X-linked congenital adrenal hypoplasia: Report of long clinical follow-up and description of a new complex variant in the NR0B1 gene
Abstract
Adrenal hypoplasia congenita, attributed to NR0B1 pathogenic variants, accounts for more than 50% of the incidence of primary adrenal insufficiency in children. Although more than 250 different deleterious variations have been described, no genotype–phenotype correlation has been defined to date. We report a case of an adopted boy who reported the onset of an adrenal crisis at 2 weeks of age, requiring replacement therapy with mineralocorticoids and glucocorticoids for 4 months. For 3 years, he did well without treatment. At almost 4 years of age, the disorder was restarted. A long follow-up showed the evolution of hypogonadotropic hypogonadism. Molecular studies on NR0B1 revealed a novel and deleterious deletion–insertion–inversion–deletion complex rearrangement sorted in the 5′-3′ direction, which is described as follows: (1) deletion of the intergenic region (between TASL and NR0B1 genes) and 5′ region, (2) insertion of a sequence containing 37 bp at the junction of the intergenic region of the TASL gene and a part of exon 1 of the NR0B1 gene, (3) inversion of a part of exon 1, (4) deletion of the final portion of exon 1 and exon 2 and beginning of the 3′UTR region, (5) maintenance of part of the intergenic sequence (between genes MAGEB1 and NR0B1, telomeric sense), (6) large posterior deletion, in the same sense. The path to molecular diagnosis was challenging and involved several molecular biology techniques. Evaluating the breakpoints in our patient, we assumed that it was a nonrecurrent rearrangement that had not yet been described. It may involve a repair mechanism known as nonhomologous end-joining (NHEJ), which joins two ends of DNA in an imprecise manner, generating an “information scar,” represented herein by the 37 bp insertion. In addition, the local Xp21 chromosome architecture with sequences capable of modifying the DNA structure could impact the formation of complex rearrangements.
1 INTRODUCTION
X-linked congenital adrenal hypoplasia (X-HipoAC) (OMIM #300200) is a disorder affecting the adrenal cortex and the hypothalamic–pituitary-gonadal axis. It is clinically characterized by early onset primary adrenal insufficiency manifested by salt loss, hypogonadotropic hypogonadism in adolescence, and infertility in adulthood (Buonocore & Achermann, 2020).
Based on the observation that patients with Duchene muscular dystrophy and glycerol-kinase deficiency also had X-HipoAC and hypogonadotropic hypogonadism, Zanaria et al. (1994) studied the X chromosome (Xp21), which is the location of the gene responsible for X-HipoAC. The authors mapped the region in individuals with isolated X-HipoAC, identified deletions and point mutations in the NR0B1 gene (Nuclear Receptor Subfamily 0, Group B, Member 1; DAX1; DSS-AHC critical region on the X chromosome 1, gene 1—OMIM *300473), and concluded that this gene was responsible for the origin of the disease (Zanaria et al., 1994). Subsequently, other studies described the role of the NR0B1 gene as that of encoding a nuclear receptor that regulates adrenal and gonadal development during fetal and adult life (Guo et al., 1995; Guo et al., 1996). Since then, several pathogenic variants of the gene have been reported, which are responsible for X-HippoAc (Buonocore & Achermann, 2020; Lin et al., 2006). However, no well-established genotype–phenotype relationship is known (Jadhav et al., 2011; Kyriakakis et al., 2017). In fact, pathogenic variants of the NR0B1 gene have been found in approximately 58% of males with primary adrenal insufficiency of unknown etiology (Mohan et al., 2019).
More than 250 pathogenic variants have been described along the two exons of the NR0B1 gene (www. Hgmd. Cf. ac. Uk-accessed—01/24/2023), including small and large deletions, insertions, missense, nonsense, frameshift, and splicing site variants (Phelan & McCabe, 2001). As previously described, large X chromosomal deletions are responsible for contiguous gene syndrome and include deletions in Duchenne muscular dystrophy (DMD) (OMIM*300377, Xp21.2-p21.1) and glycerol-kinase deficiency (GK) genes (OMIM*300474, Xp21.2) (Roucher-Boulez et al., 2018).
Here we report a case of primary adrenal insufficiency due to a complex rearrangement in the NR0B1 gene whose sequencing required several molecular genetic study strategies to reach the diagnosis.
2 CASE REPORT
A two-week-old male neonate was admitted to the emergency department and presented with severe vomiting, dehydration, hyponatremia (122 mmol/L; reference value [RV] 135–145 mmol/L), hyperkalemia (7.2 mmol/L, RV 3.5–5.0 mmol/L), and severe weight loss. The infant was adopted at 3 days of age with unknown gestational and delivery histories. Initially diagnosed with congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21OH-HAC), we collected blood samples to determine serum levels of 17OH-progesterone and androstenedione and initiated replacement therapy with hydrocortisone and fludrocortisone at doses of 20 mg/m2/day and 100 μg/day, respectively. The 17OH- progesterone value was 4.39 ng/mL (RV up to 3.2 ng/mL), and 1.6 ng/mL (RV 0.2–0.53 ng/mL) of androstenedione was recorded, ruling out the diagnosis of 21OH-HAC. Treatment was administered for 4 months and then discontinued after a progressive dose reduction. The child progressed well clinically and did not require hospital admission.
He demonstrated good progress until 30 months of age, when he began to experience low weight gain and repeated airway infections. At 3.5 years of age, the child presented with severe dehydration, hyponatremia (101 mmol/L), and hyperkalemia (7.2 mmol/L) with tonsillitis. He had pronounced salt cravings and undetectable aldosterone levels (<25 pg/mL, RV 10–160 pg/mL). Sodium supplementation and fludrocortisone were initiated. At 4 years and 3 months of age, when fludrocortisone was discontinued, he showed indosable levels of aldosterone (<25 pg/mL, VR 10–160 pg/mL) and cortisol (<1.0 μg/%, VR 5.0–25.0 μg/%) along with the elevation of ACTH (adrenocorticotropic hormone) (220 pg/mL, RV up to 46 pg/mL) and plasma renin activity (41.15 ng/mL/h, RV 0.15–2.33 ng/mL/h), without an increase in adrenal precursor levels (i.e., low 17OH-progesterone and androstenedione), confirming the diagnosis of primary adrenal insufficiency. Mineralocorticoid and glucocorticoid replacement therapy have been definitively established, and blood samples were used for the molecular study of the NR0B1 gene.
At 14 years of age, as he did not show pubertal signs, we first evaluated gonadal function with a hCG (human chorionic beta gonadotropin) stimulation test (protocol 1500 U/day for 5 days) that proved to be responsive (with an increase of 132% in testosterone levels: <0.02 and 2.62 ng/mL before and 24 h after testing, respectively) (Bertelloni et al., 2018); to assess hypothalamic–pituitary axis, a prolonged GnRH (gonadotropin- releasing hormone) stimulation test (100 mcg/day for 5 days) was performed, with a gradual increase in LH (luteinizing hormone) and FSH (follicle-stimulating hormone) levels suggesting a hypogonadotropic hypogonadism of hypothalamic origin (LH raised from 0.55 IU/L [basal level before 5th GnRH injection] to 2.17 IU/L [at 30 min after 5th dosis]) and FSH from 5.49 to 7.48 IU/L (Fraietta et al., 2013; Grinspon et al., 2010). Testosterone replacement therapy was initiated, and full pubertal development was recorded, with a final height of 176 cm.
3 RESULTS
Each exon of the NR0B1 gene and its respective flanking regions were PCR-amplified from genomic DNA (after extraction and ethanol precipitation according to standard techniques) (Phelan & McCabe, 2001), using primers designed to cover the entire length of the NR0B1 coding sequence (detailed in Table S1, Data S1). PCR identified deletions in the initial part of exon 1 (named portion 1A) and the entire exon 2. However, the end of exon 1 (named portion 1 B) was present, as illustrated by the agarose gel electrophoresis images in Figure 1a. This figure shows that the combination of primers named P1 forward and P6 reverse, which were used to amplify the first half of exon 1 and part of the 5′ flanking region, did not show an amplicon when compared to the control, indicating the deletion of this region. A similar result was obtained when P2 forward + P10 reverse and P11 forward + P12 reverse primer combinations were used to amplify exons 1 and 2, respectively. This finding confirmed both the deletion of exon 1, portion 1a, and the deletion of exon 2. Intriguingly, the combinations of primers P8 forward + P10 reverse and P5 forward + P9 reverse produced amplificons, indicating that the exon 1 portion 1B would have been preserved. To investigate if other parts of the X-chromosome has also been deleted, we used the MLPA technique, which was performed using the SALSA MLPA P185 version C3 Intersex kit (MRC-Holland, Amsterdam, Netherlands) as per the manufacturer's recommendation (Roucher-Boulez et al., 2018). The results of the two assays confirmed these findings, as illustrated in Figure 1b.
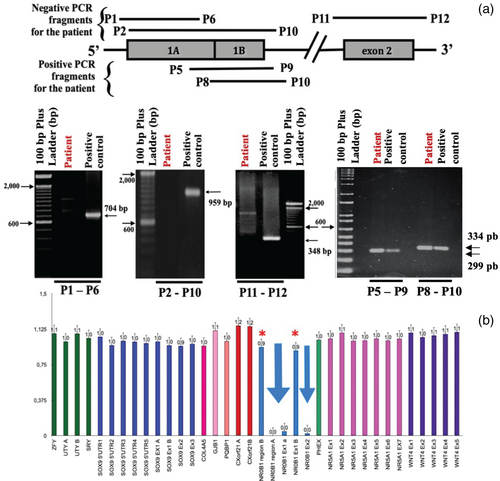
To elucidate this rearrangement, we initially investigated the remaining portion of exon 1 named portion 1B. The initial idea was to perform Sanger sequencing of the PCR product using the primer named P8 forward (described above), which was not deleted, combined with a new primer named P3′ reverse that annealed to the c.* 527_548 position at the 3 ‘UTR end (Table S1). However, this PCR did not produce an amplicon. Based on similar rearrangements in this region (Wheway et al., 2003), we hypothesized that the remaining portion 1 B had undergone inversion. To test this hypothesis, we performed a PCR reaction with the nondeleted P9 reverse primer combined with the reverse P3’ primer. This reaction yielded an amplicon of approximately 900 bp, which was purified and sequenced. The result showed that the 5′ region of the remaining exon 1 portion B was indeed connected to the 3′ UTR portion of the NR0B1 gene, assuming an inverted orientation, which suggested a complex rearrangement. Efforts were then focused on elucidating the exact breakpoint in the 5′ region. Hence, different primers were designed to tentatively get some positive amplification, but none of the primer combinations generated PCR amplicons.
Another molecular tool, array comparative genomic hybridization (aCGH) [performed with Genome-Wide Human SNP Array 6.0 and analyzed by GeneChipR Operating Software (GCOS) (Affymetrix)], was then used to identify the approximate breakpoints based on the location of probes included in the assay and, consequently, estimate the size of the deletion. The aCGH assay showed two deleted regions: a deletion of approximately 23 kb in the intergenic region between TASL and NR0B1 that includes the 5′ region of the NR0B1 gene and part of exon 1, and a second deletion of approximately 37 kb at the 3′ end located between NR0B1 and MAGEB1. The inverted region that was not deleted between them corresponded to what we call exon 1 portion B, whose breakpoint and joining were identified by sequencing. Despite knowing the breakpoint of the inversion and genomic location of deleted and nondeleted probes, we were not successful in amplifying the breakpoints of the deletions using different combinations of primer pairs located within nondeleted regions.
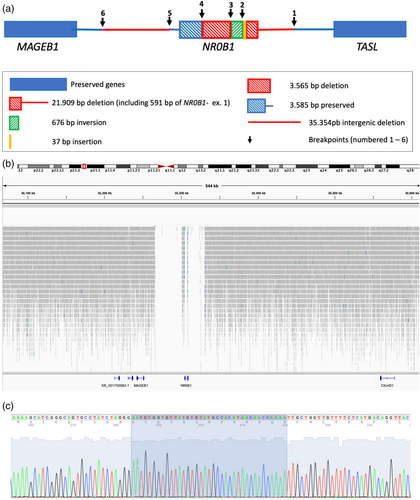
We used whole-genome sequencing (WGS) as an alternative to test the hypothesis of the rearrangement of the NR0B1 gene. This method, performed using the NovaSeq 6000 system (Illumina, San Diego, CA, USA), using pairs of reads with 30-fold coverage and analyzed by the free Integrative Genomics Viewer (IGV) software, version 2.9.2 (Thorvaldsdóttir et al., 2013), showed three deleted segments: one in the intergenic region between TASL and NR0B1 extending to the medial of exon 1 with a size of 21,909 bp, a second 3585-bp intra-genic deletion that included intron 1 and exon 2, and a third segment of 35,354 bp in the intergenic region posterior to the 3′ region of the gene located toward the MAGEB1 gene, as illustrated in Figure 2. WGS also indicated the inversion of 676 bp as part of exon 1, confirming previous Sanger sequencing data. Once all breakpoints were known, new primers were designed for sequences close to these points (Table S1). Through PCR and sequencing, we were able to confirm joining points in the 5′ region and identify a new 37-bp insertion at this point (Figure 2).
Translated with www.DeepL.com/Translator (free version).
4 DISCUSSION
X-HipoAC is a life-threatening condition characterized by adrenal insufficiency (mineralocorticoid and glucocorticoid insufficiency) that clinically manifests as vomiting, malnutrition, growth deficiency, seizures, vascular collapse, and sudden death. Laboratory findings include hyponatremia, hyperkalemia, hypoglycemia, decreased serum cortisol and aldosterone levels, and increased plasma ACTH (Lin et al., 2006). The clinical presentations are diverse and include: (1) Differences in the age of onset of adrenal insufficiency, with approximately 40% of affected boys presenting clinically with adrenal insufficiency in the first months of life or throughout childhood (Suntharalingham et al., 2015). In some cases, manifestation begins in adulthood (Jadhav et al., 2011); (2) Differences in the type of corticoid deficiency are recorded, with some cases predominantly showing mineralocorticoid deficiency while others reporting glucocorticoid deficiency (Landau et al., 2010); (3) Variation in the onset of puberty, with pubertal delay secondary to hypogonadotropic hypogonadism and, more rarely, precocious puberty (Nagel et al., 2019). Guran et al. (2016) described a bimodal pattern of clinical presentation in childhood, with one group showing initiation of the condition in the first months of life and the others after 18 months.
Here, we describe a case of a boy with X-HipoAC who showed manifestations in the first 2 weeks of his life with classic symptoms of vomiting, weight loss, dehydration, hyponatremia, and hyperkalemia, which required drug treatment for a short period (4 months). He evolved well without treatment, although his mother reported increased cravings for salt. During this period, the patient remained untreated and primarily reported allergic episodes of acute laryngitis and bronchitis requiring no hospitalization. At the age of 3 years and 6 months, the patient presented with a clinical picture suggestive of adrenal insufficiency (vomiting and severe dehydration), and replacement with hydrocortisone and fludrocortisone was reinitiated. Given the wide variation in clinical presentation, we question whether the period during which he grew and developed well without corticosteroid replacement was due to his craving for salt and the occasional use of glucocorticoids to treat allergic crises, simulating an apparent “period of remission” that lasted from 4 months to 3 years of age. Salvi et al. (2002) described a case of X-HipoAC with a clinical presentation very similar to that described herein (early onset adrenal insufficiency followed by apparent remission with subsequent recurrence associated with pubertal delay due to hypogonadotropic hypogonadism) due to a complex rearrangement in the NR0B1 gene (deletion of exon 2 associated with the insertion of 27 base pairs). As described in other studies, the initial diagnosis is challenging, with the misdiagnosis of 21OH-HAC or hypoaldosteronism, being common. In our case, not knowing the pathological history of the biological family made the diagnosis even more difficult (Landau et al., 2010).
Stem cell studies have shown that the NR0B1 gene is expressed in progenitor cells and promotes differentiation and expansion of the cell pool (Roucher-Boulez et al., 2018). The loss of NR0B1 gene function can prematurely compromise the differentiation of progenitor cells into steroidogenic cells even before the expansion of cell number occurs, preventing proper embryogenesis (Niakan & McCabe, 2005).
In the present case, we consider the rearrangement found in the NR0B1 gene to be deleterious, which has a strong potential to compromise DAX1 function as a nuclear receptor. This is because it includes a large deletion that excludes part of exon 1 (which encodes the DNA-binding domain) and the entire exon 2, which encodes the LBD domain and is associated with a severe phenotype. In this scenario, it would be interesting to consider the initial evolution of the disease presented by the child, marked initially by a severe clinical manifestation of adrenal insufficiency, followed by a stable period without corticosteroid replacement until a pronounced and definitive recurrence was reported some years later.
The steps toward molecular diagnosis were challenging and required the use of different molecular biology techniques. First, although we had no biological family history to suspect the diagnosis of X-HipoAC, phenotypic and biochemical data directed us to postulate the presence of pathogenic variants in the NR0B1 gene and tried to use Sanger sequencing as a tool to evaluate gene sequence. Parts of exon 1 (called portion 1A) and exon 2 were not amplified by PCRs. Subsequently, the MLPA technique was used to confirm the presence of deletions. Lin et al. (2006) reported that approximately one-third of patients with X-HipoAc had NR0B1 deletions, and their recognition was important but difficult because PCR of the NR0B1 gene in an affected male individual will not produce any products since the gene is characterized by a single allele on the X chromosome (Jadhav et al., 2011; Kyriakakis et al., 2017).
Similar to the PCR results, MLPA showed the presence of the final part of exon 1 (portion 1 B); however, the nondeleted portion could not be confirmed by Sanger sequencing. Wheway et al. (2003) described a rearrangement involving more than 2 Mb of Xp21 in a patient with contiguous gene syndrome where a nondeleted part within this DNA segment was inverted, and all GK, NR0B1, IL1RAPL1, and MAGEB genes were deleted. By analogy with what these authors proposed, it was hypothesized that the nondeleted region in our patient could be inverted, characterizing a deletion–insertion–inversion–deletion rearrangement. The use of aGH indicated the size of each deletion and guided us to design primers within nondeleted sequences close to breakpoints. However, attempts to amplify and sequence these regions were unsuccessful. The use of different molecular techniques to study NR0B1 has been described previously (Barbaro et al., 2012; Rojek et al., 2016; SIKL, 1948).
WGS demonstrates more potent applicability, particularly in cases of structural rearrangements (Fan et al., 2021; Onore et al., 2021). Complex structural rearrangements can be defined by the presence of more than one simple rearrangement or two or more breakpoints, generating duplications, deletions, triplications, or inversions (Weckselblatt & Rudd, 2015).
Some segments of the genome are prompt to rearrangements due to their local architecture, marked either by the presence of areas known as low copy repeats (LCR) (segments larger than 1 kb in size that have high similarity) or by other repetitive elements (RE) of the DNA (long interspersed nuclear elements–LINE, short interspersed nuclear element–SINE, Alu sequences, etc.), which, by similarity, may predispose alignment to similar sequences, either from the same or distinct chromosomes, in case of DNA molecule injury, generating nonallelic homologous recombination (NAHR) (Liu et al., 2012). NAHR causes recurrent rearrangements. The architecture of the distal portion of the X chromosome predisposes it to genomic instability (Liu et al., 2011; Stankiewicz & Lupski, 2002) because of the presence of such elements. Using the online tools RepeatMasker (https://www.repeatmasker.org) and Dfam (https://www.dfam.org/home) (Keegan et al., 2019; Chen, 2004), we identified the presence of retrotransposons at some breakpoints: at breakpoint 1, L114_orf2 (LINE); at breakpoint 5, MER68 (LTR); and at breakpoint 6, repetitive elements L2 (LINE) and MLT2C2 (LTR). Nevertheless, we believe that the origin of the deletions was not NAHR owing to the following reasons: (1) no significant similarity was recorded between the RE sequences of one breakpoint and another, which is required for NAHR; (2) it was not a recurrent rearrangement; and (3) the product of NAHR repair is usually more conservative (Liu et al., 2012).
Thus, we postulate that the rearrangement originated from another repair mechanism known as nonhomologous end-joining (NHEJ). This mechanism joins two ends of DNA (resulting from double-strand breakage) in an imprecise manner because it does not use segments of extensive homology for pairing, tolerating the loss or addition of nucleotides at the junction site and generating “information scars,” a typical finding of this type of repair (Storer et al., 2021). In the present case, the insertion of 37 base pairs between breakpoints 1 and 2 configured the scars left by NHEJ. Owing to the absence of complementarity between the broken strands, the repair required regions with small stretches of homology (microhomology) for pairing, which is often associated with large deletions (Lieber, 2008). Upon analyzing our patient's breakpoint sequences by multiple sequence alignment using the online tool ClustalW (https://www.ebi.ac.uk/Tools/msa/clustalo), we noted the presence of microhomology in these regions (size 1 to 7 base pairs), suggesting that this small similarity served as a guide for microhomology-mediated nonhomologous end joining repair (MMEJ).
As mentioned above, we believe that the RE found at some breakpoints were not directly involved in the repair mechanism but assisted in the formation of the rearrangement since they induce genomic instability and subsequent rearrangement in association with other sequences capable of modifying the DNA structure (e.g., non-B DNA) (Ma et al., 2003). An online non-B DNA motif search tool (https://nonb-abcc.ncifcrf. gov/apps/nBMST) was used to assess the presence of these sequences at the breakpoints (we defined the breakpoint region as the 160 base pairs surrounding the breakpoint, according to the genomic sequence). At breakpoints 1 and 4, we found the presence of two sequences capable of forming inverted repeats and cruciform motifs; at breakpoint 5, we found different sequences capable of generating both direct repeats and slipped motifs, as well as mirror repeats, triplex motifs, and short tandem repeats. The non-B DNA analysis of breakpoint 6 revealed sequences capable of generating mirror repeats, triplex motifs, short tandem repeats, inverted repeats, and cruciform motifs. These findings gave clues to the suggested mechanism and highlighted the impact of local genomic architecture on the formation of complex rearrangements.
In conclusion, we consider this case of great interest for the following reasons: (1) a high index of suspicion is necessary to avoid misdiagnosis, especially when family history is unknown; (2) long follow-up is essential for appropriate management and treatment; (3) molecular analysis of the NR0B1 gene, especially in boys presenting salt loss with or without cortisol deficiency, is important for appropriate diagnosis and genetic counseling; and (4) despite the large number of known pathogenic variants in NR0B1, the discovery of new variations continues to challenge the understanding of the genotype–phenotype relationship found in the disease and demands the use of several molecular tools when dealing with a complex variants.
AUTHOR CONTRIBUTIONS
AMEA performed the experiment, analyzed the data, and wrote the manuscript. SHVLM provided patients' samples and clinical data and reviewed the manuscript. VLGSL and APFA performed a CGH experiment and interpreted the results. CSCP and TNM performed DNA extraction and MLPA experiments. MSG performed WGS bioinformatic analysis. MPM and GGJ conceived, designed, and coordinated the study, analyzed and interpreted the data, and edited and reviewed the manuscript. All authors read and approved the final version of the manuscript.
FUNDING INFORMATION
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 471121/2004–5) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP # 2008/54776–1).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this case report are available from the corresponding author on request ([email protected]).