Stratification of the risk of ovarian dysfunction by studying the complexity of intermediate and premutation alleles of the FMR1 gene
Laila El Khattabi and Thierry Bienvenu contributed equally to this work.
Abstract
FMR1 premutation female carriers are at risk of developing premature/primary ovarian insufficiency (POI) with an incomplete penetrance. In this study, we determined the CGG repeat size among 1095 women with diminished ovarian reserve (DOR) / POI and characterized the CGG/AGG substructure in 44 women carrying an abnormal FMR1 repeat expansion number, compared to a group of 25 pregnant women carrying an abnormal FMR1 CGG repeat size. Allelic complexity scores of the FMR1 gene were calculated and compared between the two groups. In the DOR/POI cohort, 2.1% of women presented with an intermediate repeat size and 1.9% with a premutation. Our results suggest that the risk of POI is highest in the mid-range of CGG repeats. We observed that the allelic score is significantly higher in POI women compared to the pregnant women group (p-value = 0.02). We suggest that a high allelic score due to more than 2 AGG interspersions in the context of an intermediate number of repetitions could favor POI. Larger studies are still needed to evaluate the relevance of this new tool for the determination of the individual risk of developing POI in women with abnormal number of CGG repeats.
1 INTRODUCTION
Premature ovarian insufficiency (POI) is a heterogeneous disorder characterized by a loss of ovarian function, occurring before the age of 40. The diagnosis is based on the combination of abnormal menstrual cyclicity with oligomenorrhea during more than four consecutive months, including primary or secondary amenorrhea, and serum follicle stimulating hormone (FSH) higher than 25 IU/L, in at least two samples collected more than 4 weeks apart, in women before 40 years old (Chon et al., 2021). The incidence of POI is about 1:10,000 in women by 20 years old, 1:1000 in women under 30 years old, and 2:100 in women under 40 years old (Chon et al., 2021). Inadequate formation of the follicular pool in utero and accelerated depletion of the follicular pool seem to be two major mechanisms involved in POI (Chon et al., 2021). A variety of known causes may be involved in POI. They include iatrogenic causes such as chemotherapy, radiotherapy, or surgery, as well as autoimmune diseases, and genetic factors. So far, more than 120 candidate genes have been described. A particular genetic cause is FMR1 (the Fragile X messenger ribonucleoprotein 1 gene) premutation. However in 50%–60% of cases, the etiology of POI remains idiopathic (Chon et al., 2021). “Diminished Ovarian Reserve” (DOR) is diagnosed in an infertile woman with regular cycles, a slightly elevated FSH level, and a reduced number of ovarian follicles, identified by a low antral follicular count and/or a decreased level of AMH, generally lower than 1 ng/mL. However, there is no consensual definition for DOR (Cohen et al., 2015). The incidence of DOR is 15%–17% of women (Devine et al., 2015).
FMR1 is located on the long arm of the X chromosome. It bears dynamic CGG (Cytosine—Guanine—Guanine) triplet repeats at the 5′ untranslated region. Four allelic groups have been described, based on the number of FMR1 CGG repeats (Biancalana et al., 2015). Alleles between 6 and 44 CGG repeats are traditionally considered normal. The full mutation corresponds to alleles above 200 CGG repeats and causes the well characterized fragile X syndrome, through transcriptional silencing of FMR1 leading to the absence of the Fragile X messenger ribonucleoprotein 1 (FMRP). Between the normal and full mutation ranges, intermediate or gray zone (45–54 repetitions) and premutation (55 to 200 repetitions) ranges can be distinguished, both of which are unstable. Premutation can expand to full mutation over generations (S. Sherman et al., 2005). The prevalence of the FMR1 premutation in POI population is significantly higher than in the general one (12%–14% compared to 1%) (S. L. Sherman, 2000; S. D. Sullivan et al., 2011), and several studies have demonstrated that women carrying FMR1 premutation are at higher risk of developing POI, suggesting that FMR1 premutation could be involved in altered ovarian function and loss of fertility although with incomplete penetrance (Biancalana et al., 2015).
FMR1-linked POI occurs in approximately 20% of women who carry a premutation; these women develop hypergonadotropic hypogonadism and have absent or very irregular cycles prior to age 40. The repeat size of FMR1 is associated with age at menopause in a non-linear fashion. Several studies have shown that age at menopause is negatively correlated to premutation sizes under 80 repeats while it becomes positively correlated to larger sizes (A. K. Sullivan et al., 2005; Ennis et al., 2006; Mailick et al., 2014). Moreover, Barasoain et al. suggested that even intermediate FMR1 repeat alleles (35–54 CGG) are at higher frequency in women with DOR (Barasoain et al., 2013). However, this was not confirmed in a larger study (Bennett et al., 2010).
The molecular mechanism causing POI in the context of FMR1 premutation is not clearly understood. A recent study, published in 2022, demonstrate the sequestration of an RNA-binding protein, Sam68, in FMR1 premutation carriers. This sequestration may decrease the free fraction of Sam68, impairing FSH receptor transcript processing, responsible for reduced receptor levels and a diminished ovarian response to FSH hormonal stimulation (Boustanai et al., 2022). Several studies suggest that the secondary hairpin structure resulting from the long repeat in FMR1 mRNA may be involved (Berman et al., 2014). In the normal population, the CGG repeat is interrupted by AGG trinucleotides, typically at positions 10 and 20. The length of the CGG repeat and the AGG interruption pattern within that repeat is known to play a role in the risk of instability at parent/mother-to-child transmission (Eichler et al., 1994). AGG interruptions are known to disrupt or de-stabilize the hairpin structures and therefore may be protective against strand slippage and expansion from premutation (Napierala et al., 2005).
Recently, Lekovich et al. (Lekovich et al., 2018) examined whether repeat size and AGG interruption pattern were associated with three markers of ovarian reserve. 96 women with a premutation were evaluated for serum anti-Müllerian hormone (AMH), their antral follicle count, and the number of oocytes retrieved after ovarian stimulation for IVF. They found that premutation carriers with 70–90 CGG repeats showed significantly lower ovarian reserve than carriers with fewer or more repeats and that reduction in AGG interruptions within the repeated sequence was associated with a lower ovarian reserve.
To understand the cumulative effect of the CGG repeat tract length and its AGG interspersions, Rodriguez et al developed a mathematical model to evaluate the complexity of alleles combination (Rodrigues et al., 2020). The output of this mathematical function is a positive number called allelic score. This new tool computes the combined AGG interspersion number and pattern and the total repeat length, reflecting the CGG/AGG substructure and allowing a new characterization of this polymorphic region. According to their model, the AGG “protective effect” in females carrying premutated alleles with at least two interspersions could be visualized by a high allelic score in these alleles.
In most of the studies focusing on the link between POI and FMR1 premutation, rare are those considering both the CGG repeat tract length and its AGG interspersions. The aim of this study is, first, to compare the FMR1 allelic score between a cohort of POI women and a group of pregnant women, both carrying intermediate size or premutation alleles. In addition, we compare the results according to the level of ovarian dysfunction, POI or DOR.
2 PATIENTS AND METHODS
2.1 Study population
This is a retrospective cohort study including 1095 unrelated women with DOR or POI. These were referred from Gynecology, Obstetrics, Reproductive Endocrinology, and Reproductive medicine departments from different French university hospitals, Assistance Publique des Hôpitaux de Paris (Cochin, Saint-Antoine, Jean Verdier, and Pitié-Salpêtrière hospitals), Poissy-Saint Germain hospital, Hospices Civils de Lyon, CHU de Lille, CHU de Toulouse Larrey, CHU Toulouse Paule de Viguier and CHR Orléans. Clinical data related to the reproductive condition of each patient was collected from the referee clinicians. All patients gave their informed consent for genetic studies.
Women carrying an abnormal FMR1 CGG repeat size, either intermediate allele defined as 45–54 repeats and premutation alleles defined as 55 to 200 repeats according to the ACMG/EMQN guidelines (Biancalana et al., 2015), were selected for further analysis. Those with chromosome abnormalities, when karyotype was available, were excluded.
We also included a group of 25 pregnant women under 40 years old, carrying an abnormal FMR1 CGG repeat size and referred during the same period than the POI group. Karyotyping was not performed in this group. The mean age of patients in both groups is comparable (32.4 years old in the POI group and 31.3 years old in the pregnant women group), as is the median age.
2.2 FMR1 analysis
Genomic DNA was isolated from peripheral blood leukocytes according to standard procedures using Promega DNA Blood Kit. FMR1 CGG repeat size was first determined by a fluorescent PCR. PCR was conducted using primers PR155_F: (5′-gctcagctccgtttcggtttcacttccggt-3′) and PR156_R: (5′-agccccgcacttccaccaccctcctcca-3′). The total reaction volume of 20 μL contained OneTaq® DNA Polymerases Products (New England Biolabs, Inc. Ipswich, MA, USA) including buffer 5× OneTaq® GC Reaction, buffer OneTaq® High GC Enhancer (30%) and 2.5 U of OneTaq® DNA Polymerase, 60 μM dNTPs (Invitrogen™, Walthma, MA, USA), 10 pmol of each primer, and 100 ng of genomic DNA. The reactions used the following temperature conditions: denaturation at 95°C for 3 min; 27 cycles of 95°C for 15 s (denaturation), 62°C for 2 min (annealing), 75°C for 2 min (extension), and termination at 75°C for 10 min. The PCR product was analyzed by fragment analysis using the ABI 3130XL sequencer ((Applied Biosystems, Waltham, MA, USA). Samples with repeat size above the normal range (>44) were re-analyzed using the Amplidex PCR/CE FMR1® kit (Asuragen, Austin, TX, USA). For analysis of AmplideX FMR1 CGG-Primed PCR, 2 μL of DNA (20 ng/μL) was used. The reaction mix was prepared according to the manufacturer's instructions (11.45 μL of GC-rich Amp Buffer, 0.5 μL of FMR1 F/R FAM-Primers, 0.5 μL of FMR1 CGG Primers, 0.5 μL of diluent, and 0.05 μL of GC-Rich Polymerase Mix). The PCR program consisted of an initial stage of denaturation step at 95°C for 5 min; 10 cycles of 97°C for 35 s, 62°C for 35 s, and 68°C for 4 min; 20 cycles of 97°C for 35 s, 62°C for 35 s, and 68°C for 4 min, including a 20-s increment for each cycle; and a final extension at 72°C for 10 min. PCR products were separated by capillary electrophoresis (CE) using an ABI3130XL Genetic Analyzer sequencer (Applied Biosystems, Foster City, CA, USA). Data were analyzed using the Genescan v5.0 software (Applied Biosystems, Waltham, MA, USA).
The AGG interruption pattern within the FMR1 repeat sequence was determined using Xpansion Interpreter® (Asuragen, Austin, TX, USA). We used the recently developed mathematical model (Rodrigues et al., 2020) to define the CGG/AGG substructure and calculated the allelic score for each allele (Rodrigues et al., 2020). Following the nomenclature defined by Gleicher et al. (Gleicher et al., 2012), the allele with the lower number of CGG repeats is defined as allele-1, whereas the one with the higher number was defined as allele-2.
2.3 Statistical analysis
For the comparison between FMR1 allelic scores in the POI and pregnant women groups, we used the analysis of variance (ANOVA). To compare the subgroup size in premutation, Pearson's Chi-squared test with Yates' continuity correction was used to test the independence. Statistical analyses were performed with R studio and statistical significance was set at p ≤ 0.05.
3 RESULTS
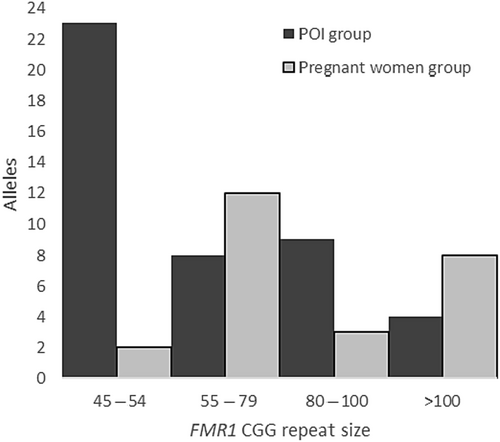
Among the 1095 women with POI, 46 presented with an abnormal repeat size. Two patients were excluded because of a known karyotype abnormality in addition to a premutation of the FMR1 gene. Among the remaining 44 women, 23 (2.10%) presented with an intermediate allele ranging from 45 to 53 repeats, and 21 (1.92%) with a premutation allele ranging from 57 to 115 repeats (Table 1 and Figure 1). In this subgroup, 8 women had a low size premutation allele, defined as 55–79 repeats (0.73%), 9 a mid-size premutation allele, defined as 80–100 repeats (0.82%), and 4 a high size of premutation allele, defined as >100 repeats (0.37%). Among the 25 pregnant women, 2 displayed an intermediate allele (45 and 48 CGG repeats) and 23 a premutation allele (12 low, 3 medium, and 8 high) ranging from 60 to 135 repeats (Table 1). In the POI group, more patients had a medium premutation [80–100 CGG] (9/21) than a high [>100 CGG] (4/21) compared to the pregnant women group (3/23 and 8/23, respectively), but this did not reach statistical significance (p-value = 0.1013). Considering the allele-1, it ranged from 19 to 43 CGG and from 20 to 48 CGG in the POI and the pregnant women groups respectively. The comparison of both medians did not show any statistical significance. The most common structures of allele-1 in POI group are (CGG)10AGG(CGG)9AGG(CGG)9 (22.9%) and (CGG)9AGG(CGG)9AGG(CGG)9 (20.0%), whereas the most common structures of allele-1 in the pregnant women group are (CGG)9AGG(CGG)9AGG(CGG)9 (28.6%) and (CGG)9AGG(CGG)9AGG(CGG)10 (23.8%). (Tables 2 and 3, Tables S1 and S2).
| POI group | Pregnant women group | ||
|---|---|---|---|
| Intermediate size allele [45–54] | Number of patients | 23 | 2 |
| CGG repeats [range] | [45–53] | [45–48] | |
| Premutation allele [55–200] | Number of patients | 21 | 23 |
| CGG repeats [range] | [57–115] | [60–135] | |
| – | |||
| Low range [55–79] | 8 | 12 | |
| Medium range [80–100] | 9 | 3 | |
| High range [>100] | 4 | 8 | |
| Total | – | 44 | 25 |
| Patient | POI type | Karyotype | Allele 1 | Allele 2 | Allelic score of allele 1 | Allelic score of allele 2 |
|---|---|---|---|---|---|---|
| 1 | unk | unk | (CGG)9AGG(CGG)35 | 71 | ||
| 2 | Secondary amenorrhea | Normal | (CGG)9AGG(CGG)9AGG(CGG)9AGG(CGG)7 | (CGG)9AGG(CGG)7AGG(CGG)9AGG(CGG)8AGG(CGG)9 | 763 | 2937 |
| 3 | Secondary amenorrhea | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)25 | 189 | 205 |
| 4 | DOR | Normal | (CGG)14AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)25 | 65 | 205 |
| 5 | unk | Normal | (CGG)10AGG(CGG)9AGG(CGG)10 | (CGG)9AGG(CGG)9AGG(CGG)25 | 206 | 205 |
| 6 | Secondary amenorrhea | Normal | (CGG)20AGG(CGG)7AGG(CGG)9 | (CGG)10AGG(CGG)35 | 357 | 75 |
| 7 | unk | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)26 | 189 | 206 |
| 8 | DOR | unk | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)10AGG(CGG)37 | 189 | 77 |
| 9 | Primary amenorrhea | Normal | ||||
| 10 | DOR | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)29 | 205 | 209 |
| 11 | DOR | Normal | ||||
| 12 | DOR | unk | (CGG)10AGG(CGG)9AGG(CGG)14 | (CGG)49 | 210 | 49 |
| 13 | Secondary amenorrhea | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)10AGG(CGG)9AGG(CGG)9AGG(CGG)9AGG(CGG)9 | 189 | 3325 |
| 14 | DOR | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)39AGG(CGG)9 | 205 | 165 |
| 15 | Primary amenorrhea | Normal | ||||
| 16 | unk | unk | (CGG)10AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)30 | 49 | 210 |
| 17 | unk | unk | (CGG)10AGG(CGG)20 | (CGG)9AGG(CGG)9AGG(CGG)31 | 60 | 211 |
| 18 | unk | unk | (CGG)9AGG(CGG)23 | (CGG)9AGG(CGG)9AGG(CGG)31 | 59 | 211 |
| 19 | Secondary amenorrhea | Normal | (CGG)8AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)9AGG(CGG)22 | 173 | 778 |
| 20 | Secondary amenorrhea | Normal | ||||
| 21 | DOR | Normal | (CGG)9AGG(CGG)10AGG(CGG)8 | (CGG)9AGG(CGG)41 | 192 | 77 |
| 22 | DOR | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)41 | 205 | 77 |
| 23 | Secondary amenorrhea | Normal | (CGG)24 | (CGG)9AGG(CGG)9AGG(CGG)33 | 24 | 213 |
| 24 | DOR | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)10AGG(CGG)9AGG(CGG)36 | 189 | 232 |
| 25 | Secondary amenorrhea | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)40 | 205 | 220 |
| 26 | unk | unk | (CGG)9AGG(CGG)9AGG(CGG)22 | (CGG)9AGG(CGG)9AGG(CGG)40 | 202 | 220 |
| 27 | DOR | unk | ||||
| 28 | Secondary amenorrhea | unk | ||||
| 29 | unk | unk | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)73 | 205 | 73 |
| 30 | DOR | Normal | (CGG)10AGG(CGG)10AGG(CGG)9 | (CGG)73 | 209 | 73 |
| 31 | unk | unk | (CGG)41 ou (CGG)42 | (CGG)77 | 42 | 77 |
| 32 | Secondary amenorrhea | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)81 | 205 | 81 |
| 33 | DOR | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)72 | 189 | 108 |
| 34 | Secondary amenorrhea | unk | CGG(40) | (CGG)83 | 40 | 83 |
| 35 | Secondary amenorrhea | Normal | ||||
| 36 | DOR | Normal | (CGG)9AGG(CGG)9 | (CGG)85 | 45 | 85 |
| 37 | Secondary amenorrhea | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)86 | 205 | 86 |
| 38 | unk | Normal | ||||
| 39 | Secondary amenorrhea | Normal | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)67 | 189 | 247 |
| 40 | Secondary amenorrhea | Normal | (CGG)10AGG(CGG)10AGG(CGG)9 | (CGG)90 | 209 | 90 |
| 41 | DOR | Normal | (CGG)10AGG(CGG)18 | (CGG)102 | 58 | 102 |
| 42 | Secondary amenorrhea | unk | (CGG)15AGG(CGG)9 | (CGG)86AGG(CGG)19 | 69 | 363 |
| 43 | Secondary amenorrhea | Normal | (CGG)10AGG(CGG)30 | (CGG)111 | 70 | 111 |
| 44 | DOR | Normal | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)115 | 205 | 115 |
| Patient | Allele 1 | Allele 2 | Allelic score of allele 1 | Allelic score of allele 2 |
|---|---|---|---|---|
| 1 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)19AGG(CGG)25 | 189 | 101 |
| 2 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)9AGG(CGG)28 | 189 | 208 |
| 3 | (CGG)9AGG(CGG)25 | (CGG)60 | 61 | 60 |
| 4 | (CGG)9AGG(CGG)10 | (CGG)62 | 46 | 62 |
| 5 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)9AGG(CGG)51 | 189 | 87 |
| 6 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)8AGG(CGG)45 | 189 | 77 |
| 7 | (CGG)9AGG(CGG)10 | (CGG)9AGG(CGG)9AGG(CGG)47 | 46 | 227 |
| 8 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)69 | 189 | 69 |
| 9 | (CGG)10AGG(CGG)19 | (CGG)72 | 59 | 72 |
| 10 | (CGG)9AGG(CGG)29 | (CGG)74 | 65 | 74 |
| 11 | (CGG)9AGG(CGG)9AGG(CGG)10 | (CGG)75 | 190 | 75 |
| 12 | (CGG)9AGG(CGG)9AGG(CGG)10 | (CGG)9AGG(CGG)66 | 190 | 102 |
| 13 | (CGG)9AGG(CGG)10 | (CGG)9AGG(CGG)9AGG(CGG)53 | 46 | 233 |
| 14 | (CGG)9AGG(CGG)9AGG(CGG)10 | (CGG)79 | 190 | 79 |
| 15 | ||||
| 16 | ||||
| 17 | (CGG)9AGG(CGG)10 | (CGG)89 | 46 | 89 |
| 18 | (CGG)9AGG(CGG)9AGG(CGG)10 | (CGG)9AGG(CGG)9AGG(CGG)82 | 190 | 262 |
| 19 | (CGG)9AGG(CGG)9AGG(CGG)9 | (CGG)105 | 189 | 105 |
| 20 | ||||
| 21 | (CGG)9AGG(CGG)9AGG(CGG)10 | (CGG)109 | 190 | 109 |
| 22 | ||||
| 23 | (CGG)10AGG(CGG)9AGG(CGG)9 | (CGG)122 | 205 | 122 |
| 24 | (CGG)10AGG(CGG)9AGG(CGG)10 | (CGG)130 | 206 | 130 |
| 25 | (CGG)9AGG(CGG)10 | (CGG)135 | 46 | 135 |
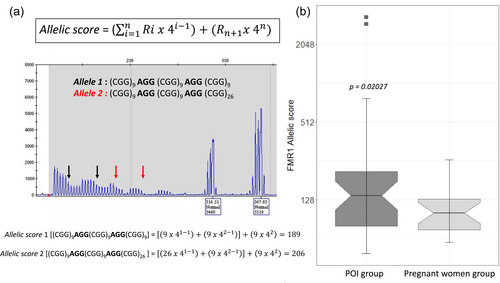
For each woman, we calculated the FMR1 allelic complexity score developed by Rodrigues et al. (Rodrigues et al., 2020), which integrates the AGG interspersion number and pattern but also the total repeat length, to reflect the polymorphic CGG/AGG substructure of the 5′UTR of FMR1. We were not able to calculate the allele score for 8 patients in the POI group and 4 in the pregnant women group because the graphs were not available. For 36 women with POI, allelic scores of “allele 2” ranged from 49 to 3325 with a median of 140 (Figure 2 and Table 2). In this group, three patients have more than 2 interspersions [one with three AGG interspersions (1/36) and two with four interspersions (2/36)] and these three women were in the intermediate size allele (gray zone) group. The presence of four interspersions increases considerably the value of the allelic score, since the mathematical model gives higher weight to the number of interspersion than the number of CGG repeats. For 21 women in the pregnant women group, allelic scores of “allele 2” ranged from 60 to 262 with a median of 101 and none of them had more than 2 interspersions. Hence, the allelic score was significantly higher in the POI group compared to the other one (p-value = 0.02027, ANOVA). (Figure 2 and Table 3).
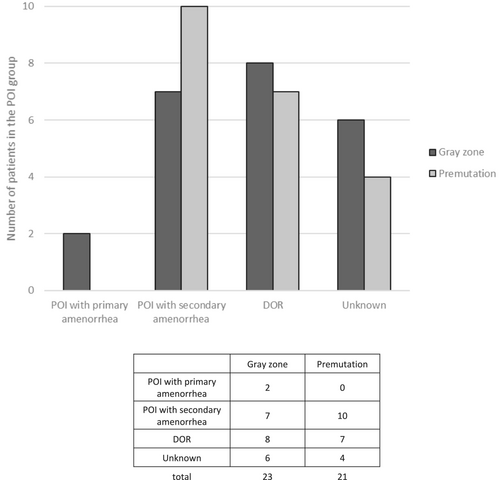
We retrospectively classified the POI group according to whether the POI was primary or secondary, or if it was actually a DOR based on the clinical data that were collected (FSH, AMH, and LH measurements; age of menses; amenorrhea; pregnancy) (Figure 3). For 10 patients, we could not determine the status of the POI due to lack of clinical information. Two patients had primary ovarian failure (2/23), among the gray zone subgroup, but none in the premutation subgroup (0/21). Seven and ten patients from the gray zone and the premutation subgroups, respectively, displayed secondary POI. Finally, eight patients had DOR in the gray zone, compared to seven in the premutation group. The distribution seems to be similar between the different POI/DOR categories, but the numbers are too small for statistical analysis.
4 DISCUSSION
Our study compared a group of 44 women with a premutation or an intermediate size of FMR1 CGG repeats in the context of fertility disorder, to a group of 25 pregnant women with a premutation or an intermediate size repeat referred for personal history of abnormal FMR1 repeat sequence size.
In this study, among all women tested for the FMR1 gene for ovarian dysfunction (n = 1095), an intermediate repeat size or a premutation was found in 4.02% of cases (2.10% and 1.92% respectively). The frequency of the premutation in our POI cohort is higher than in the general population, estimated at 0.7% for females and 0.2% for males in a large US population (Seltzer et al., 2012). Moreover, the frequency of FMR1 premutation in our cohort is comparable to what was reported in previous POI cohorts, ranging from 0.8% to 7.5% (Bachelot et al., 2009; Bretherick et al., 2005; Conway et al., 1998; Gersak et al., 2003; Wittenberger et al., 2007), although it seems to be lower in Asian (0.5% and 1.6%) compared to Caucasian (Ishizuka et al., 2011; Komaravalli et al., 2020; Lu et al., 2017). Regarding the gray zone expansion, the frequency observed in our study (2.10%) is lower than in a control US population [2.8% in females (98/3474) and 2.4% in males (78/3273)] but slightly higher than in another but smaller control population from Canada (1.64%, 3/182 females) (Bretherick et al., 2005). However, our POI cohort shows a similar frequency of intermediate size repeat compared to other POI cohorts from Brazil (1.92%) (Ramos et al., 2022) or China (2.9%) (Guo et al., 2014). Given that the prevalence of FMR1 alleles varies considerably between geographical areas and according to the assessed population, it suggests founder effects.
Although statistical significance was not reached, likely because of small numbers, higher premutation range alleles were over-represented in the pregnant women group compared to the POI group. This is consistent with previous observations showing that the FMR1 repeat size is associated with POI in a non-linear fashion where higher ranges of premutation repeat sizes are associated with lower risk of POI. (Ennis et al., 2006) The number of triplets associated with the highest risk of POI are between 80 and 100 triplets.
Regarding the allelic score, our study shows that it is significantly higher in POI women compared to the other studied group. In the light of this result, we can hypothesize that a high allelic score due to more than 2 AGG interspersions in the context of an intermediate number of repetitions, corresponding to the gray zone, may favor POI. However, this result can also be explained by the mathematical model, which gives higher relevance to the number of interspersion than number of CGG repeats. In fact, in the POI cohort, 3 patients have more than 2 interruptions in the gray zone (against none in the pregnant women group), which considerably increases the value of the allelic score. Currently in genetic laboratories, the number of AGG interspersions is not reported to the clinician. A better evaluation of the individual risk of developing POI in women with a CGG triplet expansion would be ideal to provide the best genetic counseling. The allelic score could potentially be a good parameter for such risk estimation, but this still needs to be demonstrated on larger cohorts. Furthermore, large studies in control populations of non-POI women are needed to determine the proportion of gray zone alleles in the fertile population.
At the DNA level, AGG interruptions within the CGG repeats play an important stabilizing and protective role (Napierala et al., 2005). However, from a clinical perspective, several conflicting results have been obtained. Friedman-Gohas et al. showed that the number of AGG interruptions within the CGG repeat region did not protect patients, or improve ovarian response to ovarian hyperstimulation (Friedman-Gohas, Kirshenbaum, et al., 2020). Allen et al. found no association between the number of AGG interruptions and the age of secondary amenorrhea in premutation carriers (Allen et al., 2018). On the other hand, Lekovich et al. found higher ovarian reserve in women with two AGGs compared to none or one AGG interruption. The differences between ours and the other studies could be explained by the differences in the parameters studied (age of secondary amenorrhea, anti-müllerian hormone level, pregnancy), sample size, and the different patient characteristics (AGG interruptions associated with CGG repeats). At the molecular level, AGG interruptions in the CGG tracts play an important role in the formation of the FMR1 mRNA structure, and that both the length of the CGG tracts and their AGG interruptions may have clinical implications for POI. A single AGG interruption destabilizes the CGG hairpin in a position-dependent manner. We hypothesize that the number of AGG interruptions in an intermediate number of repetitions may affect the FMR1 mRNA and/or FMR polyglycine-containing protein (FMRpolyG) accumulation in oocytes, leading to a decline in ovarian reserve (Friedman-Gohas, Elizur, et al., 2020; Napierala et al., 2005). To verify this hypothesis, further clinical studies are needed to correlate the POI phenotype with allelic variations in the premutation carriers, the FMR1 mRNA structure, and the FMR1 mRNA levels.
5 LIMITATIONS
Although such other studies are needed to better understand the link between FMR1 allele structure and POI, we acknowledge some limitations. First, there is a phenotype heterogeneity in the group of women with ovarian disorders; some displayed primary POI, others secondary and some other patients had a DOR. Besides, we have not been able to collect the karyotype results for 30% of women from the POI group. Second, data on ovarian function until the age of 40 in the group of pregnant women was not available. Thirdly, recent reports showed that very low CGG repeats (<26 CGG) had lower FMR1 expression than higher repeat numbers but still within a normal range (Wang et al., 2018). In our study, the allele score for allele-1 was comparable in both groups (median equal to 189) (Supplemental tables). However, it would be interesting to analyze more allelic substructures in larger cohorts to fully understand the involvement of the FMR1 gene in POI. Although we observed no difference of CGG lengths and allelic complexity in normal alleles (i.e., allele 1), further investigation of this complexity in normal FMR1 alleles should lead to an improved understanding of contributions of the FMR1 gene to POI.
6 CONCLUSION
To conclude, our study highlights that expansions of FMR1 CGG repeats may explain at least 2% of clinically diagnosed POI/DOR. As demonstrated in previous studies, we confirm that the risk of POI is lower in women with a premutation at the higher range, above the threshold of 100 CGG repetitions. This study suggests that the allelic score could be an additional parameter to help stratify the risk of POI/DOR. However, larger studies are still needed to conclude.
AUTHOR CONTRIBUTIONS
Laila El Khattabi and Thierry Bienvenu co-conceptulized and designed the study. Sandrine Perol, Sarah Grotto, Pénélope Jordan, Delphine Héron, Denise Molina-Gomes, Eva Pipiras, Michael Grynberg, Sophie Catteau-Jonard, Philippe Tourraine, Sophie Christin-Maître, and Geneviève Plu-Bureau reviewed medical records and collected patient data. Juliette Quilichini, Corinne Fouveaut, Laurence Cuisset and Jean Claude Barbot performed molecular analysis, and provided data analysis, tables and figures. Juliette Quilichini, Thierry Bienvenu, Camille Verebi, and Laila El Khattabi written content toward the first draft of the manuscript. All authors reviewed and revised the manuscript and approved the final version as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors would like to sincerely acknowledge all clinicians for their continuous support in providing the blood samples and clinical information of the patients. We extend our thanks to all the study participants for their valuable involvement to execute this study successfully.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




