Arterial tortuosity in pediatric Loeys-Dietz syndrome patients
Abstract
Loeys-Dietz syndrome (LDS) is an autosomal connective tissue disorder commonly presenting with hypertelorism, bifid uvula, aortic aneurysms, and arterial tortuosity. The aim of the present study was to investigate differences in tortuosity index (TI) between genotypes of LDS, possible progression over time and its use as an adjunctive prognostic tool alongside aortic dimensions to aid timely surgical planning in pediatric patients. A retrospective observational study of pediatric LDS patients referred to our center (November 2012–February 2021) was conducted. Using magnetic resonance angiography (MRA) with 3D maximum intensity projection volume-rendered angiogram, arterial TI was measured. Twenty three patients had genetically confirmed LDS with at least one head and neck MRA and 19 had no less than one follow-up MRA available. All patients presented arterial tortuosity. Patients with TGFBR2 variants had greater values of TI compared to patients with TGFB2 variants (p = 0.041). For patients who did not undergo surgery (n = 18), z-scores at the level of the sinus of Valsalva showed a significant correlation with vertebral TI (rs = 0.547). There was one death during follow-up. This study demonstrates that patients with LDS and TGFBR2 variants have greater values of TI than patients with TGFB2 variants and that greatest values of TI are associated with increased aortic root z-scores. Furthermore, as TI decreases over time, less frequent neuroimaging follow-up can be considered. Nevertheless, additional studies are needed to better define more accurate risk stratification and long-term surveillance in these patients.
1 INTRODUCTION
Loeys-Dietz syndrome (LDS) is an autosomal connective tissue disorder commonly presenting with hypertelorism, bifid or broad uvula or cleft palatae, aortic aneurysms, and generalized arterial tortuosity (Loeys et al., 2005; MacCarrick et al., 2014). LDS is caused by variants in the transforming growth factor β (TGFβ) receptor I (TGFBR1) and II (TGFBR2) genes, in the mothers against decapentaplegic homolog 2 (SMAD2) and 3 (SMAD3) genes, and in the transforming growth factor β 2 (TGFB2) and 3 ligand (TGFB3) genes (MacCarrick et al., 2014; Schepers et al., 2018; Zhang et al., 2017).
LDS has been associated with vascular anomalies with an increased risk of neurovascular pathology such as intracranial aneurysms (LoPresti et al., 2020). Arterial tortuosity has been reported in 80%–100% of LDS patients and is generally most prominent in the carotid and vertebrobasilar system (Kono et al., 2010; LoPresti et al., 2020; Morris et al., 2011). Current adult guidelines include head to pelvis magnetic resonance angiography (MRA) or computed tomography angiography (CTA) at the time of diagnosis of LDS to assess for arterial aneurysms and tortuosity and to repeat imaging on a yearly basis (Servatius et al., 2018). Nevertheless, pediatric guidelines are lacking with respect to surveillance. Moreover, there are scarce data regarding potential associations between the extent of cerebrovascular tortuosity and its use as a prognostic marker for elective surgery before adverse cardiovascular outcomes namely aneurysm, dissection, or even death occur (LoPresti et al., 2020). Hence, the aim of this study was to evaluate these associations.
2 MATERIALS AND METHODS
2.1 Data collection
A retrospective observational study was conducted of pediatric individuals (aged ≤18 years) with a diagnosis of LDS referred to Great Ormond Street Hospital between November 2012 and February 2021. The study was approved by the Research Board and consent waived in view of the retrospective data collection. Electronic patient records were systematically reviewed.
2.2 Clinical evaluation
LDS diagnosis was determined by the presence of clinical characteristics of LDS (craniofacial features, aortic dilatation and/or tortuosity) alongside a documented TGFβ variant (LoPresti et al., 2020). All patients underwent clinical evaluation including personal and family medical history, physical examination, resting 12-lead ECG, echocardiography, cardiac magnetic resonance imaging (MRI), and MRA of the head and neck.
2.3 Study population
Patients that had a confirmed LDS diagnosis and had undergone at least one cardiac MRI and a head and neck MRA were retrospectively reviewed.
2.4 Genetic testing
A thorough clinical evaluation by a medical geneticist specialized in the diagnosis of connective tissue disorders was performed in patients with clinically suspected LDS. In all patients, a subsequent diagnostic genetic panel including genes associated with heritable aortopathies was sent.
2.5 MRA analysis and tortuosity index
MRA studies were performed using a 1.5-Tesla or 3 Tesla Siemens scanner with a suitable coil for each patient size. In patients <6 years of age, the study was performed under general anesthesia or deep sedation as they could not cooperate with the procedure.
The initial and the most recent follow-up MRA scans, if available, were collected from each patient's electronic medical records. They were analyzed by a blinded single investigator. Using a free and open source software application for medical image computing (3D Slicer) (Morris et al., 2011), a 3D volume-rendered angiogram model was generated from the source gadolinium-enhanced MRA for each patient for both the initial and the most recent MRA separately (Figure 1) (Fedorov et al., 2012). The software allows a user to rotate the 3D volume-rendered data set in any arbitrary direction in order to obtain a more accurate measurement of tortuosity and simultaneously plot a path that follows the tortuous vessel in 3D space (LoPresti et al., 2020). The patient's head and neck vessels needed to be segmented from the MRA images, were subsequently uploaded onto the database of 3D Slicer in order to create a 3D volume rendered model. It allowed to select arterial tissue and make a robust separation of the vessels, distinguishing arterial tissue from bone and providing a preliminary 3D segment. Unnecessary fragments and artifacts were removed. The final 3D segmentation was exported as a 3D model node to carry out the center-line and straight-line measurements (Figure 1a). These 3D volume rendered models allowed accurate measurements of both internal carotid and vertebral arteries alongside petrous segments of the carotid artery. The internal carotid and vertebral arteries were measured bilaterally to determine the arterial tortuosity index (TI) which was used to quantify the excess vessel length using the previously reported distance factor: ([center-line length/straight-line length] – 1) × 100 (Figure 1b) (LoPresti et al., 2020; Morris et al., 2011).
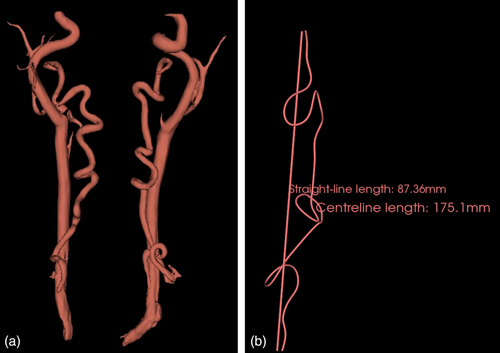
Center-line and straight-line distances of each vertebral artery were measured from the origin of the vessel to a point before the normal bend of the vertebral artery at the level of C2 (Morris, 2015). Each patient was assigned with the TI of the most tortuous vertebral artery. The interval percentage change in TI over time was measured for each patient with follow-up MRA. It was calculated by subtracting the initial raw score TI from the most recent raw score TI and dividing by the initial raw score and then multiplied by 100: (initial TI−recent TI/initial TI) × 100. A vertebral artery TI of 0–10 was used as the normal reference range as previously reported (Morris et al., 2011). The petrous carotid artery was measured bilaterally for horizontal elongation using linear measurements. When analyzing the data with regards to genetic variants and association to tortuosity, the greatest TI between the right and left arteries was selected for comparison.
2.6 MRI analysis and aortic measurements
Cardiac MRI images were blindly analyzed by an experienced investigator according to current guidelines (Kaiser et al., 2008; Kawel-Boehm et al., 2015). Aortic diameters were standardized to account for body height and weight and presented as aortic z-score values (Kaiser et al., 2008). The most recent aortic diameter (millimeters) and z-score or the latest measurements prior to surgery (when applicable) were obtained for each patient.
2.7 Statistics
Data were explored for normality by a Kolmogorov–Smirnov test/Saphira-Wilson and further analyzed as appropriate. Normally distributed data are presented as mean values (± standard deviation) and non-normally distributed variables as a median (interquartile range [IQR]). Categorical variables are expressed as number (n) and percentages (%).
Spearman rank correlation coefficient (rs), Student's t-test, Friedman, and Kruskal–Wallis tests were used when appropriate to compare continuous variables. Post hoc Wilcoxon signed-rank tests using Benferroni adjustment were also applied when appropriate. We performed receiver operating characteristics (ROC) curve analysis for vertebral TI and cardiac surgery. A p-value <0.05 was considered to be statistically significant for all data. Statistical analysis was performed using Statistical Package of Social Sciences (SPSS, version 28.0, IBM inc, Chicago, Illinois).
3 RESULTS
We identified 43 patients at our center institution with confirmed LDS diagnosis. The final study cohort consisted of 23 patients (53.5%) who underwent one neurovascular MRA and one cardiac MRI at the minimum. Among them, 19 patients (82.6%) underwent at least one follow-up head and neck MRA although it was available for analysis in 16 (69.5%). Table 1 shows baseline characteristics of LDS patients.
| Baseline information | Total cohort (n = 23) |
|---|---|
| Female, n (%) | 6 (26.1) |
| De novo genetic variant, n (%) | 12 (52.2) |
| LDS genetic variant, n (%) | |
| TGβR 1, n (%) | 4 (17.4) |
| TGβR 2, n (%) | 12 (52.2) |
| SMAD3, n (%) | 3 (13.0) |
| TGFβ2, n (%) | 3 (13.0) |
| TGFβ3, n (%) | 1 (4.3) |
| Age at the time of first available cerebral MRA, years [IQR] | 10.3 [6.5–12.9] |
| Patients with an available follow-up MRA, n (%) | 16 (69.6) |
| Follow-up MRA from first MRA, years [IQR] | 3.0 [2.0–4.3] |
| Aortic surgery, n (%) | 5 (21.7) |
| Deaths, n (%) | 1 (4.3) |
- Abbreviations: IQR, interquartile range; LDS, Loeys-Dietz syndrome; MRA, magnetic resonance angiography.
3.1 Cerebral imaging analysis and arterial tortuosity
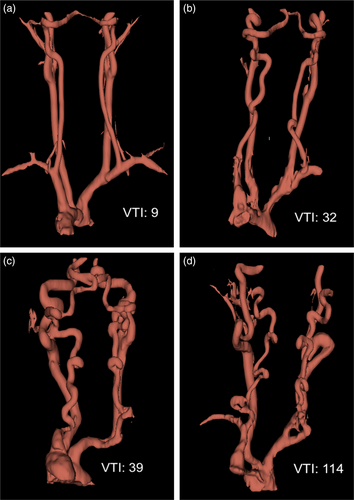
All patients in our cohort had arterial tortuosity when using a cut off of 10 (Figure 2a). Table 2 includes the arterial tortuosity for bilateral vertebral and internal carotid arteries from our LDS cohort.
| Arterial tortuosity index | Total cohort (n = 23) |
|---|---|
| Right vertebral artery, [IQR] | 39 [20.2–84.9] |
| Left vertebral artery, [IQR] | 32.1 [13.4–78.9] |
| Right Internal carotid artery, [IQR] | 22.4 [8.5–29.6] |
| Left Internal carotid artery, [IQR] | 17.5 [9.9–31.6] |
- Abbreviations: IQR, interquartile range; LDS, Loeys-Dietz syndrome; MRA, magnetic resonance angiography.
There were no significant differences in tortuosity between the right (RICA) and left internal carotid artery (LICA) (p = 0.563). However, there was a statistically significant difference in tortuosity between the right (RVA) and left vertebral arteries (LVA), the RVA and RICA, the RVA and LICA, the LVA and RICA, and the LVA and LICA (p = 0.024; p = <0.001; p = <0.001; p = 0.003; p < 0.001, respectively).
Considering the greatest TI score out of the RVA and LVA for each patient at the time of the first head and neck MRA, 91.3% of the patients (n = 21) had increased vertebral artery tortuosity (TI > 10). The follow-up head and neck MRA was available in 16 patients (69.6%). Of those, 100% had increased vertebral artery tortuosity.
Table S1 shows interval of change in TI for bilateral vertebral and internal carotid arteries. There was no statistically significant difference between the interval changes in TI in the aforementioned arteries [χ2(3) = 1.275, p = 0.735]. However, there was a significant difference between the interval change in TI for both the right and left petrous carotid artery: [t(14)=3.083, p = 0.008] and [t(14)=2.731, p = 0.016], respectively. In our cohort, no patients were found to have aneurysms nor other vascular anomalies or intracranial lesions.
3.2 Genetic variants
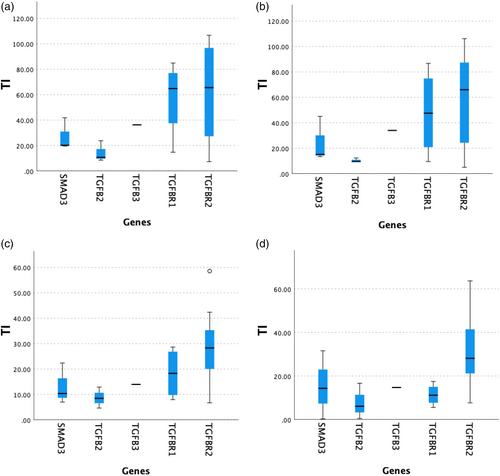
There was a statistically significant difference between median TI in the RICA, LICA, and ICA in patients with the TGFBR2 variant (28.3 [18.5–36.6], 28.2 [20.9–41.7], and 34.0 [25.9–47.8], respectively) compared with the remaining genetic variants (11.6 [8.0–22.4], 12.5 [5.6–16.7], and 14.3 [8.5–25.0]) (p = 0.014; p = 0.003; p = 0.003, respectively). There was no significant difference between median TI in RVA, LVA, or VA in patients with the TGFBR2 variant with respect to the rest of genetic variants (p = 0.065, p = 0.056; p = 0.056, respectively). Figure 3 depicts the box plots of TI by genetic variants.
3.3 Cardiac outcomes and follow-up
Five patients (21.7%) underwent a total of six cardiac surgical interventions with a median age of 13 [10.3–17.3] years: aortic root replacement with valve sparing technique (n = 2; 40%), Bentall procedure (n = 2, 40%), personalized external aortic root support (PEARS), and coartectomy surgery (n = 1, 20%) (Table S2). The median z-score at the level of the sinus of Valsalva before surgery was +7.5 [7.2–8.3].
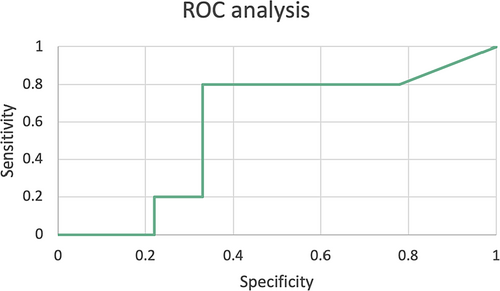
In our cohort, using the initial TI scores and carrying out ROC analysis for vertebral TI and cardiac surgery, a vertebral TI of 50 provided a sensitivity of 80% and a specificity of 67% as a predictive threshold for cardiac surgery (Figure 4).
Regarding the 18 patients (78.3%) that did not undergo surgery, the median z-score was 4.5 [2.9–6.4] with increasing z-scores showing a significant correlation with vertebral TI (rs = 0.547, p < 0.001). On analysis of the annual change of the z-score at the level of the sinus of Valsalva among unoperated patients, the median change per year was 0.5 [0.2–0.5]. No correlation was found with annual change in z-score and vertebral TI.
There was one death (4.3%) over follow-up. This patient underwent a Bentall procedure 2 months earlier and was subsequently on anticoagulant therapy. He suddenly died at the age of 19 years, with no available data on death circumstances and no post-mortem examination performed.
4 DISCUSSION
This study reports the arterial tortuosity analysis of the head and neck vessels of an exclusively LDS pediatric cohort by producing 3D volume rendered models of MRA images. We noted that patients with TGFBR2 variants have greater values of TI in vertebral and internal carotid arteries than patients with TGFB2 variants. Furthermore, we demonstrated that greatest values of TI were associated with higher aortic root z-scores.
4.1 Arterial tortuosity and intracranial pathology
In line with previous studies, 100% of our patients presented arterial tortuosity (Kono et al., 2010; LoPresti et al., 2020; Morris et al., 2011). Furthermore, our results showed stability or even a decrease of the head and neck arterial tortuosity among pediatric LDS. In line with Lo Presti et al., while there was a significant difference between the interval change in TI for petrous carotid arteries, there was no statistical difference between the interval changes in tortuosity in vertebral and internal carotid arteries in our cohort. Nevertheless, there was a trend toward improvement in tortuosity during follow-up in these latter arteries (LoPresti et al., 2020). This subjective amelioration could be attributed to the young age of our patients implying that their necks elongated over time as they aged and grew in height, thus diminishing the degree of arterial tortuosity over surveillance (LoPresti et al., 2020). This lack of progression in tortuosity raises the question of which is the most appropriate time frame for follow-up imaging of younger patients with LDS in order to minimize risks associated with MRA and maximize clinical benefit. In this context, LoPresti et al. recently described a low rate of TI progression among their LDS cohort highlighting the need to determine patients that require a closer follow-up to lessen avoidable screening and exposure to anesthesia, contrast, and radiation (LoPresti et al., 2020). The present study further strengthens the suggested screening recommendations by LoPresti et al. that LDS patients without high-risk characteristics, aneurysms nor artery tortuosity on first or consecutive follow-up imaging, can be monitored safely with less frequent neuroimaging (LoPresti et al., 2020).
Specifically, LoPresti et al. suggested an initial neurovascular image either by MRA or CTA during the first year of diagnosis with neurovascular imaging surveillance in patients ≤25 years depending on the findings of the first neurovascular imaging: in 6 months and referral to neurosurgery in the setting of an intracranial aneurysm, in 2 years if arterial tortuosity is present and in 5 years in the absence of neurovascular pathology (LoPresti et al., 2020).
As aforementioned, surveillance of arterial tortuosity is required as development of intracranial aneurysm has been reported (Kim et al., 2016, 2018). Precisely, LoPresti et al. described an incidence of intracranial aneurysms of 5.1% in their LDS cohort (LoPresti et al., 2020). Additionally, intracranial hemorrhage has been reported as the third cause of mortality in LDS patients due to intracranial aneurysms rupture (Kono et al., 2010; LoPresti et al., 2020). Nevertheless, in our cohort, no patients presented aneurysms nor other vascular lesions.
4.2 Genetic variants
In our study population, patients with TGFBR2 variants had statistically significant greater values of TI in the RICA, LICA, and ICA compared to patients with the remaining genetic variants. Morris et al. reported a trend toward a greater vertebral TI among patients with TGFBR2 variants in comparison with patients with TGFBR1 variants, although this difference was not statistically significant, likewise our results (Morris et al., 2011). Our greater values of TI in these arteries could be related to the fact that patients with TGFBR2 variants have more severe phenotype of LDS (LoPresti et al., 2020; Morris et al., 2011). Precisely, Tran-Fadulu et al. demonstrated that patients with TGFBR2 variants were more likely to have dissections at lower aortic diameters than patients with TGFBR1 variants (Tran-Fadulu et al., 2009). It could be speculated that different genetic variants affect the level of TGF-β activation to varying extents which may be reflected in the degree of arterial tortuosity or different cardiovascular risk.
4.3 Cardiac outcomes
Using the initial TI scores and carrying out ROC analysis for vertebral TI and cardiac surgery, we found that a vertebral TI of 50 provides a sensitivity of 80% and a specificity of 67% as a predictive threshold for cardiac surgery. Moreover, we found a significant correlation between vertebral TI and aortic root z-score. This concurs with Morris et al. who demonstrated that higher vertebral TI in patients with LDS was associated with greater adverse outcomes, namely increased rate of cardiac surgery, more significantly dilated aortic root, and a younger age at dissection, cardiac intervention and death (Morris et al., 2011). Specifically, they noted that a higher TI was associated with a younger age at surgery (Morris et al., 2011). Earlier age at dissection and death was associated with vertebral TI ≥50 respect to TI <50 (p = 0.001 vs. p < 0.001) (Morris et al., 2011). It could be hypothesized that vertebral TI alongside aortic root z-score can be used as a measure of vascular fragility and a prognostic tool.
Even though the real correlation between vertebral arterial tortuosity and cardiac outcome is elusive, it has been speculated that arterial tortuosity, dilatation, and dissection of vessels are a manifestation of arterial wall fragility in patients with LDS caused by abnormalities in the TGF-β signaling pathway which leads to a disturbance in the elastic fibers and subsequent weakening of the wall of the vessels (Loeys et al., 2006; Spinardi et al., 2020).
5 LIMITATIONS
The study is limited by the small cohort of patients which not only limits the comparison between LDS patients with different genetic variants but also the power of the statistical analysis. The present trial is limited by its retrospective design with intrinsic limitations that could lead to inherent ascertainment bias, mainly in that consensus guidelines for and timing of MRA have not been reported. Therefore, in our study, they were not standardized.
Furthermore, the follow-up periods between MRAs are erratic. Hence, interval changes in vertebral TI are variable. Consequently, they could not be compared between subgroups. Moreover, as 3D reconstruction requires many steps of post-processing, there could be loss and misinformation; however, the consistency within the study was maintained as a repeated technique and a standard protocol was followed for each measurement.
6 CONCLUSION
This study demonstrates that patients with LDS and TGFBR2 variants have greater values of TI in vertebral and internal carotid arteries than patients with TGFB2 variants. Besides, it shows that greatest values of TI are associated with increased aortic root z-scores. Furthermore, it strengthens the previously suggested surveillance of LDS patients without high-risk characteristics, aneurysms, or artery tortuosity with less frequent neuroimaging. Nevertheless, additional studies with larger cohorts and further long-term prospective follow-up are required in order to define more accurate risk stratification and long-term surveillance of arterial tortuosity in LDS patients.
AUTHOR CONTRIBUTIONS
Laia Brunet-Garcia: primary author, drafted the manuscript, writing the bulk of the manuscript and responsible for the overall content. Pirasuja Prabaharan: data collection, image acquisition, statistical study and critical revision of the manuscript. Luc Bruyndonckx: statistical study and critical revision of the manuscript. Ella Field: data collection and critical revision of the manuscript. Felice D'Arco: coordinated and supervised data collection and critical revision of the manuscript. Claudio Capelli: coordinated and supervised data collection and critical revision of the manuscript. Elena Cervi: coordinated and supervised data collection, editing and critical revision of the manuscript. All authors have read and approved the final manuscript.
FUNDING INFORMATION
Luc Bruyndonckx received a personal grant from the Mathilde Horlait-Dapsens Foundation for a clinical fellowship at Great Ormond Street Hospital.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




