Hearing loss and history of otolaryngological conditions in adults with microdeletion 22q11.2
Abstract
Previous studies have shown that the 22q11.2 microdeletion, associated with 22q11.2 deletion syndrome (22q11.2DS), conveys an increased risk of chronic otitis media, and hearing loss at young age. This study reports on hearing loss and history of otolaryngological conditions in adults with 22q11.2DS. We conducted a retrospective study of 60 adults with 22q11.2DS (41.7% male) at median age 25 (range 16–74) years who had visited an otolaryngologist and audiologist for routine assessment at a 22q11.2 expert center. Demographic, genetic, audiometric, and otolaryngological data were systematically extracted from the medical files. Regression analysis was used to evaluate the effect of age, sex, full-scale intelligence quotient, and history of chronic otitis media on the severity of hearing loss. Hearing loss, mostly high-frequency sensorineural, was found in 78.3% of adults. Higher age and history of chronic otitis media were associated with more severe hearing loss. Otolaryngological conditions with possible treatment implications included chronic otitis media (56.7%), globus pharyngeus (18.3%), balance problems (16.7%), and obstructive sleep apnea (8.3%). The results suggest that in 22q11.2DS, high-frequency hearing loss appears to be common from a young adult age, and often unrecognized. Therefore, we recommend periodic audiometric screening in all adults, including high-frequency ranges.
1 INTRODUCTION
The 22q11.2 deletion syndrome (22q11.2DS) is a multisystem disorder with an estimated prevalence of 1 in 2148 live births, and is caused by recurrent heterozygous microdeletions on chromosome 22q11.2 (Blagojevic et al., 2021; McDonald-McGinn et al., 2015). The majority (~85%) of patients with 22q11.2DS have a de novo deletion (McDonald-McGinn et al., 2015). Patients with 22q11.2DS show a marked variability in clinical manifestations, that may include palatal abnormalities, congenital heart defects, intellectual disability, and an increased risk of developing psychiatric disorders (e.g., schizophrenia) and neurodegenerative disorders that may present at young age (e.g., early-onset Parkinson's disease) (Bassett et al., 2005; Boot et al., 2019; Campbell et al., 2018; Schneider et al., 2014). Previous studies in 22q11.2DS, mainly performed in children, have indicated an increased prevalence of hearing loss (6%–60%), with a predominance of the conductive type (6%–53%), most likely related to otitis media and Eustachian tube dysfunction (Verheij et al., 2017). However, little is known about hearing loss in adults with 22q11.2DS (Bassett et al., 2005; Persson et al., 2012).
Therefore, we aimed to study the prevalence and predictors of hearing loss in adults with 22q11.2DS. We hypothesized that the severity of hearing loss would be greater in those with higher age, with history of chronic otitis media, and with lower full-scale IQ (FSIQ), based on previous research (Golub et al., 2020a, 2020b; Zarchi et al., 2011). Secondary aims were to investigate lifetime history of other otolaryngological conditions in 22q11.2DS.
2 METHODS
2.1 Editorial policies and ethical considerations
The study was approved by the Medical Ethics Review Board of MUMC+ (#2019-1440, METC 19-044/NL70681.068.19) that waived the need for written consent to use pseudonymized clinical data.
2.2 Study design and setting
This was a retrospective study of otolaryngological conditions in patients who visited the Dutch expert clinic for adults with 22q11.2DS at Maastricht University Medical Center+ (MUMC+), The Netherlands, between January 2016 and April 2023.
2.3 Participants and data sources
We included 60 adults (25 males) aged 16 years and older with a molecularly confirmed 22q11.2 deletion that at least included the LCR22A-LCR22B region, using standard methods (McDonald-McGinn et al., 2015). Patients were ascertained through referrals from general practice (n = 21), pediatrics (n = 15), intellectual disability medicine (n = 11), clinical genetics (n = 5), psychiatry (n = 5), neurology (n = 1), internal medicine (n = 1), and otolaryngology (n = 1). In one adult, reasons for referral included an otolaryngological problem, that is, globus pharyngeus. We excluded adults with no audiometry data (n = 3), including one 39-year-old female with history of hearing loss and hearing aids.
We used available medical information to record data on demographics, molecular diagnosis, lifetime history of otolaryngological conditions, and most recent FSIQ score. All patients were routinely evaluated by an otolaryngologist and audiologist. Standard examinations included a semi-structured interview, a complete ear, nose, and throat examination, and audiometric testing. Two had their most recent audiogram at MUMC+ before 2016.
2.4 Audiological assessments
Data for audiological assessments included pure-tone air and bone conduction (BC) audiometry. Unaided ear-specific hearing thresholds were measured from 0.25 to 8 kHz with pure-tone audiometry. Presence/absence and severity of hearing loss were classified with the Muenster classification because this classification includes criteria for high-frequency hearing loss (Table 1) (Schmidt et al., 2007), that was often found in adults with 22q11.2DS. Thus, hearing loss was defined as having loss with a severity of grade two or higher (Schmidt et al., 2007). Type and laterality of hearing loss, frequency ranges, and audiometric configuration were classified using the European Working Group on the Genetics of Hearing Impairment definitions (Mazzoli et al., 2009). Because most of the audiometric abnormalities in the 22q11.2DS sample concerned the high frequencies, adaptations were made to these definitions, that is: (1) we averaged the pure-tone hearing thresholds over 0.5, 1, 2, and 4 kHz instead of 0.5, 1, and 2 kHz, to define type and laterality of hearing loss, (2) we introduced n-shaped configurations in order to classify patients that performed best at the mid-frequencies, and (3) in addition to the standard criteria for gently and steeply sloping, we considered audiometric configurations showing a decrease in ≥15–29 dB HL or ≥30 dB HL between 4 and 8 kHz gently- and steeply-sloping as well (Table 1).
| Hearing loss, grading of severitya | |
| Normal or borderline hearing (0–1) | ≤20 dB HL over all frequencies |
| Loss, limited to high-frequencies (2) | >20 dB HL, not affecting frequencies ≤2 kHz |
| Mild (2a) | >20 ≤ 40 dB HL |
| Moderate (2b) | >40 ≤ 60 dB HL |
| Severe (2c) | >60 dB HL |
| Loss, corrigible with hearing aids (3) | >20 dB HL, affecting frequencies ≤2 kHz |
| Mild (3a) | >20 ≤ 40 dB HL |
| Moderate (3b) | >40 ≤ 60 dB HL |
| Severe (3c) | >60 dB HL |
| Type of hearing lossb | |
| Conductive | Normal BC thresholds (<20 dB HL) and ABG >15 dB averaged over 0.5, 1, 2, and 4 kHz |
| Sensorineural | BC thresholds >20 dB HL and ABG <15 dB HL averaged over 0.5, 1, 2, and 4 kHz |
| Mixed | BC thresholds >20 dB HL and ABG >15 dB averaged over 0.5, 1, 2, and 4 kHz |
| Audiometric configurationb | |
| Low-frequency ascending | >15 dB HL difference between the worst low-frequency thresholds and the better high-frequency thresholds |
| Mid-frequency n-shaped | >15 dB HL difference between the best mid-frequency thresholds and the worse low and high-frequency thresholds |
| High-frequency | |
| Gently sloping | 15–29 dB HL difference between the mean of 0.5 and 1 kHz and the mean of 4 and 8 kHz, or 15–29 dB HL between 4 and 8 kHz |
| Steeply sloping | ≥30 dB HL difference between the above frequencies or ≥30 dB HL between 4 and 8 kHz |
| Flat | <15 dB HL difference between the mean of 0.25 and 1 kHz, the mean of 1 and 2 kHz, and the mean of 4 and 8 kHz |
| Frequency ranges | |
| Low-frequencies | ≤0.5 kHz |
| Mid-frequencies | >0.5 kHz and ≤2 kHz |
| High-frequencies | >2 kHz and ≤8 kHz |
| Symmetry of hearing impairment | |
| Unilateral | >10 dB HL difference between the ears in at least two frequencies <20 dB HL average over 0.5, 1, 2, and 4 kHz of the better ear |
| Bilateral symmetrical | <10 dB HL difference between the ears in at least two frequencies. Average over 0.5, 1, 2, and 4 kHz of both ears >20 dB |
| Bilateral asymmetrical | >10 dB HL difference between the ears in at least two frequencies >20 dB HL average over 0.5, 1,2, and 4 kHz of the better ear |
- Note: Adapted from the definitions by the European Working Group on the Genetics of Hearing Impairment (see Section 2 for details) (Mazzoli et al., 2009).
- Abbreviations: ABG, air-bone gap; BC, bone conduction; dB, decibel; HL, hearing level; kHz, kilo Hertz.
- a Severity of hearing loss according to the Muenster classification (Schmidt et al., 2007).
- b Of the better-hearing ear.
2.5 Tympanometry
If deemed to be indicated, tympanometry was used to measure the tympanic membrane's response to changes in pressure in order to detect effusion or depression in the middle ear. Abnormal responses were classified using the Jerger classification that was adapted for adults (Margolis & Heller, 1987).
2.6 Statistical analyses
For hearing loss and history of otolaryngological conditions, we calculated prevalence rates and related 95% confidence intervals (CIs) using the formula . We calculated the prevalence of hearing loss of at least one ear, as well as of the better-hearing ear. We used ordinal regression analysis to evaluate the effect of age, sex, FSIQ, and history of chronic otitis media on the severity of hearing loss according to the Muenster classification. For this analysis, we used the better-hearing ear, in line with the European Working Group on the Genetics of Hearing Impairment definitions (Mazzoli et al., 2009). Mathematica (Wolfgang mathematica 13.2, Oxfordshire, United Kingdom) was used to plot the sensory thresholds (dB HL) in adults with 22q11.2DS at 0.25, 0.5, 1, 2, 4, and 8 kHz, relative to 50th and 90th percentiles in the general population (ISO, 2017). Two-tailed p-values <0.05 were considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics 25 (SPSS Inc., Chicago, Illinois, USA).
3 RESULTS
3.1 Demographics
Sixty adults (25 males, 41.7%) with 22q11.2DS were included. The median age at audiometry was 24.5 (range 16–62) years and the median age at otolaryngological examination was 25 (range 16–74) years. Mean FSIQ scores, available for 57 adults, were 65.3 ± 11.1 at median age 23 (range 13–57) years.
3.2 Audiometry
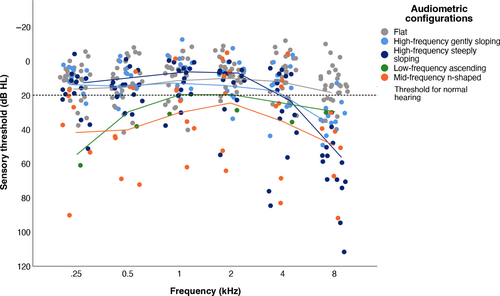
Table 2 shows data on audiological assessments. Hearing loss in at least 1 ear was found in 47 adults (78.3%) with 22q11.2DS of whom 8 had unilateral hearing loss. Of the better-hearing ear, 39 adults (65.0%) showed hearing loss that was mostly limited to the high frequencies (n = 25, 41.7%). In 14 adults (23.3%), more severe hearing loss was found that also included loss at the mid-frequencies. Because of missing BC thresholds that were not measured at 6 and 8 kHz, the type of hearing loss could not be established in 18 of 39 adults (46.2%). Of those with hearing loss, sensorineural loss was most common (n = 14 out of 21 with data, 66.7%). The audiometric configuration that was observed most, was flat (n = 27, 45.0%, Figure 1).
| Prevalence of hearing loss | Number | % | 95% CI |
|---|---|---|---|
| Hearing loss, ≥1 eara | 47 | 78.3 | 67.9–88.8 |
| Hearing loss, better-hearing eara | 39 | 65.0 | 52.9–77.1 |
| Limited to high-frequencies (2) | 25 | 41.7 | 29.2–54.1 |
| Mild (2a) | 16 | 26.7 | 15.5–37.9 |
| Moderate (2b) | 7 | 11.7 | 3.5–19.8 |
| Severe (2c) | 2 | 4.3 | 0.0–7.9 |
| Corrigible with hearing aids (3) | 14 | 23.3 | 12.6–34.0 |
| Mild (3a) | 12 | 20.0 | 9.9–30.1 |
| Moderate (3b) | 1 | 1.7 | 0.0–4.9 |
| Severe (3c) | 1 | 1.7 | 0.0–4.9 |
| Laterality of hearing loss | |||
| Bilateral, symmetrical | 24 | 40.0 | 27.6–52.4 |
| Bilateral, asymmetrical | 15 | 25.0 | 14.0–36.0 |
| Unilateral | 8 | 13.3 | 4.7–21.9 |
| Type of hearing lossb | |||
| Sensorineural | 14 | 66.7 | 46.5–86.8 |
| Conductive | 5 | 23.8 | 5.6–42.0 |
| Mixed | 2 | 9.5 | 0.0–22.1 |
| Audiometric configuration | |||
| Flat | 27 | 45.0 | 32.4–57.6 |
| High-frequency steeply sloping | 14 | 23.3 | 12.6–34.0 |
| High-frequency gently sloping | 11 | 18.3 | 8.5–28.1 |
| Mid-frequency n-shaped | 7 | 11.7 | 3.5–19.8 |
| Low-frequency ascending | 1 | 1.7 | 0.0–4.9 |
- Abbreviation: CI, confidence interval.
- a Severity according to the Muenster classification (Schmidt et al., 2007).
- b Proportions of a total of 21 out of 39 adults with hearing loss of the better-hearing ear; hearing loss limited to the high-frequencies could not be classified in the other 18 adults mainly due to missing BC thresholds at 6 and 8 kHz.
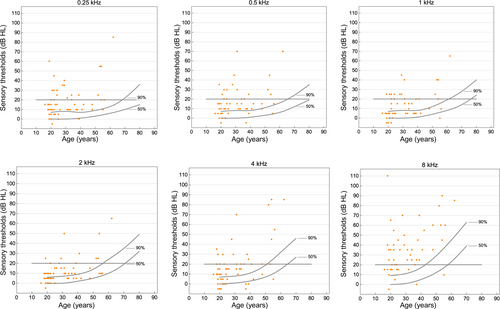
Plots of individual sensory thresholds showed that the majority of adults with 22q11.2DS had sensory thresholds that were higher, meaning the stimulus needed to be stronger, than 90% of the norm population (Figure 2). For the mid-frequency of 1 kHz, 52 adults (86.7%) had sensory thresholds higher than 50%, and 25 (41.7%) had sensory thresholds higher than 90% of the norm population. At 8 kHz, 56 adults (93.3%) had sensory threshold higher than 50%, and 55 (91.7%) had sensory thresholds higher than 90% of the norm population.
3.3 Tympanometry
Tympanometry was performed in 24 adults (40.0%) for the better-hearing ear. Most (n = 13, 54.2%) had a normal tympanogram (type A) as per the Jerger classification for adults (Margolis & Heller, 1987), 5 (20.8%) had normal admittance with the peak occurring at a negative middle ear pressure (type C), 3 (12.5%) had abnormally high admittance with normal tympanometric pressure (type Ad), 2 (8.3%) had abnormally low admittance with no discernable peak (type B), and 1 (4.2%) had abnormally low admittance with normal tympanometric pressure (type As).
3.4 Otolaryngological conditions and treatments
The majority of the study sample had history of one or more otolaryngological conditions (Table 3). Chronic/recurrent otitis media was reported most often (n = 34, 56.7%), followed by hearing loss (n = 19, 31.7%), globus pharyngeus (n = 11, 18.3%), balance problems (n = 10, 16.7%), swallowing difficulties (n = 6, 10.0), obstructive sleep apnea (OSA, n = 5, 8.3%), and nasal regurgitation (n = 5, 8.3%).
| Number | % | 95% CI | |
|---|---|---|---|
| Conditions | |||
| Chronic/recurrent otitis media | 34 | 56.7 | 44.1–69.2 |
| Hearing loss | 19 | 31.7 | 19.9–43.4 |
| Globus pharyngeus | 11 | 18.3 | 8.5–28.1 |
| Balance problems | 10 | 16.7 | 7.2–26.1 |
| Swallowing difficulties | 6 | 10.0 | 2.4–17.6 |
| Obstructive sleep apnea | 5 | 8.3 | 1.3–15.3 |
| Nasal regurgitation | 5 | 8.3 | 1.3–15.3 |
| Treatments | |||
| Speech-language therapya | 47 | 78.3 | 67.9–88.8 |
| Speech | 40 | 66.7 | 54.7–78.6 |
| Feeding/swallowing | 7 | 11.7 | 3.5–19.8 |
| Indication not specified | 7 | 11.7 | 3.5–19.8 |
| ENT-surgery | 43 | 71.7 | 60.3–83.1 |
| Ear tube placement | 23 | 38.3 | 26.0–50.6 |
| Pharyngoplasty | 17 | 28.3 | 16.9–39.7 |
| Tonsillectomy | 9 | 15.0 | 6.0–24.0 |
| Tympanoplasty/myringoplasty | 9 | 15.0 | 6.0–24.0 |
| Palatoplasty | 7 | 11.7 | 3.5–19.8 |
| Adenotomy | 6 | 10.0 | 2.4–17.6 |
| Nose/septum surgery | 2 | 3.3 | 0.0–7.9 |
| Ear reconstruction surgery | 2 | 3.3 | 0.0–7.9 |
| Laryngeal web resection | 1 | 1.7 | 0.0–4.9 |
| Tracheotomy and vocal cord surgery | 1 | 1.7 | 0.0–4.9 |
| Mastoidectomy | 1 | 1.7 | 0.0–4.9 |
| Hearing aid | 5 | 8.3 | 1.3–15.3 |
| Bilateral | 4 | 6.7 | 0.3–13.0 |
| Unilateral | 1 | 1.7 | 0.0–4.9 |
- Abbreviations: CI, confidence interval; ENT, ear-nose-throat.
- a Seven adults with 22q11.2DS received speech-language therapy for both speech and feeding/swallowing issues.
Most adults had received speech therapy (n = 47, 78.3%). Ear tube placement was the most reported surgical intervention (n = 23, 38.3%). Five adults (8.3%) had hearing aids prescribed prior to their visit (at age 57, 55, 32, 21, and 19 years), of whom 4 (6.7%) bilateral (Table 3). Another five adults (8.3%) were advised to consider hearing aids after audiological examination. Treatments that were recommended after routine examination included muscle relaxation therapy for globus (n = 4, 6.7%) and dysphagia (n = 1, 1.7%), and balance exercises for benign paroxysmal positional vertigo (n = 1, 1.7%). Additional examinations were recommended for sleep disorders (n = 2, 3.3%; i.e., polysomnography) and vestibular dysfunction (n = 1, 1.7%). Five adults were advised to start medication for otitis externa (n = 2, 3.3%), rhinitis (n = 2, 3.3%), and otitis media (n = 1, 1.7%), respectively. Eight adults were advised to start (n = 6, 10%) or continue (n = 2, 3.3%) aural toileting for ear wax. Two adults (3.3%) qualified for surgical correction of nasal septum deviation.
3.5 Factors related to severity of hearing loss
The regression model predicting severity of hearing loss in 22q11.2DS was significant (p = 0.002, Nagelkerke = 0.29). Higher age (OR = 1.08, 95% CI = 1.03–1.12, Wald = 11.30, p = 0.001) and a history of chronic otitis media (OR = 2.87, 95% CI = 1.07–7.67, Wald = 4.43, p = 0.04), but not male sex (OR = 0.65, 95% CI = 0.24–1.73, Wald = 0.74, p = 0.39) or FSIQ (OR = 0.99, 95% CI = 0.95–1.03, Wald = 0.26, p = 0.61), were related to the severity of hearing loss.
4 DISCUSSION
The results of this study involving a relatively young adult sample of 22q11.2DS indicate that a large proportion of adults with 22q11.2DS have hearing loss, mostly limited to the higher frequencies, and often unrecognized. Age and a history of chronic otitis media, but not sex or FSIQ, were positively related to the severity of hearing loss. Lifetime history of otolaryngological conditions that were frequently reported included chronic otitis media, globus pharyngeus, swallowing difficulties, balance problems, and OSA.
4.1 Hearing loss
The proportion of adults with hearing loss (78.3%) was much higher in comparison to two previous studies in adults with 22q11.2DS that reported hearing loss in 28.5% and 40.9%, respectively (Bassett et al., 2005; Persson et al., 2012). However, when interpreting the findings, it is important to take into account the study designs, definitions for hearing loss, and age and size of the samples in the different studies. The first study was a retrospective chart review in 78 adults at a mean age of 31.5 ± 10.5 years, and included those with hearing loss reported in the medical records (Bassett et al., 2005). No audiological assessments were reported. The proportion of adults with a documented history of hearing loss was similar to this study (28.5% vs 31.7%). In the second study, 22 of 24 adults underwent an audiological assessment at a mean age of 25 (range 19–38) years (Persson et al., 2012). The same definition for hearing loss was used as in this study. However, in the current study hearing was measured up to eight, instead of six, kHz. Unilateral conductive hearing loss was found in a much higher proportion of the patients (44.4% vs. 12.8%) compared to this study. However, absolute numbers were low affecting reliability of those findings, and results were possibly influenced by a few younger participants (Persson et al., 2012).
In contrast to findings in children (Digilio et al., 1999; Jiramongkolchai et al., 2016; Weir et al., 2017), adults showed predominantly sensorineural hearing loss instead of conductive or mixed hearing loss in this study. Also, the prevalence rates of hearing loss in adults in this study were higher compared to prevalence rates reported in children (Digilio et al., 1999; Jiramongkolchai et al., 2016; Weir et al., 2017). This suggests that the type and severity of hearing loss evolve over time.
4.2 Potential contributors to hearing loss in 22q11.2DS
There are several potential contributors to hearing loss in adults with 22q11DS (Tian & Johnson, 2020; Verheij et al., 2017). First, chronic otitis media, with or without congenital inner ear abnormalities (Loos et al., 2016), may result in the cochlea being more vulnerable in patients with 22q11.2DS compared to the general population (van Eynde et al., 2016; Verheij et al., 2017). Second, early physiological degeneration and other medical conditions and their treatment, may lead to hearing loss (Huang & Tang, 2010). Age-related disorders such as Parkinson's disease and type 2 diabetes mellitus (Boot et al., 2018; Van et al., 2020), and multimorbidity and polypharmacy are often seen in 22q11.2DS at a relatively young age (Malecki et al., 2020). Third, genetic susceptibility should be considered (Huang & Tang, 2010). For example, young-onset and age-related hearing loss has been reported in other genetic neurodevelopmental disorders, such as in Down syndrome (Picciotti et al., 2017), and Turner syndrome (Bonnard et al., 2017). Importantly, findings may be specific per genetic variant. When comparing the sensory thresholds found in Turner syndrome to those reported in 22q11.2DS it is striking that adults with Turner syndrome scored worst at the mid-frequency (2 kHz) (King et al., 2007), whereas adults with 22q11.2DS performed best at the mid-frequencies and worst at the highest and lowest frequencies.
Genes that may possibly play a role in 22q11.2DS include TBX1 and genes involved in mitochondrial function (Keithley, 2020; Tian & Johnson, 2020). TBX1 is involved in the development of the vascular system including the stria vascularis (an important cochlear structure that is rich in vascular tissue), the central nervous system, and the semicircular canal (Baldini et al., 2017; Tian & Johnson, 2020). In mice with a homozygous missense mutation in Tbx1 inner ear malformations, an undeveloped stria vascularis, and deafness have been found (Tian & Johnson, 2020). Other candidate genes include those involved in mitochondrial function; at least six lie within the 22q11.2 region (Motahari et al., 2019). Mitochondrial dysfunction and oxidative stress have been proposed as possible contributors to degenerative changes in the cochlear duct (i.e., in the stria vascularis, hair cells, and neurons) which may result in age-related hearing loss (Keithley, 2020).
4.3 Clinical implications of the hearing findings
High-frequency hearing loss makes it more difficult to understand speech with background noise, for example, during group conversations, and to localize sounds (Huang & Tang, 2010). It often precedes and is a predictor of, hearing loss at the lower frequencies (Dubno et al., 2013). Similarly, loss at the mid-frequencies may negatively affect daily life functioning by reduced understanding of speech or hearing in traffic. If diagnosed late, hearing loss may contribute to the development of depressive symptoms, stress, anxiety, social isolation, and reduced quality of life (Chia et al., 2007; Jayakody et al., 2018). Early identification of hearing loss enables interventions through adjustments to someone's work or living environment, and/or the prescription of hearing aids, if indicated. Therefore, given the high proportion of adults with 22q11.2DS with high-frequency hearing loss, we recommend periodic audiological screening (e.g., every 5 years), including high-frequency ranges (8 kHz), in all adults with 22q11.2DS.
4.4 History of otolaryngological conditions
In this study, chronic otitis media was the most prevalent otolaryngological condition, with an occurrence comparable to what has been previously reported in 22q11.2DS (Grasso et al., 2018; Verheij et al., 2017). The results of this study are also in line with the existing literature that indicates that swallowing issues, relating to globus pharyngeus in some, are an important feature of 22q11.2DS across the lifespan (Eicher et al., 2000; Grasso et al., 2018; Welby et al., 2020; Wong et al., 2020). Because individuals with 22q11.2DS may not complain, careful history taking for swallowing issues is important.
Balance problems, reported in some adults in this and a previous study (Willaert et al., 2019), may relate to abnormalities of the vestibulum, cerebellum, and/or proprioceptive system, lower muscle strength, and/or visual problems (Enkelaar et al., 2012; Loos et al., 2016; Rogdaki et al., 2020; Willaert et al., 2019). Movement disorders, such as Parkinson's disease, may also be considered in older adults with 22q11.2DS (Boot et al., 2022; Willaert et al., 2019). The occurrence of OSA in this study was comparable to that previously reported in children and adults with 22q11.2DS (Cancelliere et al., 2023; Kennedy et al., 2014). OSA can have a negative effect on quality of life, and may increase the risk of several diseases including cardiovascular and pulmonary systems (Gottlieb & Punjabi, 2020).
We recommend otolaryngological examination at least once for adults with 22q11.2DS. Special attention should be paid to conditions that may present at adult age and may have treatment implications, such as OSA (Boot et al., 2023; Cancelliere et al., 2023), globus pharyngeus (Khalil et al., 2003), dysphagia, and vestibular dysfunction.
4.5 Strengths and limitations
To our knowledge, this is the largest sample of adults with 22q11.2DS who received audiometric testing and standardized otolaryngological examinations. Limitations of the study mainly relate to the retrospective design. On the one hand, data concerning the medical history may not be complete resulting in an underestimation of some otolaryngological conditions. On the other hand, referral bias may have resulted in higher prevalence rates because adults with 22q11.2DS with a more severe clinical presentation may be more likely to be referred to a 22q11 specialty clinic. In addition, some adults did not have audiometric results because of difficulties with performing the test or noncooperation, which may have influenced results. Due to the study design and sample size, it was not possible to assess for possible contributions of other causes of hearing loss such as noise exposure and impact on daily living. Last, given the relatively small number of adults with data regarding type of hearing loss (21 of 39 adults with hearing loss), further studies are needed to confirm the proportions of the types of hearing loss in this study.
5 CONCLUSIONS
Hearing loss at a relatively young age, especially at the higher frequencies, appears to be common in adults with 22q11.2DS. Higher age and history of chronic otitis media are associated with more severe hearing loss. Therefore, we recommend periodic audiometric screening, including high-frequency testing (8 kHz), from early adulthood in all adults with 22q11.2DS.
AUTHOR CONTRIBUTIONS
Emma N. M. M. von Scheibler: Conceptualization, organization, data—inclusion, interpretation and analysis, writing—original draft, review, and editing. Josine C. C. Widdershoven: Clinical examinations, methodology, interpretation and analysis, writing—review and editing. Denise C. P. B. M. van Barneveld: Clinical examinations, methodology, interpretation and analysis, writing—review and editing. Nina Schröder: Data—inclusion and interpretation, writing—review and editing. Agnies M. van Eeghen: conceptualization, writing—review and editing. Thérèse A. M. J. van Amelsvoort: Conceptualization, writing—review, and editing. Erik Boot: Conceptualization, organization, interpretation and analysis, writing—review and editing.
ACKNOWLEDGMENT
AvE is member of European Reference Network ITHACA.
FUNDING INFORMATION
This work was supported financially by Stichting Wetenschappelijk Onderzoek, 's Heeren Loo (#2210100). The funder had no role in the design and conduct of the study, or approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Any data requests can be directed to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




