Cause, severity, and efficacy of treatment for hearing loss in children with Trisomy 18: A single institution-based retrospective study
Ririko Sato and Hidekane Yoshimura contributed equally to this study.
Abstract
Trisomy 18 is a common chromosomal aberration syndrome, characterized by variable clinical manifestations, including cardiovascular, pulmonary, genitourinary, and musculoskeletal findings, leading to a shorter survival and severe developmental delay in survivors. However, recently, intensive therapeutic intervention has allowed for prolonging survival. In terms of otological complications, only a limited number of relevant reports have been published. To demonstrate the characteristic of hearing loss (HL) in children with Trisomy 18, we retrospectively evaluated 22 patients (44 ears) by comprehensive auditory evaluation with the auditory steady-state response (ASSR) test and temporal bone computed tomography (CT). ASSR revealed that 20 patients (91%) had bilateral moderate to profound HL, more frequent and severe than that in Trisomy 21; among 42 ears having HL, 12 ears (29%) had conductive HL, and 26 ears (62%) had mixed HL. CT scans of 38 ears revealed that 34 ears (89%) had an external and middle ear malformation. Hearing aids (HA) were fitted in 17 patients (air and bone-conduction HAs). The threshold hearing with HA was improved in all of them. Accurate otological evaluation using ASSR and CT and intervention by HAs could be a feasible choice for children with Trisomy 18.
1 INTRODUCTION
Trisomy 18 is a common chromosomal aberration syndrome caused by the duplication of the entire length or part of Chromosome 18 (Edwards et al., 1960). The estimated prevalence was reported as 1 in 3500 to 8500 births (Kosho et al., 2013). Major complications observed with Trisomy 18 include congenital heart defects (such as atrial septal defect, ventricular septal defect, patent ductus arteriosus, and pulmonary stenosis), pulmonary hypertension, apnea, respiratory, digestive, urinary system-related disorders, musculoskeletal symptoms (such as polydactyly, scoliosis, and hypotonia), and malignant tumors. Many facilities refrain from intensive pediatric treatment of Trisomy 18 because of a shorter survival and profound psychomotor developmental delay in survivors (Carey & Kosho, 2016). However, in these two decades, perinatal and pediatric care for Trisomy 18 has been re-evaluated, and intensive therapeutic intervention has been considered to be implemented mainly in Japan and the United States (Dereddy et al., 2017; Kosho, 2008; Kosho et al., 2006; Kosho & Carey, 2016). Although 1-year survival was 5.6% in the most commonly cited report (Rasmussen et al., 2003), standard neonatal and pediatric treatments have enabled prolonging survival in two children's hospitals in Japan: up to 25% at Nagano Children's Hospital from 1994 to 2003 (Kosho et al., 2006) and up to 59% at the Hyogo Prefectural Kobe Children's Hospital from 2013 to 2017 (Tamaki et al., 2022). In addition, two studies reported higher discharge rates of 21% and 81%, respectively (Kosho et al., 2006; Tamaki et al., 2022).
Otological complications include hearing loss (HL), microtia, and aural atresia/stenosis (Karimnejad & Costa, 2015); however, only a limited number of relevant reports have been published, and the otological features and preferable management in children with Trisomy 18 remain unclear (Carey & Kosho, 2016; Cereda & Carey, 2012). Recently, characteristics of HL in 14 children with Trisomy 18 have been reported from the Hyogo Prefectural Kobe Children's Hospital (Tamaki et al., 2023).
Here, we performed comprehensive auditory evaluation on 22 patients with Trisomy 18 including the effectiveness of intervention with hearing aid (HA).
2 MATERIALS AND METHODS
2.1 Patients studied
Of the 74 children diagnosed with Trisomy 18 who visited Nagano Children's Hospital from January 2000 to April 2021, 22 children (9 boys and 13 girls) who had undergone comprehensive auditory evaluation including the auditory steady-state response (ASSR) test were recruited in this study. Table 1 provides a summary of clinical characteristics and complications of the patients. The study was approved by the Nagano Children's Hospital Ethics Committee (approval no.: S-03-46). We obtained written informed consent from the patients' parents following the Ethics Committee's regulations.
| Characteristic | Number |
|---|---|
| Sex | |
| Male | 9 |
| Female | 13 |
| Gestational age | |
| Preterm (<37 weeks) | 3 |
| Term (37–41 weeks) | 18 |
| Post-term (≥42 weeks) | 1 |
| Birth weight | |
| Very low-birth weight (1000–1500 g) | 1 |
| Low-birth weight (1500–2500 g) | 21 |
| Nutritional Methods | |
| Gastrostomy feeding | 18 |
| Nasogastric feeding | 4 |
| Associated diseases | |
| Artificial respiration or oxygenation | 22 |
| Cardiac diseases | 22 |
| Pulmonary diseases | 19 |
| Gastrointestinal disorders | 5 |
| Malignant tumors | 3 |
2.2 Audiometric assessment
The ASSR test was performed in 44 ears (22 patients) under sedation. Both air- and bone-conduction hearing ability was examined. Air-conduction hearing was calculated as the average of 0.5, 1, 2, and 4 kHz, whereas bone-conduction hearing was calculated as the average of 1, 2, and 4 kHz. Air-conducted hearing at a threshold as 41–70 dB normal hearing level (nHL) was defined as moderate HL; 71–90 dB nHL, severe HL; ≥91 dB nHL, profound HL. As mild HL (25–40 dB nHL) was difficult to differentiate from normal hearing, patients with mild HL was excluded in this study. HL according to bone-conduction hearing was defined as ≥30 dB nHL. The assessment was conducted based on the initial ASSR testing.
2.3 Temporal bone computed tomography
Among the 44 ears (22 patients) with Trisomy 18, temporal bone computed tomography (CT) scans were obtained for 38 ears (19 patients; 8 boys and 11 girls). The tests were performed at 5 months to 7 years of age (mean: 35.6 months) under sedation. The external auditory canal, middle ear, and inner ear were evaluated. Regarding inner ear, internal auditory canal (IAC) stenosis was diagnosed when the IAC width was 3 mm or less in the horizontal dimension, and bony cochlear nerve canal (BCNC) stenosis was defined as a BCNC diameter of ≤1.4 mm. In addition, enlarged vestibular aqueduct (VA) was defined as a VA diameter of ≥2 mm.
2.4 Treatment
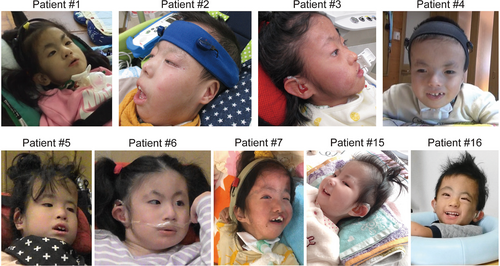
HA fitting was initiated in accordance with their parents' requests. The HA fitting was conducted based on the results of the ASSR test. The age to start wearing HA and HA type (air-conduction-HA or bone-conduction-HA) was examined. To evaluate the effectiveness of HA, aided- and unaided-hearing thresholds were tested by conditioned orientation response (COR) audiometry. Pictures of children with Trisomy 18 wearing HA were provided by their parents.
2.5 Statistical analysis
Two groups were compared using unpaired two-tailed Student's t-test. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Frequency, degree, and type of HL in Trisomy 18
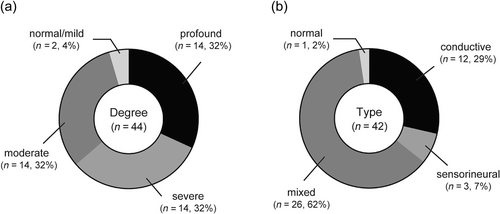
Among the 44 ears from 22 patients with Trisomy 18, 20 patients (91%) had bilateral moderate to profound HL (Supplementary Table S1). The degree of HL was profound in 14 ears (32%), severe in 14 ears (32%), moderate in 14 ears (32%), and mild-normal in 2 ears (4%) (Figure 1a). In terms of HL type, among 42 ears from 21 patients (1 patient who could not complete the bone-conduction hearing assessment was excluded in this assessment), 12 ears (29%) had conductive HL, 3 ears (7%) had sensorineural HL, 26 ears (62%) had mixed HL, and 1 ear (2%) had normal hearing (Figure 1b).
3.2 Assessment by temporal bone CT findings (Figure 2)
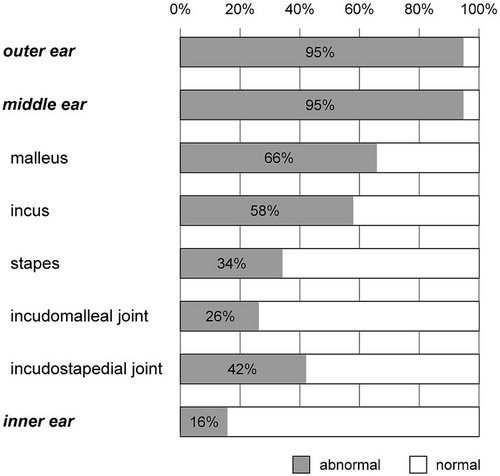
Among 38 ears whose CT data were available, 16 ears (42%) had microtia and 9 ears (24%) had microtia-atresia. Of the 16 ears microtia, 15 were classified as Grade I (were smaller than normal), and only I was classified as Grade IV (anotia). Furthermore, 12 ears (32%) had congenital aural atresia (CAA); 24 ears (63%) had congenital aural stenosis (CAS); and 2 ears (5%) were normal.
Middle ear effusion and ossicular malformations (ossicles, incudomalleolar joints, and incudostapedial joints) were evaluated. Overall, 36 ears (95%) had abnormalities in the middle ear. The effusion was observed in 18 ears (47%). The frequency of malformations in malleus, incus, and stapes were 25 (66%), 22 (58%), and 13 (34%) ears, respectively. In addition, 10 ears (26%) had incudomalleolar abnormalities and 16 ears (42%) had incudostapedial abnormalities.
Inner ear malformations were found in six ears (16%); cochlear hypoplasia Type III was found in two ears, and the remaining four ears had BCNC.
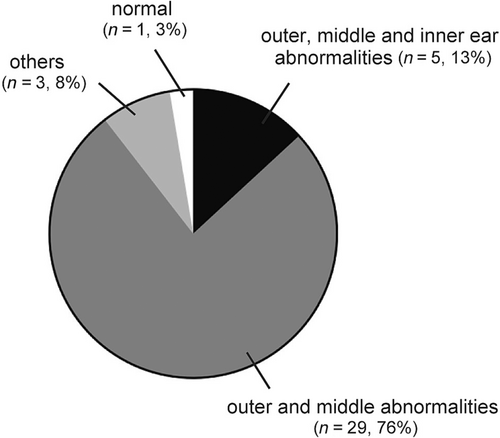
Taken together, the frequency of causes of HL based on the CT is shown in Figure 3. The most common cause was a combination of the external canal and middle ear abnormalities in 29 ears (76%), followed by a combination of the external canal, middle ear, and inner ear abnormalities in 5 ears (13%). Therefore, 34 ears (89%) had an external and middle ear malformation.
3.3 HA effectiveness
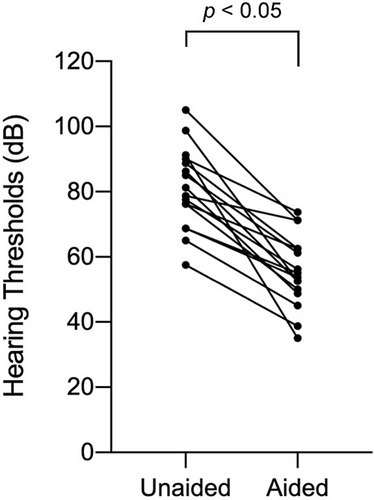
Among the 22 patients, 17 patients eventually used HA (Supplementary Table S1). Three patients who did not receive HAs died before the initiation of HA; two patients had moderate HL, but their families did not agree to the initiation of HAs. The age to start HA ranged from 5 to 48 months (mean: 19 months). Regarding the type of HA, nine patients used bone-conduction HAs and eight patients used air-conduction HAs. Figure 4 shows photographs of patients with Trisomy 18 wearing HA. In aided ears, we observed statistically better hearing thresholds (from 35 to 73.8 dB HL [mean: 55.6]) as compared with unaided ears (from 57.5 to 105 dB HL [mean: 80.9]) (Figure 5). In Figure 5 one patient (two ears) was excluded because he was too sick to undergo the hearing test.
4 DISCUSSION
The current report represents the second and largest cohort to address characteristics of HL in children with Trisomy 18 (Tamaki et al., 2023) and the first to attempt formal evaluation of the efficacy of HA.
Our findings reported that most of children with Trisomy 18 had moderate to profound HL and conductive or mixed HL, indicating that it is one of the significant symptoms of Trisomy 18. However, as the timing of initial hearing test varied, the frequency of HL was likely overestimated, which was one of the limitations of this study. In more than half of the patients, the degree of HL was profound to severe, and no correlation was noted between the severity of HL and the degree of other malformations such as cardiac disease, gastrointestinal disease, and other visceral and skeletal malformations. All 14 patients evaluated in the Hyogo Prefectural Kobe Children's Hospital showed more than or moderate HL: moderate in 1, moderately severe in 7, severe in 3, and profound in 3 (Tamaki et al., 2023). All 10 patients who underwent both air-conduction and bone-conduction study showed mixed-type HL (Tamaki et al., 2023).
Temporal bone CT assessments also revealed external and middle ear malformations in 89% of patients, supporting our findings that most of the patients had conductive or mixed HL, not sensorineural HL. In addition to our CT findings, Tadaki et al. (2003) and Saito et al. (1987) reported partial absence of the superior semicircular canal, enlargement of the endolymphatic duct sac, and abnormalities of the vestibule using temporal bone pathology of Trisomy 18 fetuses. In the cohort of the Hyogo Prefectural Kobe Children's Hospital, all nine patients underwent temporal bone CT showed external auditory canal atresia or stenosis (Tamaki et al., 2023). Compared with these reports, our study documented that Trisomy 18-related HL is not only due to the malformations of the ossicles but also due to otitis media with effusion (OME) and abnormal joint of the ossicles. However, as OME and CAS might be cured over time, follow-up was required. Moreover, movement of the ossicles was not evaluated, which was one of limitations in this study.
Similar to children with Trisomy 18 and 21, 50%–75% of children with Down syndrome (DS) have HL (Earley et al., 2022; Park et al., 2012). Of them, many children with DS have a conductive and mixed HL; by contrast, sensorineural HL is observed in 4%–28% (Lau et al., 2015; Sait et al., 2022). However, while more than half the children with Trisomy 18 had profound to severe HL, 50%–70% of children with DS have mild HL (Kreicher et al., 2018). While the cause of HL in children with DS is frequently associated with OME or CAS, children with Trisomy 18 have a higher rate of aural atresia and middle ear malformations, indicating that the HL due to Trisomy 18 is considered more severe than that due to DS.
As mentioned earlier, the common type of HL in Trisomy 18 is conductive and mixed; however, considering the patient's age and general condition, the primary treatment is HAs rather than surgery. In general, children with congenital HL should start wearing HAs before 6 months of age, but the current study documented that the use of HA started later in Trisomy 18, which was not surprising because resolving other Trisomy 18-related severe problems takes priority over resolving a hearing issue.
In the current study, patients with Trisomy 18 wore approximately 50% bone-conduction HA and 50% air-conduction HA. When bone-conduction hearing was preserved, bone-conduction HA was proffered due to CAA or CAS. However, as children with Trisomy 18 are often bedridden, a head band of bone-conduction HA easily detach owing to head movement, leading to the feedback. Therefore, even in cases of CAA or CAS, if a HA mold can be inserted, an air-conduction HA was sometimes selected.
For children with Trisomy 18 typically showing severe to profound developmental delay, judging HA effectiveness by functional gain seems challenging. The current study showed improved threshold in all the 16 patients whose COR data were available before and after the initiation of HA. In our previous study, their parents were asked to fill in some questionnaire about their child's daily auditory behavior when wearing HAs. As a result, the child's reactions to the voices of family members and other people improved, and more than half of the family members answered that benefits by HAs were observed (Sato et al., 2020). Although any words were not observed following wearing HAs, many children showed good responses, such as turning around when called, smiling, and projecting their voice, indicating the feasibility of HAs in children with Trisomy 18. In the Hyogo Prefectural Kobe Children's Hospital study, improved responses were suggested in four patients with HA through parent interviews (Tamaki et al., 2023).
Trisomy 18 is associated with significant cognitive developmental delays; however, some children were reported to express emotions and communicate using signs and other methods (Liang et al., 2015). In addition, children with Trisomy 18 can continue to develop slowly but steadily and will continue to interact with others in support group-based studies (Baty et al., 1994; Kosho et al., 2013). Hence, we believe that for children with Trisomy 18, HAs could help improve communication, and would be a reasonable approach for children with Trisomy 18 following comprehensive hearing screening including ASSR and CT, where active engagement of pediatric otolaryngologists would be recommended.
5 CONCLUSION
We studied HL in children with Trisomy 18 that is mainly caused by CAS, CAA, and/or middle ear malformations. To the best of our knowledge, this study included the largest number of cases reported to date. HL is more frequent and severe in Trisomy 18 than in Trisomy 21. Air- and bone-conduction HAs are effective in HL in children with Trisomy 18, suggesting that wearing HAs is a reasonable intervention in view of comprehensive developmental support.
AUTHOR CONTRIBUTIONS
Ririko Sato: Conceptualization, data curation, formal analysis, writing—original draft, and writing—review and editing. Hidekane Yoshimura: Data curation, formal analysis, and writing—original draft, and writing—review and editing. Tomoki Kosho: Conceptualization and writing—review and editing. Yutaka Takumi: Conceptualization, data curation, formal analysis, and writing—review and editing. All authors approved the final manuscript.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to the patients and their parents for their cooperation to this study. We are also thankful to colleagues of Nagano Children's Hospital: Dr. Tsukasa Higuchi and Dr. Kisei Minami of the Department of General Pediatrics, Dr. Yuji Inaba of the Department of Pediatric Neurology, Dr. Ryojun Takeda of the Division of Medical Genetics, and Dr. Takehiko Hiroma of the Department of Neonatology for their invaluable cooperation and critical suggestions in this study. We thank Editage (www.editage.com) for English language editing.
FUNDING INFORMATION
No funding has been received for this study.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare in this study. Tomoki Kosho is a member of an endowed chair named “Division of Clinical Sequencing, Shinshu University School of Medicine” sponsored by BML, Inc. and Life Technologies Japan Ltd. of Thermo Fisher Scientific Inc.
ETHICS STATEMENT
The study was approved by the Nagano Children's Hospital Ethics Committee (approval no.: S-03-46).
PATIENT CONSENT STATEMENT
Written informed consent was obtained from all patients or their guardians.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




