Patent ductus arteriosus and coarctation of the aorta in association with PRDM6 variants
Alanna Strong and Balram Gangaram contributed equally to this work.
Abstract
Patent ductus arteriosus (PDA) and coarctation of the aorta (CoA) are relatively common congenital heart defects. Pathogenic variants in PRDM6, which encodes a smooth-muscle-cell-specific transcription factor, have now been etiologically associated with non-syndromic PDA. We present three patients with PDA and CoA found to harbor PRDM6 variants, including a novel, likely-pathogenic variant.
Abbreviations
-
- CNCC
-
- cardiac neural crest cell
-
- CoA
-
- coarctation of the aorta
-
- DA
-
- ductus arteriosus
-
- PDA
-
- patent ductus arteriosus
-
- VUS
-
- variant of unknown significance
1 INTRODUCTION
Patent ductus arteriosus (PDA) and coarctation of the aorta (CoA) are common congenital heart defects, which can occur in isolation or as part of a syndrome and can lead to heart failure when undiagnosed. The genetic bases of non-syndromic PDA and CoA are incompletely understood, with PRDM6 (OMIM 616982) representing one of the few known genetic causes of familial non-syndromic PDA (Li et al., 2016). PRDM6 encodes PR domain-containing protein 6, a smooth-muscle-cell-specific putative histone methyltransferase and transcriptional repressor. Studies of the mouse homolog suggest that PRDM6 directs cardiac neural crest cell (CNCC) differentiation and migration and is critical to ductus arteriosus (DA) closure in mammals after birth (Hong et al., 2022; Zou et al., 2023). We present two unrelated families with PDA and CoA found to harbor PRDM6 variants, representing an expansion of PRDM6-related disease to include CoA. In addition, we share a novel, likely disease-causing loss-of-function PRDM6 variant.
2 CLINICAL REPORTS
Consent for publication was obtained from all patients or their legal guardian.
2.1 Family 1: Patient A, B, and C
2.1.1 Patient A
Patient A is a now 18-year-old female who presented to outpatient cardiology at 10 days of life for evaluation of a murmur. On presentation, she was tachycardic with a 21 mmHg blood pressure gradient from right arm to right leg. Echocardiography showed mild transverse arch hypoplasia, severe CoA, and right-to-left PDA shunting. She underwent PDA ligation and CoA repair with end-to-end anastomosis via left thoracotomy 3 days later. She developed intermittent systolic hypertension at 4 years of age. Evaluation for extra-cardiac causes of hypertension, including renal vascular imaging, was non-diagnostic. Her hypertension progressed, necessitating beta blocker initiation at 7 years of age; it is currently well-controlled with amlodipine and losartan. She underwent balloon angioplasty at 10 years of age for moderate residual coarctation, without improvement. Routine magnetic resonance imaging was performed at 16 years of age, showing stable moderate residual coarctation (Figure 1a). She has café-au-lait macules and is clinically well with normal growth and development.
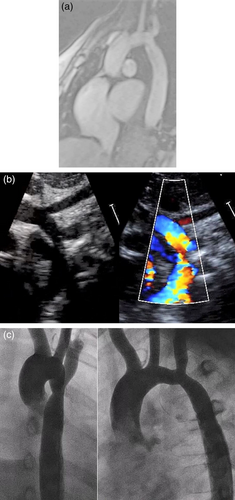
2.1.2 Patient B
Patient B is the 14-year-old full sister of Patient A. She was diagnosed prenatally with mild aortic arch hypoplasia. Echocardiography demonstrated a large PDA with CoA (Figure 1b), and she underwent PDA ligation and CoA repair with extended end-to-end anastomosis at 2 weeks of life. She developed intermittent systolic hypertension at 3 years of age. Renal vascular imaging demonstrated a small caliber left renal artery without focal stenosis, likely not contributing to her hypertension. Atenolol was initiated at 6 years of age. She underwent balloon angioplasty for residual coarctation at 6 years old, without significant improvement (Figure 1c). She has café-au-lait macules and is clinically well with normal growth and development.
2.1.3 Patient C
Patient C is the 55-year-old father of Patients A and B. He was incidentally diagnosed with PDA in his fourth decade during evaluation of palpitations and underwent surgical repair shortly thereafter. He has café au lait macules and mild hypertension diagnosed in adulthood.
2.2 Family 2: Patients D, E, and F
2.2.1 Patient D
Patient D is a 2-year-old male diagnosed with PDA at 2 weeks of life because of a heart murmur. He became symptomatic with poor feeding 1 month later and repeat Echocardiography demonstrated CoA, mild aortic arch hypoplasia, moderate PDA, and left heart dilation. He underwent CoA repair with end-to-end anastomosis and PDA ligation. His medical history is notable for mild developmental delay with normal growth parameters.
2.2.2 Patient E
Patient E is the 3-year-old full brother of Patient D. A murmur was noted after birth, and he was diagnosed with a restrictive PDA during his second week of life. He underwent transcatheter device closure 4 months after developing left ventricular enlargement. He has mild developmental delay with normal growth parameters.
2.2.3 Patient F
Patient F is the mother of Patients D and E. She is a 20-year-old female with PDA diagnosed and repaired surgically in childhood. Her paternal half-brother and his son had PDAs repaired surgically, and her father may have had a PDA (history unconfirmed). They are not known to have other medical concerns.
3 GENETIC TESTING
Family 1 underwent quad whole exome sequencing notable in both sisters for a previously-reported, likely-pathogenic PRDM6 variant (NM_001136239.4: c.1646G>A; p.(Arg549Gln)) that was paternally-inherited and a variant of uncertain significance (VUS, c.767C>G; p.(Pro256Arg)) that was maternally-inherited (Figure 2a). Mother had a normal echocardiogram.
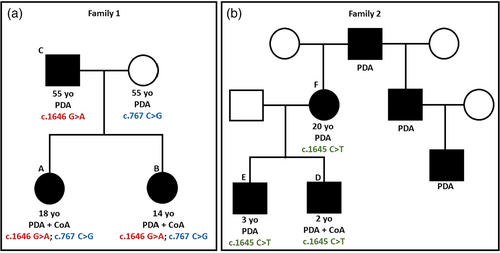
Family 2 underwent quad whole exome sequencing, revealing a heterozygous VUS in exon 7 of the PRDM6 gene (NM_001136239.1: c.1645 C>T, p.(Arg549Ter)) in Patients D, E, and F, and absent in Patient D's father (Figure 2b). Additional gene variants unrelated to their cardiac phenotype are described in the supplemental material.
4 DISCUSSION
During fetal life, the ductus arteriosus (DA) shunts blood from the pulmonary artery to the aorta in order to bypass the lungs. Closure of the DA after birth is a complex process of vascular remodeling that requires CNCC migration to the DA, transformation to vascular smooth muscle cells (SMC), and contraction. PRDM6 is one of the few known causes of familial non-syndromic PDA (Li et al., 2016). PRDM6 encodes a transcription factor (also called PRISM) expressed in the cardiac outflow tract and DA that promotes SMC differentiation (Davis et al., 2006; Hong et al., 2022; Zou et al., 2023). Functional evidence from in vitro cell lines together with in vivo mouse model data suggest a loss-of-function mechanism; gene depletion leads to dysregulation of genes critical for SMC differentiation with resultant disruption of vascular remodeling, decreased DA tone and contractility, and persistent ductal patency (Hong et al., 2022; Li et al., 2016; Zou et al., 2023).
Human gene-disease association studies for PRDM6 are limited. The likely-pathogenic missense variant in Family 1, c.1646G>A; p.(Arg549Gln), was previously identified (Li et al., 2016; Lynch et al., 1965). The Arg549 amino acid residue falls in a zinc finger domain of PRDM6 that is critical for PRDM6 nuclear localization (Davis et al., 2006).
Family 2's heterozygous nonsense variant involving the functionally-important Arg549 residue is not previously reported in the literature and is predicted to cause premature truncation with loss of the terminal 47 amino acids. Although classified as a VUS due to limited published PRDM6 gene-level association, the p.(Arg549Ter) variant is likely causative for Family 2's cardiac phenotype. This variant has an extremely low allele frequency of 0.0006% (1/156180 alleles) in the genome aggregation database (gnomAD, v.2), has a very high CADD score of 40 (https://cadd.gs.washington.edu), and co-segregates with disease in three family members (Karczewski et al., 2020).
CoA in association with PRDM6 variants has not been reported. Non-syndromic CoA is observed to have autosomal dominant inheritance with variable expressivity (Beekman & Robinow, 1985). Though candidate genes such as NOTCH1 and MCTP2 have been suggested, there are no clear single genes directly associated with CoA (Parker & Landstrom, 2021). The presence of PRDM6 expression in the cardiac outflow tract and its role in regulating CNCC migration and SMC contraction supports an association between PRDM6 and CoA. These findings require additional investigation.
Family 1 highlights the phenotypic diversity of PRDM6-related disease with both isolated PDA and PDA with CoA. The siblings' more severe phenotype may be related to the maternally-inherited missense variant (c.767C>G; p.(Pro256Arg)), perhaps a hypomorphic allele, though its location (exon 3) outside of the known functional domain makes it more likely benign. Alternatively, the likely-pathogenic heterozygous nonsense variant could have phenotypic variation, causing PDA both with and without CoA.
It is notable that both siblings in Family 1 have systemic hypertension. Though hypertension is a known finding after coarctation repair, mouse models suggest PRDM6 may regulate blood pressure via effects on renin-producing cells (Gunawardhana et al., 2023). PRDM6 variants may thus affect vascular tone and blood pressure control, potentiating hypertension in CoA patients or more generally in the population.
5 CONCLUSION
We describe three cases of PDA and CoA in the setting of PRDM6 variants, identifying a novel association between PRDM6 and CoA. We additionally describe the first reported cases of biallelic PRDM6 variants as well as a new likely-disease-causing nonsense variant (p.(Arg549Ter)). The identification of additional probands will further delineate the full phenotypic spectrum of PRDM6-related disease, facilitate early diagnosis, and improve recurrence counseling in congenital heart disease. Genetic testing for PRDM6 variants should be considered in patients with PDA, especially in conjunction with CoA or hypertension.
AUTHOR CONTRIBUTIONS
The authors confirm contribution to the paper as follows: study conception and design: Helen M. Stanley, Alanna Strong, and Balram Gangaram. Analysis and interpretation of results: All authors contributed equally to the analysis and interpretation of the results. Draft manuscript preparation: Helen M. Stanley, Brian R. White, Alanna Strong, and Balram Gangaram. All authors reviewed, edited, and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no financial conflicts to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




