Optical genome mapping with genome sequencing identifies subtelomeric Xq28 deletion and inserted 7p22.3 duplication in a male with multisystem developmental disorder
Abstract
We report a 17-year-old male with supravalvular stenosis, initial failure to thrive and delayed early development, short stature, acromelia, dysmorphic facial features, hypertelorism, macrocephaly, syringomyelia, hypertension, and anxiety disorder. Fluorescent in situ hybridization (FISH), chromosomal microarray analysis (CMA), and exome sequencing (ES) were nondiagnostic. Combined optical genome mapping (OGM) and genome sequencing (GS) showed a complex rearrangement including an X chromosome with a 22.5 kb deletion in band Xq28 replaced by a 61.4 kb insertion of duplicated chromosome 7p22.3 material. The deletion removes the distal 3′ untranslated region (UTR) of FUNDC2, the entire CMC4 and MTCP1, and the first five exons of BRCC3. Transcriptome analysis revealed absent expression of CMC4 and MTCP1 and BRCC3 with normal transcript level of FUNDC2. The inserted duplication includes only one known gene: UNCX. Similar overlapping Xq28 deletions have been reported to be associated with Moyamoya disease (MMD), short stature, hypergonadotropic hypogonadism (HH), and facial dysmorphism. Although he has short stature, our patient does not have signs of Moyamoya arteriopathy or hypogonadism. The structurally abnormal X chromosome was present in his mother, but not in his unaffected brother, maternal uncle, or maternal grandparents. We propose that the combination of his absent Xq28 and duplicated 7p22.3 genomic material is responsible for his phenotype. This case highlights the potential of combined OGM and GS for detecting complex structural variants compared with standard of care genetic testing such as CMA and ES.
1 INTRODUCTION
Constitutional chromosomal structural abnormalities, such as deletions, duplications, and translocations, give rise to numerous birth defects, complex multisystem disorders, and even Mendelian disorders (McFadden & Friedman, 1997; Shaffer & Lupski, 2000; Truty et al., 2019). Their contribution has significantly grown with the improvement of resolution in methods that detect them, from karyotyping of banded chromosomes to fluorescent in situ hybridization (FISH), and to microarray-based comparative genomic hybridization (CGH), now commonly known as chromosomal microarray analysis (CMA; Dave & Sanger, 2007). CMA detects quantitative changes, such as loss or gain of chromosomal material (Pinkel et al., 1998). Such copy-number variants (CNVs) are common in the genome, they are defined as >1 kb in size and may represent benign polymorphic variation or be disease-causing (Girirajan et al., 2011). CMA provides much higher diagnostic yield (15%–20%) than G-banded karyotyping and has been recommended as first-tier diagnostic test for patients with unexplained developmental disabilities or congenital anomalies (Miller et al., 2010). The resolution of CMA to detect CNVs, however, is limited to about 5–100 kb depending on the region of the genome and the probe density on the array (Huang et al., 2021). CMA diagnostic testing does not detect balanced rearrangements, such as inversions or translocations, and cannot determine the location of inserted sequences and the orientation of duplicated segments. In addition, low levels of mosaicism may also be missed (Martin & Warburton, 2015).
With advances in DNA sequencing technologies, exome sequencing (ES) and genome sequencing (GS) have become available as diagnostic tests (Alvarez-Mora et al., 2023; Manickam et al., 2021; Marshall et al., 2020). As a result, the diagnostic yield of previously undiagnosed genetic disorders has greatly increased. Currently, the American College of Medical Genetics (ACMG) recommends ES/GS as first or second tier in the genetic work-up of pediatric patients with birth defects or developmental/intellectual disability (Manickam et al., 2021). Bioinformatics analysis of sequencing data can also provide some information about structural variants, duplications, and deletions (Balachandran & Beck, 2020).
However, GS based on short-read technology and alignment to a reference sequence generated using short-read technology has shortcomings at specific locations in the genome including tandem repeats, paralogous genes, and other repeat sequences (Mascher & Stein, 2014; Torresen et al., 2019). For example, GS using even the latest Manta version of Dragen v4.3 is unlikely to allow identification of balanced rearrangements and inversions unless special informatics tools are applied to identify them in a high background of false positive calls (Loomis et al., 2013; Treangen & Salzberg, 2011; Yang, 2020). These are considered significant limitations since repetitive regions are often associated with the formation of SVs (Rudd et al., 2009). Given this, there is a need for alternative methods that are less sensitive to low information content regions of the genome and can aid detection of SVs.
Optical genome mapping (OGM), as developed by Bionano Genomics Inc., offers a completely independent approach (Sahajpal et al., 2021). This technique uses long DNA molecules (>150 kbp) that are fluorescently labeled at a particular 6 bp motif, linearized and aligned in nanoarrays that are imaged. Alignment of the unique labeling patterns allows a de novo assembly of the genome and detection of nearly all types of structural variants. Extensive validation studies of samples with known chromosome abnormalities have documented the power of OGM for constitutional postnatal cases, reaching close to 100% concordance for cases with noncentromeric breakpoints (Iqbal et al., 2023; Mantere et al., 2021). In addition, the potential of OGM to detect unknown pathogenic SVs previously missed by standard-of-care testing has also been reported (Shieh et al., 2021). OGM can also identify mosaic SVs (Cope et al., 2021). While OGM locates structural variants, confirmatory GS is used to pinpoint the breakpoints at the nucleotide level. Given these results, we propose OGM/GS to be used as a clinical test to detect SVs currently being missed by standard-of-care testing.
Here, we present a 17-year-old male with nondiagnostic FISH, CMA, and ES testing who later was diagnosed with a novel complex chromosomal rearrangement by OGM/GS. The rearrangement includes an abnormal X chromosome with a 22.5 kb subtelomeric deletion in band Xq28 replaced by an insertion of an extra copy of chromosome 7p22.3 material.
1.1 Clinical history
The proband initially presented to our Multidisciplinary Cardiovascular Connective Tissue Disease Clinic with a history of supravalvar aortic stenosis, hypercalcemia, and failure to thrive concerning for Williams–Beuren syndrome (OMIM: 194050). He also had a history of developmental delay, growth delay, short stature, hypertension, syringomyelia, headaches, tinnitus, general anxiety disorder, and attention-deficit/hyperactivity disorder (ADHD). More recently, he was diagnosed with autism spectrum disorder.
He was delivered by Cesarean-section at 36 weeks gestational age, due to leaking amniotic fluid and low fetal heart rate in the setting of partial placental calcification. Birth weight was 1.9 kg indicating intrauterine growth retardation (IUGR). A heart murmur noted at 1 month of age led to the diagnosis of supravalvar aortic stenosis, which was surgically corrected. At age 4 years, aortic valve stenosis was treated with balloon valvuloplasty followed by a three-patch sinus aortoplasty with excision of supernumerary coronary minimal ridge tissue. A previously diagnosed supravalvar pulmonary stenosis had resolved at the time. A recent echocardiogram revealed left ventricle enlargement, a mildly thickened aortic valve, no valvar aortic stenosis, and moderate secondary aortic insufficiency. Since his cardiac surgery, he had intermittent episodes of hypertension, controlled by multiple antihypertensive medications.
His postnatal growth was slow and followed the third percentile in height. Initial endocrine work-up included normal growth hormone and IGF-1 levels. At age 14 years, his height percentile had decreased even further due to lack of adolescent growth spurt (below first percentile). At 17 years of age, he had undergone puberty noted by Tanner 4 penis and pubic hair with testes descended bilaterally (Emmanuel & Bokor, 2024). Axillary hair was also present. He had normal testosterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), and Inhibin B levels, but low anti-mullerian hormone (AMH).
Early developmental milestones were delayed. He sat independently at 12 months and walked alone at 28 months. More recently, he attended mainstream classroom, and was considered to have average cognition and above average memory.
A work-up for bilateral leg pain and spasticity with magnetic resonance imaging (MRI) revealed non-progressive syringomyelia from C3 to the thoracic cord. A brain MRI performed to evaluate his complaints of headaches and tinnitus showed a short thick corpus callosum and a thick eighth cranial nerve within the internal auditory canals without associated enhancement. Olfactory bulbs and tracts were not identified. There was prominent fluid within the optic nerve sheaths, protrusion of the optic disc into the vitreous, prominent CSF within the pars nervosa and bilateral narrowing at the transverse-sigmoid sinus junctions. This constellation of findings suggested idiopathic intracranial hypertension. On lumbar puncture (LP), the opening pressure was elevated. His tinnitus, headaches, and agitation/anxiety significantly improved post-LP for a few days.
Ophthalmological evaluation showed optic nerve elevation with no edema. Retinal nerve fiber layer thickness was not responsive to Diamox or methazolamide suggesting pseudo-papilledema. The patient has worn glasses for nearsightedness and had a history of esotropia and diplopia.
A nephrology work-up for complaints of polydipsia and polyuria revealed normal renal function. A renal ultrasound showed echogenic kidneys most likely consistent with mild nephrocalcinosis, possibly related to his previous history of hypercalcemia.
Orthopedic work-up for occasional joint pain, mostly in elbows and hips, led to the diagnosis of Rhesus factor (Rh)-negative, antinuclear antigen (ANA), and C-reactive (CR) -protein-positive pauciarticular juvenile rheumatoid arthritis.
Due to complaints of exercise intolerance, lung function tests were performed and showed mild obstruction. He also had bilateral inguinal hernia repair when he was 1 month old, as well as tonsillectomy and adenoidectomy due to narrow airways shown by bronchoscopy.
Pertinent family history included an older brother who is tall and thin with a history of migraines, a depressive disorder, and ADHD. Father (height 5′8″) has history of hypertension. Mother (height 5′1″) had a heart murmur with a normal echocardiogram and hypothyroidism requiring medication. She was previously diagnosed with rheumatoid arthritis and cemento-osseus dysplasia, a benign condition of the jaws. Recently, the probable diagnosis of relapsing polychondritis was added. Maternal uncle (height 5′10″) had seizures as a toddler, he now has low gamma globulin levels and enamel dysplasia of his teeth. Maternal grandmother had a history of frequent migraines and grand mal seizures throughout adulthood, requiring antiepileptic medications. She also had breast cancer. Maternal great aunt also had a history of epilepsy and breast cancer. Ancestry was northern European on both sides, consanguinity was denied.
On physical exam at age 17 years his height was 156 cm (less than first percentile), with normal proportions (upper to lower segment ratio: 1.05) and his weight was 53 kg (sixth percentile). He had a few dysmorphic features (Figure 1a,b): Macrocephaly (occipitofrontal circumference [OFC] = 59 cm, +2SD) with high forehead, arched eyebrows, mild ptosis bilaterally, and a congenital melanocytic nevus on the left cheek. He also had hypertelorism, a deep-grooved long philtrum, full lips with high wide peaks to vermilion of upper lip and a short, upturned nose with fleshiness of the tip and nasal alae. His ears were small and posteriorly rotated. The hairline in the back of his neck was low. Teeth were widely spaced, with hypoplastic enamel, he wore orthodontal braces. He had brachydactyly of his hands with all distal phalanges appearing short (Figure 1c,d). The left second toe was contracted and overlapped by hallux.
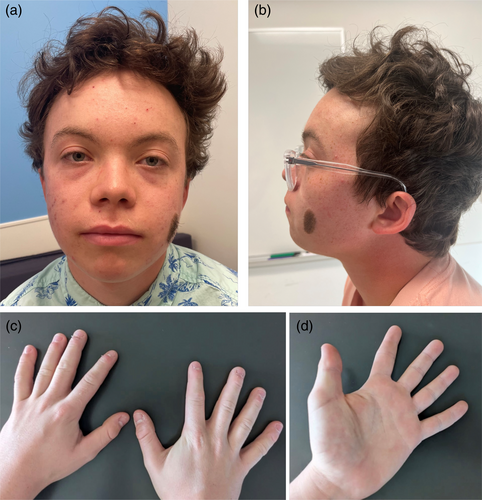
1.2 Prior genetic testing
The initially suspected diagnosis of Williams–Beuren syndrome (OMIM: 194050), typically caused by a 7q11.23 deletion, had been excluded by FISH and CGH on SNP array. Genetic supravalvular aortic stenosis was excluded by normal ELN gene sequence analysis. ES of the patient, both parents and the unaffected brother (ARUP laboratory), revealed de novo variants of unknown significance (VUS) in three genes: THSD7A (c.1402C > T, p.Gln468Ter) (GRCh38: chr7:11590511), HRH1 (c.625 T > C, p.Phe209Leu) (GRCh38:chr3:11259662) (rs201101191), and WRAP73 (c.172G > T, p.Ala58Ser) (GRCh38: chr1:3647458) (rs138332031). By using trio GS, we have now confirmed the de novo status of these variants. As of July 2024, all three variants are still classified as VUS. The missense variants are present in gnomAD at allele frequencies of 0.000003098 (allele count 5) for (rs201101191) and 0.000009295 (allele count 15), respectively within Admixed American and South Asian and African American populations. The truncating variant in THSD7A (Thrombospondin type 1 domain containing 7A) has not been previously described and seemed to be the most promising finding. The soluble form of THSD7A promotes endothelial cell migration and filopodia formation during sprouting angiogenesis via a FAK-dependent mechanism (Kuo et al., 2011). It is known that autoantibodies against this protein induce membranous nephropathy (Tomas et al., 2016). The (c.1402C > T, p.Gln468Ter) variant is absent from gnomAD and does not have a single nucleotide polymorphism database (dbSNP) identification number. While it is extremely rare (PM2) at least in the well-studied human populations, we find little evidence for its pathogenicity by using the ACMG criteria for classifying pathogenic variants (Richards et al., 2015). Although it is a null variant and predicted to undergo nonsense-mediated decay, the PVS1 criterion does not apply since no loss of function variants have been described in this gene to cause disease. Since phenotype consistency cannot be assessed, the PS2 criterion does not apply. Posting of the variant on GeneMatcher did not return informative results.
2 METHODS
Ultrahigh-molecular-weight DNA was isolated from blood samples of the proband and consenting family members, labeled using Bionano Prep SP version 2 DLS kits (Bionano Genomics Inc.) and loaded on a Bionano Genomics Saphyr Genome Imaging instrument. Details of the procedures have been described previously (Iqbal et al., 2023). De novo assembly and data analysis were performed using Bionano Access v1.6.
Genome and transcriptome sequencing was performed using short-read technology (2 × 150 bp) using a Novaseq 6000 and Complete Genomics DNBSEQ-T7 instruments. Long read genome/methylome sequencing was performed as well for the mother using Oxford Nanopore Technologies device and analyzed using https://github.com/epi2me-labs/wf-human-variation. Read alignment, variant calling and annotation (genome-wide single nucleotide variant (SNV), CNV, mitochondrial genome SNV, deletion/depletion, repeat expansion screening including FXS) are performed using Dragen (v4.3; https://emea.illumina.com/products/by-type/informatics-products/dragen-secondary-analysis.html). Differential expression analysis was done using edgeR (v3.30.3; Robinson et al., 2010). Annotation was done using Genoox (v60) (https://franklin.genoox.com/clinical-db/home) and mitochondrial deletion analysis was done using MitoSalt (v2.0.0) (Basu et al., 2020). Analysis pipeline was developed internally by Praxis Genomics (v2.0.0).
Result interpretation: variants detected by OGM, GS, and transcriptome analyses were interpreted together. Variants were classified based on ACMG criteria (Richards et al., 2015).
3 RESULTS
Given the nondiagnostic prior genetic testing, we recommended additional testing using combined OGM and GS to the family offered as a clinical test by Praxis Genomics LLC. GS data analysis using Dragen v4.03 identified over 4,019,085 SNVs, 137 CNVs (72 duplications, 65 deletions) and over 10,000 SVs (4050 deletions, 5818 insertions, 50 duplications, and 877 breakend pairs). Assessment of the SNVs confirmed the previously described variants but no additional variants were identified that would have explained the patient's phenotype or would have fulfilled the ACMG criteria for likely pathogenic classification (Richards et al., 2015). Based on the Dragen CNV calls, a 22.5 kb deletion near the tip of the X chromosome long arm (in band Xq28) was deemed of clinical interest. A 61.4 kb long duplication of the short arm of chromosome 7 (p22.3) was also noted. OGM demonstrated that the chromosome 7 duplication was not in situ and that the duplicated segment, based on its labeling pattern, was inserted into the X chromosome at the site of the deletion identified by GS (Figure 2a). The Manta analysis breakpoint output was filtered for sample specific translocations from chr7 to chrX. Of the two breakpoints thus identified one matched the approximate coordinates provided by the CNV analysis of Dragen. The exact insertion junction coordinates were established using visual analysis of unmatched sequences flanking the insertion as chrX:155057239-chr7:1196268 and chrX:155079766-chr7:1257633. The deletion, designated as GRCh38 Xq28 (chrX:155057239-155079766)×0 (GRCh37/hg19 chrX:154285514-154308041) removes the distal 2/3 of the 3′ untranslated region (UTR) of the FUNDC2 gene, the CMC4 and MTCP1 genes and the first five exons of BRCC3 (Figure 2b). The inserted 7p.22.3 duplication (7p22.3 [chr7:1196268-1257633]×3) includes only one known gene: UNCX (Figure 2c).
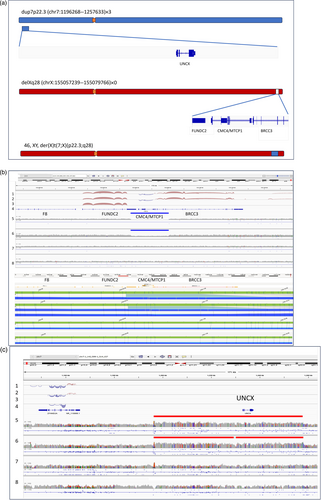
Test results of the proband's mother showed heterozygosity for the same abnormal X chromosome, while the proband's unaffected brother, maternal uncle, and maternal grandparents (not shown) did not carry the abnormal X chromosome (Figure 2b).
To further test the effects of these results, transcriptome analysis was performed on the proband, which showed the loss of expression of BRCC3, CMC4, and MTCP1 as expected from the genomic data. Transcriptome analysis of the mother showed an ~50% decrease in the expression of the genes affected by the deletion and no bias in the expression of heterozygous variants over the length of the X chromosome. This is consistent with the long read methylation studies which do not support a biased X chromosome inactivation in peripheral blood. The expression of adjacent genes FUNDC2 and VBP1 was unaltered in both samples (Figure 2b). Of note, the transcriptional effect of the triplication of UNCX could not be assessed as this gene is not expressed in blood (Figure 2c).
4 DISCUSSION
Clinically, identification of SVs is essential for genome interpretation as they are a major cause of birth defects and developmental disorders (Shaikh, 2017). Despite significant advances in molecular testing, the identification of SVs continues to be a challenge. Standard-of-care testing, which includes traditional cytogenetic techniques such as karyotyping and FISH are limited by their low resolution. CMA and ES have an inherent inability to detect balanced events or complex inversions. GS using short-read technologies is limited by the length of DNA fragments analyzed that often fail to bridge repetitive regions flanking structural variants. Manta, an analysis software designed to detect structural variants in the 50–10,000 bp range is part of the most recent NGS analysis suite (Dragen v4.3) from Illumina (Chen et al., 2016). On average, it outputs about 10,000 structural variants per genome. Even after filtering out recurrent variants, there are dozens of variants unique to the sample left to consider. To properly evaluate these variants, an alternative method is needed that can bridge repetitive regions. Novel methods, such as OGM, combined with GS, can overcome these limitations (Chen et al., 2020; Iqbal et al., 2023; Mantere et al., 2021; Sabatella et al., 2021; Schnause et al., 2021).
In this report, we present a 17-year-old male with a complex medical history and prior nondiagnostic genetic testing including FISH, CMA, and ES. Our studies by OGM/GS discovered a novel chromosomal rearrangement highlighting the potential of OGM/GS as a diagnostic test in the clinical setting.
The proband's unbalanced rearrangement includes a 22.5 kb deletion of Xq28 and insertion of a 61.4 kb segment of 7p.22.3 at the site of the deletion, in the presence of two structurally normal chromosomes 7. The structural variant was assessed for pathogenicity using the ACMG classification for such variants (Riggs et al., 2020). The following conditions were fulfilled for the chr7 duplication and the chrX deletion as well: (1) 1A (0.00) contains protein-coding or other known functionally important elements; (2) 3A (0.00) number of protein-coding RefSeq genes wholly or partially included in the CNV region is between 0 and 34. In addition, the chrX deletion also satisfied the: Overlap with established haploinsufficiency (HI)/loss of function (LOF)-sensitive genes or genomic regions 2A (+0.10). However, independently both variants were classified by the ACMG criteria as of uncertain significance.
We further investigated the significance of the der(X) variant in our patient by testing other members of the family and by functional characterization using transcriptome analysis.
The patient's mother did not have a balanced (X;7) translocation as OGM/GS analysis revealed that she is only heterozygous for the derivative X, der(X), chromosome with two structurally normal chromosomes 7. We showed that the der(X) was not inherited in the maternal lineage from previous generations and thus can be classified as de novo occurrence, the reported phenotype is consistent with the gene/genomic region, but not “highly specific and/or with high genetic heterogeneity” (4C (+0.15)). In order to determine the relevance of the genomic imbalance for our proband's phenotype, we tested at-risk males from the maternal lineage by OGM/GS. The unaffected older brother and maternal uncle did not inherit the der(X).
Transcriptome analysis of the proband's whole blood revealed loss of expression of two genes in the affected Xq28 region: BRCC3 and CMC4. MTCP1 is not expressed in blood. Thirty to fifty percent decrease in BRCC3 and CMC4 expression was noted in the mother's peripheral blood consistent with lack of evidence from methylation studies for biased X-inactivation.
Similar deletions of this region have been reported to be associated with Moyamoya Disease (MMD) type 4 (MYMY4) consisting of moyamoya arteriopathy, short stature, hypergonadotropic hypogonadism (HH) and facial dysmorphism (OMIM: 300845). Patients with deletions that extend into the clotting factor VIII gene (F8) also have severe hemophilia A (Jourdy et al., 2017; Pyra et al., 2021; Tzeravini et al., 2022).
Miskinyte et al. (2011) reported three unrelated families with nine affected males. Their overlapping Xq28 deletions suggested a critical interval for MYMY4 including exon 1 of MTCP1/CMC4 and exons 1–3 of BRCC3.
Our patient shares some of the physical features reported in these cases, such as short stature, hypertelorism, ptosis, long philtrum, low-set ears, short fingers, hypertension, and developmental delay in walking, speaking, and social behavior. Most cases report evidence of MMD angiopathy. Upon reevaluation of previous brain vascular imaging, our patient did not show any findings consistent with moyamoya or other vascular abnormalities. One of the reported cases also did not show any signs of angiopathy at age 28 years. The true frequency of angiopathy in BRCC3 deletion cases that are not ascertained via MMD has yet to be established.
We hypothesize the molecular etiology of this vascular phenotype is most likely associated with BRCC3 as animal studies have shown an essential role in angiogenesis (Miskinyte et al., 2011). ClinVar lists nine likely benign or benign SNVs in the BRCC3 gene. No deletions, duplication, indels, or insertions have been reported within the gene.
Previous cases also had cardiac involvement which varied from left ventricular enlargement to severe dilated cardiomyopathy (Herve et al., 2010). Our patient had supravalvar aortic stenosis that was surgically corrected. His recent echocardiograms have documented left ventricular enlargement.
Our patient also had delayed puberty and lack of pubertal growth spurt, but genital exam and serum hormone levels of testosterone, LH, and FSH were normal, except for AMH that was very low. Based on previous reports of deletions in the Xq28 region, both FUNDC2 and CMC4 are hypothesized to be associated with HH and short stature in male patients (Deng et al., 2020). CMC4 is mostly expressed in mitochondria, with highest expression in fetal testes and its function remains unknown (Deng et al., 2020). HH is also reported in severe hemophilia A and MMD caused by deletions disrupting the Factor VIII (F8) gene and extending to BRCC3 including FUNDC2 (Lavin et al., 2016). In our patient, who does not manifest HH, CMC4 is deleted resulting in loss of expression while FUNDC2 expression is not affected. This finding suggests different functions for these two genes: the loss of FUNDC2 is responsible for the HH phenotype, while CMC4 is associated with slow growth since birth leading to short stature.
The mature T cell proliferation 1 protein encoded by MTCP1 was also affected in our patient. Both MTCP1 and CMC4 share the 5′UTR and the first noncoding exon 1. Alternative usage of the 6 coding exons results in two entirely different protein products: CMC4 (8 kDa) and MTCP1 (13 kDa), the latter being only expressed in hematologic malignancies (Madani et al., 1996; Walker et al., 2021). Deficiencies of these gene products have not been reported in ClinVar.
Females heterozygous for Xq28 deletions who are X-inactivation mosaics need to be carefully evaluated for subtle phenotypes. Reduced adult height has repeatedly been reported (Janczar et al., 2014), our patient's mother is 155 cm tall. No heterozygote for the Xq28 deletion has been reported to have MMD angiopathy, but it is unclear how many underwent brain vascular imaging. Our patient's mother reported intermittent headaches, dizziness/vertigo, and upper and lower extremities tingling. Noncontrast CT and CT angiograms of head and neck did not detect any stenoses of the carotid and intracranial arteries. On serial brain MRIs, monofocal leukoaraiosis in the periventricular white matter was described as stable. Both the patient and his mother are clinically followed for development of MMD manifestations.
The 61.4 kb duplication of chromosome 7 includes the UNCX gene, which encodes a homeobox transcription factor involved in somitogenesis and neurogenesis in animal studies (Sammeta et al., 2010). This gene is also required for the maintenance and differentiation of specific elements of the axial skeleton. Other roles include controlling the development of connections of hypothalamic neurons to pituitary elements, allowing central neurons to reach the peripheral blood circulation and deliver hormones that control peripheral functions (Nittoli et al., 2019).
In humans, UNCX expression is limited to cerebellum (GTEx Release V.8). The significance of an increased dosage of this gene remains unclear as there is currently no known disease association. In addition, a similar 7p22.3 duplication as the one identified in our patient and his mother has not been previously described.
In this article, we highlight the advantages of OGM in the diagnosis of structural variants that may remain undetected with current standard testing as was the case for our patient. In our patient, we were able to detect a complex SV including a deletion at Xq28 that was replaced by an extra copy of a segment of chromosome 7. The structurally abnormal X chromosome was present in his mother, but not in his unaffected brother, maternal uncle and maternal grandparents. Therefore, we propose that the combination of his absent Xq28 and triplicated 7p22.3 genomic material is responsible for his phenotype. Overall, our data suggest OGM is essential for proper interpretation and orthologous confirmation of complex structural rearrangements previously unreported based on standard-of care-testing. OGM is best used in combination with GS, to help evaluate the true positives in the large number of structural variants reported by Manta. However, Manta is useful to precisely identify the breakpoints although, as in this case, only one junction could be unequivocally localized by Manta, the other junction had to be visually assessed because of the low information content sequence surrounding that breakpoint. We recommend OGM/GS combination testing for general use, since it allows independent review, prioritization and orthologous verification of structural variants.
ACKNOWLEDGMENTS
We thank the patient and his family for their cooperation. Michael Fidel Nagy and Nicholas Rouse performed the molecular analyses done by Praxis Genomics. The following health care providers took care of the patient at Stanford: Shannon Jeanine Beres, Hilary Hoffman Seeley, Ronnie Thomas Collins, Claudia Alejandra Algaze Yojay, Abanti Chaudhuri, Miguel Andres Moreno, Sarah Lee, Allan Leonard Reiss, Tracy Lauren Jordan, Kirstie MacMillan Lechich, Megan June Wu, Suha Bachir, Aiste Narkeviciute, and Sharmila Ravi.
CONFLICT OF INTEREST STATEMENT
Peter L. Nagy is the founder and owner of Praxis Genomics LLC.
Open Research
DATA AVAILABILITY STATEMENT
FASTQ, BAM, and VCF files as well as Bionano Access data outputs for all four individuals studied are available at: https://www.praxisgenomics.com (to be determined based on submission requirements of journal).


