manner and can be added to the HPO database.
5 Conclusions
Our study used an international consensus-based dataset for AMC to systematically map the phenotypic traits and to encode HPO ontology. Use of real-world data from the AMC registry ensured representativeness of the findings for the AMC community. Our encoding and annotation approach exemplified the application of a standardized knowledge base with significant improvement in phenotypic-driven classification in rare diseases. Our approach may be used as a blueprint for systematic HPO (re)annotations for musculoskeletal and non-musculoskeletal phenotypic traits of AMC and other types of rare diseases.
Author Contributions
All authors have reviewed, discussed, and agreed to their individual contributions. The author contributions are as follows: Shahrzad Nematollahi: conceptualization, formal analysis, project administration, original draft, review and editing. Reggie C. Hamdy: conceptualization, methodology, review and editing. Harold van Bosse: conceptualization, methodology, review and editing. Joyce Li: project administration, data curation, review and editing. Daniel Blanshay-Goldberg: project administration, data curation, review and editing. Johanna I. P. de Vries: conceptualization, methodology, original draft, review and editing. Klaus Dieterich: conceptualization, original draft, review and editing. Isabel Filges: conceptualization, original draft, review and editing. Tanya Bedard: conceptualization, original draft, review and editing. Melissa Haendel: methodology, data curation, review and editing. Monica Munoz Torres: methodology, data curation, review and editing. Peter N. Robinson: conceptualization, methodology, original draft, review and editing. Noémi Dahan-Oliel: conceptualization, funding acquisition, methodology, original draft, review and editing.
Acknowledgments
The authors are grateful to all the AMC experts participating in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of the mapping and encoding processes are available in the Supporting Information of this article. The data that support the findings of the pilot testing process of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




