45,X/46,XY mosaicism: Clinical manifestations and long term follow-up
Ebba Alkhunaizi and Jenna Plamondon Albrecht contributed equally to this study.
Abstract
45,X/46,XY chromosomal mosaicism presents a range of clinical manifestations, including phenotypes from Turner syndrome through genital abnormalities to apparently unaffected phenotypic males; however, the full clinical spectrum has not yet been fully delineated since prior studies on the clinical phenotype and associated risk of gonadal tumors included small cohorts and limited follow-up. To better describe the clinical manifestations and long-term outcome of patients with 45,X/46,XY mosaicism. We conducted a retrospective chart review of patients with 45,X/46,XY from three health centers (Hospital for Sick Children and Mount Sinai Hospital in Canada, and University of Pittsburgh Medical Center in United States). Of 100 patients with 45,X/46,XY karyotype, 47 were raised as females and 53 as males. Females were significantly shorter than males (p = 0.04) and height Z-score was significantly decreased with age for both genders (p = 0.02). Growth hormone (GH) treatment did not result in a significant height increase compared to the untreated group (p = 0.5). All females required puberty induction in contrast to majority of males. Five females were diagnosed with gonadal tumors, while no males were affected. Around 58% of patients exhibited at least one Turner syndrome stigmata. This study expands the clinical spectrum, long-term outcomes, and associated tumor risk in a large cohort of patients with 45,X/46,XY mosaicism. Additionally, it highlights our experience with GH therapy and prophylactic gonadectomy.
1 INTRODUCTION
45,X/46,XY chromosomal mosaicism is a disorder of sex development that can present with a spectrum of urogenital and gonadal abnormalities. The incidence of 45,X/46,XY mosaicism is estimated to be 1.7 per 10,000 as detected following karyotype analysis of over 548,000 amniotic fluid specimens (Chang et al., 1990). A number of different mechanisms of pathogenesis have been proposed including the post-conception mitotic error leading to Y chromosome loss in a portion of cells in the developing embryo (Gardner et al., 2011).
The gonadal sex in individuals with 45,X/46,XY mosaicism largely depends on the ratio and the distribution pattern of the Y-containing cells in the genital ridge. This can vary from streak gonad to normal testes (Chemes et al., 2003; Cools et al., 2011; Reddy & Sulcova, 1998), but most commonly presents as mixed gonadal dysgenesis, a form of asymmetrical gonad development with a dysgenetic testis on one side and a streak gonad on the other (Andrade et al., 2010; Davidoff & Federman, 1973).
The ratio of XY cells to cells with monosomy X also influences the risk of developing gonadal tumors, with an estimated frequency of 15%–35% for individuals with intra-abdominal gonadal dysgenesis and 12% for females with streak gonads (Lee et al., 2006). The risk could be altered by the expression status of the testis-specific protein (TSPY) coded by the SRY located on the Y chromosome or the position of the gonads, though mosaic males born with descended testes have a small theoretical risk of gonadal tumors (Chang et al., 1990; Lee et al., 2006; Müller et al., 1999).
A limited number of studies have delineated the clinical features of individuals with 45,X/46,XY, which can overlap with the well-described features of girls with Turner syndrome (Ayuso et al., 2008; Farrugia et al., 2013; Gantt et al., 1980; Knudtzon & Aarskog, 1987; Lindhardt Johansen et al., 2012; Martinerie et al., 2012; Richter-Unruh et al., 2004; Telvi et al., 1999; Tosson et al., 2012; Yang et al., 2023). However, the clinical management of girls with Turner syndrome is different than the recommendations for patients with 45,X/46,XY (Gravholt et al., 2017; Guzewicz et al., 2021; Tosson et al., 2012).
Short stature is a widely recognized feature of Turner syndrome in patients with 45,X/46,XY, though data on the efficacy of growth hormone (GH) therapy in these individuals is limited (Gantt et al., 1980; Knudtzon & Aarskog, 1987; Lindhardt Johansen et al., 2012; Martinerie et al., 2012; Richter-Unruh et al., 2004; Rosa et al., 2014; Telvi et al., 1999; Tosson et al., 2012; Walker et al., 1990; Yang et al., 2023). The prevalence of other clinical features in this patient population is not well studied due to inadequate long-term follow-up of a sizable cohort of patients with both female and male phenotypes (Knudtzon & Aarskog, 1987; Lindhardt Johansen et al., 2012; Ljubicic et al., 2019; Martinerie et al., 2012; Richter-Unruh et al., 2004; Telvi et al., 1999; Walker et al., 1990). Likewise, the long-term outcomes are limited regarding developmental, educational, and psychological implications including learning and intellectual disabilities, autism spectrum disorder, and anxiety disorder (Fontenelle et al., 2004; Telvi et al., 1999; Tosson et al., 2012). In addition, the sex of rearing has been identified as a challenge in this population, especially in those infants born with ambiguous genitalia (Houk et al., 2004; Man et al., 2022; Ocal et al., 2009; Pesmatzoglou et al., 2019; Rosa et al., 2014).
The presence of 45,X/46,XY can be detected on noninvasive prenatal testing and microarray analysis subsequently undertaken on amniocentesis or chorionic villi samples (CVS). Of those detected cases, about 90%–95% presented as phenotypically normal males on fetal ultrasound, postnatal physical examination, or autopsy (Chang et al., 1990; Hsu, 1989; Wilson et al., 1989). Therefore, the inclusion of findings noted in prenatally diagnosed cases serves to mitigate bias associated with postnatally ascertained individuals.
In this study, we collected clinical, cytogenetic, histological data, and long-term outcomes on 100 individuals with 45,X/46,XY, diagnosed pre- and postnatally. This study was designed to address the limitations noted in previously published studies including analysis of a small number of affected individuals, ascertainment bias, and incomplete long-term follow-up.
2 METHODS
The study was performed following approval by Research Ethics Boards (REB) at Mount Sinai Hospital (MSH) for prenatally diagnosed patients, the Hospital for Sick Children (SickKids) for both prenatally and postnatally diagnosed patients (University of Toronto, Toronto, Canada), and the University of Pittsburgh Medical Center (UPMC, STUDY20050212).
2.1 Patient cohort and data collection
Data were collected retrospectively between January 1984 to March 2019, for the Toronto cohort, and January 1990 to May 2021, for the UPMC cohort, and included patients with pre- or postnatal diagnosis of 45,X/46,XY. Patients were identified through the Multidisciplinary Urogenital (MUG) clinic database, the internal database in the Division of Clinical and Metabolic Genetics (Toronto) and the databases of the cytogenetics laboratories at all health centers. The histopathological findings on all cases that underwent gonadectomy were reviewed. A query of the pathology laboratory database for patients with gonadoblastoma and a subsequent diagnosis of 45,X/46,XY mosaicism yielded additional patients (Toronto). Patients were excluded if they had an additional chromosome abnormality, insufficient follow-up data, were diagnosed in adulthood with infertility, or if their hospital charts were inaccessible. Seventy-two patients were ascertained from Toronto (7 patients from MSH and 65 patients from SickKids), and 28 patients were ascertained from UPMC.
Reviewed data included age, birth growth parameters, karyotype result, abdominal and testicular ultrasounds, gonadal biopsy/pathology, hormonal testing, growth parameters, puberty and psychological assessment, surgical history including gonadectomy or other surgeries, and surveillance for gonadal malignancy/gonadoblastoma. Overall, the referral to “female” or “males” were based on the phenotypic sex, which was determined by hormonal influence on external and internal genitalia. Detailed data collection information for each patient is provided in the Appendices S1 and S2.
2.2 Cytogenetic analysis
Classical G-banded karyotype analysis was performed on peripheral blood PHA-stimulated lymphocyte cultures, amniotic fluid, or CVS. Fluorescence in situ hybridization (FISH) studies were carried out using centromeric probes (Vysis, Inc.) specific for the X (DXZ1), the Y (DYZ3), and the SRY region (Yp11.31). FISH analyses were performed on cultured cells, and, when available, on cell pellets derived from the uncultured whole blood, buccal swabs, urine, and gonadal samples.
2.3 Statistical analysis
Descriptive statistics, specifically means, standard deviations (SD), medians, frequency distributions, and proportions with 95% confidence intervals were used. All means are presented as mean ± SD (range). All height measurements were standardized for age and sex using CDC norms. We used PROC LIFETEST in SAS 9.3 to create Kaplan–Meier survival curves of time from diagnosis to age at gonadectomy for males and females, and a log rank test was used to compare the two groups. Mixed model regression analysis using PROC MIXED in SAS 9.3 was used to model the Z-score for height for each gender separately, controlling for age and GH therapy use and treating subject as a random effect. Mean Z-scores for height and their 95% confidence limit were estimated from these models at age 5, 10, and 15. We also ran the models on the complete sample to test for differences in mean Z-score for height and the rate of change across age between males and females and between observations while on and off GH therapy.
3 RESULTS
3.1 Study population
A total of 100 charts with a confirmed karyotype of 45,X/46,XY mosaicism met the inclusion criteria for long-term retrospective analysis. Among the included subjects, 53 were reared as males (53%) and 47 were reared as females (47%).
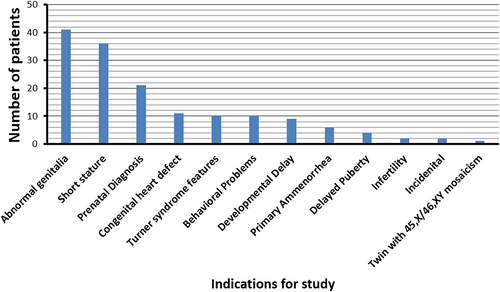
Median age at diagnosis was 2 months (1st and 3rd quartiles: 0, 6 years), and one subject was diagnosed at 28 years of age. The reason for referral in the large majority of patients was related to the associated manifestations including abnormal genitalia (40/100), short stature (36/100), and prenatally diagnosed abnormal fetal genitalia (21/100) (Figure 1). Interestingly, three patients were identified incidentally; two after a discordant noninvasive prenatal testing result showing a Y-chromosome and finding of female genitalia at birth, and another after identifying Y-chromosome material on a blood sample sent for a molecular panel of genes associated with ketotic hypoglycemia in a phenotypic female patient.
3.2 Chromosomal analysis
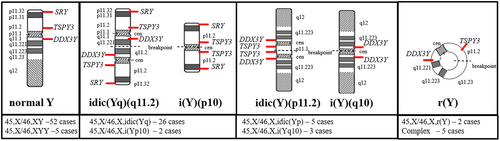
There were 52 patients with 45,X/46,XY karyotype and 5 patients with 45,X/47,XYY karyotype. Forty-three patients had a structurally abnormal Y chromosome (Figure 2), including 28 individuals with a rearranged Y chromosome including the SRY gene sequences, 8 patients with the Y chromosome material presumably negative for the SRY gene, and 7 patients with a complex Y chromosome rearrangement. The karyotype of each patient in our study population is listed (Table S1).
3.3 Genitalia and gonads evaluation
Abnormal external genitalia were observed in 47/100 patients (47%); 12 patients with mild abnormalities (e.g., isolated hypospadias, cryptorchidism or isolated clitoromegaly), and 35 patients had severe abnormalities (>1 abnormality).
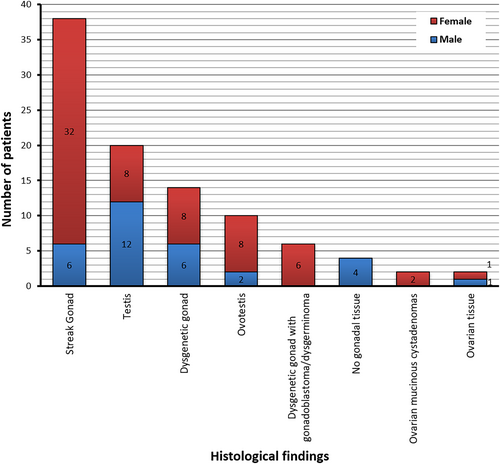
Out of 100 patients, 53 patients (17 reared as males, 35 reared as females) had either biopsy or gonadectomy of at least one of their gonads, providing histology data for 96 gonads (Figure 3). Streak gonad and testis were the most observed histological findings. No male with normal genitalia underwent gonadal biopsy or gonadectomy. Descriptions of the external genitalia at birth and histological evaluation of the gonads for each patient are listed (Table S1).
3.4 Timing of gonadectomy
Gonadectomy was common in those with abnormal and apparently female genitalia due to the reported risk of gonadal tumors. The time between diagnosis and gonadectomy differed significantly between apparently male and female external genitalia (p = 0.004); males with abnormal external genitalia underwent gonadectomy within the first couple of years following the diagnosis, while the timing in the female group varied greatly. A Kaplan–Meier survival curve was used to evaluate the timing of the gonadectomy (Figure S1).
3.5 Gonadal tumors
A total of five females (T-003, T-036, T-056, T-057, P-084) were found to have gonadal tumor histology following gonadectomy. Table 1 summarizes the information regarding the patients with gonadal tumors.
| ID | Reared sex | Age | Reason for evaluation | Ultrasound: uterus | Ultrasound: ovaries | Histology | Follow up |
|---|---|---|---|---|---|---|---|
| T-003 | Female | 16 | Primary amenorrhea | Small | Right: normal Left: non-visualized |
Right: dysgenetic with gonadoblastoma and dysgerminoma Left: dysgenetic with gonadoblastoma |
Normal CT and tumor markers |
| T-036 | Female | 13 | Short stature | Hypoplastic | Non-visualized | Bilateral ovarian mucinous cystadenomas and epithelial tumors | None |
| T-056 | Female | 15 | Delayed puberty, short stature | Normal | Right: non-visualized Left: hypoplastic |
Right: dysgenetic gonad with gonadoblastoma Left: dysgenetic gonad |
None |
| T-057 | Female | 6 | Behavioral problems | Normal | Unilateral ovarian mass | Bilateral dysgenetic with gonadoblastoma and dysgerminoma | None |
| P-084 | Female | 8 | Short stature, behavioral issues | Normal | Non-visualized | Right: predominant ovarian elements Left: gonadoblastoma |
None |
No male was diagnosed with a gonadal tumor during our study period. In total, 17 males had gonads remaining in situ and at least one follow-up testicular ultrasound. The frequency of testicular ultrasounds in this group was evaluated (Figure S2).
3.6 Puberty
Pubertal development was recorded for all patients who were above 14 years old and had at least one gonad preserved (Table S2). Puberty data was available on 4 out of 5 females identified and 20 males. All 4 females required puberty induction by hormone replacement therapy whereas 19/20 males had spontaneous puberty regardless of the status of the external genitalia at birth. Notably, 2/19 males required maintenance with testosterone therapy at mid-puberty. Additionally, a trend of a high follicle-stimulating hormone (FSH) level is observed in these males. Fertility data was available on one male, ascertained through the cytogenetics database, who was diagnosed at a fertility clinic due to primary infertility.
3.7 Postnatal growth
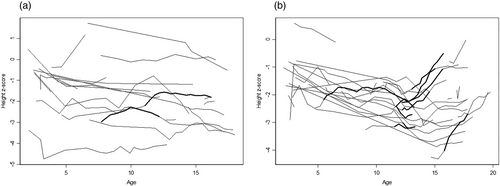
Mean Z-scores for height and 95% confidence limits at age 5, 10, and 15 were estimated using mixed model regression analysis (Table S3). The difference between the overall height Z-score in the male and female groups was −0.93 SD, demonstrating that the female group was shorter than the male group (p = 0.04). In addition, there was a reduction in the height Z-score (p = 0.02) with increasing age (Figure 4a,b and Table S3). Growth parameters prior to age 2 years were excluded due to the inaccuracy of measurements. Of note, a Z-score of −2.0 SD is equivalent to the 2.5th percentile. The growth pattern for males and females after age 2 years was plotted in Figure 4a,b.
3.8 Near-adult height
Twenty-one patients were evaluated for near-adult height, which was defined as less than 1 cm of growth per year (Table S4). The mean adult height for females was 147.95 ± 5.4 (139–157) cm and for males was 157.24 ± 10.12 (149–174.5) cm. This is equivalent to a Z-score in females and males of −2.4 and − 2.7, respectively.
3.9 GH therapy
Twenty-one patients received recombinant GH therapy at a mean age of 11.25 ± 2.8 (5.5–15.8) years, for a duration of 37 ± 24 (9–96) months (Table S5). Sixteen of 21 patients had an increase in their height Z-score following treatment, but height Z-scores were not significantly different than in untreated patients (p = 0.51).
3.10 Turner syndrome stigmata
Fifty-five of 95 (58%) patients had at least one physical finding associated with Turner syndrome other than short stature, renal anomalies, or cardiac anomalies (Figure S3). Such findings included short neck, abnormal upper:lower segment ratio, broad chest, widely spaced nipples, cubitus valgus, short 4th and 5th metacarpals, Madelung deformity, scoliosis, genu valgum, micrognathia, high arched palate, leg length discrepancy, webbed neck, low posterior hairline, posteriorly rotated ears, edema of hands and feet, hypoplastic nails, pigmented nevi, otitis media, hypothyroidism, Hashimoto thyroiditis, and alopecia.
Renal abnormalities were detected by ultrasound in 15/78 patients (19.2%); 18/61 patients (29.5%) were found to have a cardiac abnormality on echocardiography. Bicuspid aortic valve, left-sided cardiac lesions, and coarctation of the aorta were the predominant findings (Table 2).
| Renal abnormalities | Cardiac abnormalities | ||
|---|---|---|---|
| Abnormality | No. of patients | Abnormality | No. of patients |
| Duplex collecting system | 4 | Coarctation of the aorta | 4 |
| Horseshoe kidney | 7 | Bicuspid aortic valve | 6 |
| Vesicoureteral reflux | 1 | Hypoplastic left heart | 4 |
| Pelvic kidney | 1 | Dysplastic aortic valve | 1 |
| Ectopic kidney | 1 | Parachute and hypoplastic mitral valve | 1 |
| Malrotated right kidney with moderate hydronephrosis | 1 | Hypoplasia of transverse arch | 2 |
| Mitral and aortic valve stenosis | 1 | ||
| Ventricular septal defect | 2 | ||
| Ventricular tachycardia | 1 | ||
| Right coronary artery fistula | 1 | ||
- Note: Each cardiac abnormality is listed separately, and number of patients is more than 18 because some patients had more than one abnormality.
3.11 Psychological and educational issues
Psychological or educational issues were documented for 26 patients including attention deficit disorder, learning disabilities, developmental delays, and others (Table S6). In addition, 2 patients had IQ scores available; one patient had an IQ of 78 and the other had a verbal IQ of 50. The latter was also born with hypoplastic left heart syndrome, had a cardiac transplantation, and subsequent multiple strokes.
3.12 Prenatally diagnosed cases of 45,X/46,XY mosaicism
A total of 16 prenatally diagnosed patients were included in this study. These patients were diagnosed through either CVS or amniocentesis. The indication for the procedures included an increased risk for trisomy 21 on maternal serum screening in 4/16, increased nuchal thickening and pleural effusions in 2/16, late maternal age in 12/16, and a positive family history for a genetic condition in 4/16. The clinical findings of these patients are summarized in Table S7. Of note, prenatal chromosome analysis in an additional three cases revealed 45,X as the sole chromosome anomaly (P-093, P-094, P-100). However, follow-up chromosome and FISH analysis after birth showed the presence of a Y chromosome with abnormal structure in a mosaic state in these cases.
4 DISCUSSION
Our study presents the long-term follow-up of the largest published cohort of patients with 45,X/46,XY mosaicism and highlights our experience with GH therapy and prophylactic gonadectomy in both genders. It also demonstrates multiple cytogenetic abnormalities, congenital anomalies, histological characteristics, hormonal dysregulations, and suggests future management protocols.
4.1 Gonadal differentiation and tumor risk
In our study population, five females in their second decade were found to have gonadal tumors originating from dysgenetic gonads. Ultrasound failed to identify premalignant masses in their gonads except for one patient with advanced dysgerminoma.
Although the lack of virilization in females could indicate the presence of streak gonads and a low risk of developing a gonadal tumor, our study supports previous observations on the risk of gonadal tumors in females with normal genitalia (Dabrowski et al., 2020; Juul et al., 2023; Karila et al., 2022; Lindhardt Johansen et al., 2012; Tosson et al., 2012). This contradicts another observation regarding the low risk of gonadal tumors in the presence of normal genitalia of either gender (Cools et al., 2011).
None of the males under 18 years old were found to have gonadal tumors including the males with abnormal genitalia who had testicular biopsies and ultrasounds. Moreover, most of our male patients (19/20) with either normal or abnormal genitalia had spontaneous puberty. However, two males experienced gonadal failure and required testosterone therapy to complete puberty.
In addition, while LH levels were within normal limits in several male patients, the FSH levels were elevated which could suggest impaired Sertoli function and intact Leydig cells leading to limited maturation of spermatogenesis and possible infertility (Lindhardt Johansen et al., 2012; Martinerie et al., 2012).
Therefore, as no male in our study population was diagnosed with a gonadal tumor, we were unable to assess the frequency of gonadal tumors or the efficacy of tumor screening by gonadal ultrasound. However, considering the limited resolution of testicular ultrasounds in assessing gonadal histology, follow-up with annual repeat testicular ultrasounds may not be indicated in this group of patients. We continue to suggest elective gonadectomy of streak or dysgenetic gonads in males with gonadal failure who are likely infertile, and females with normal genitalia, given the limited tools for tumor screening and the combination with nonfunctional gonads in initiating puberty in the females. Pubertal development in males should be closely monitored, as some males may require maintenance with testosterone therapy. Our data also supports the published guidelines for management in this population (Cools et al., 2011).
4.2 Postnatal growth
Our study showed that patients with 45,X/46,XY mosaicism have growth deceleration in both genders throughout childhood and puberty (Pan et al., 2019; Rosa et al., 2014; Tosson et al., 2012), which has also been observed in patients with Turner syndrome (Gravholt et al., 2017). In our study, both genders had a significant reduction in the height Z-score over time but majority of males continued to stay within the normal range (>−2 SD), while majority of females were below −2 SD. Moreover, the mean adult height Z-score calculated for females and males was −2.4 and −2.7, respectively. The mean adult height Z-score was markedly reduced from the estimate of the entire population at age 15 for males (−1.32), possibly due to incomplete growth in our subgroup of males or a bias toward severely affected males within our study population.
We noted that 16/21 individuals in the GH-treated group had an improvement in their Z-score but the success rate was not as high as reported in other publications (Bertelloni et al., 2015; Huang et al., 2019; Lindhardt Johansen et al., 2012; Richter-Unruh et al., 2004). This could be influenced by non-randomization as the patients in our cohort elected to have treatment once a short stature was noted and the treated individuals were started on GH at an older age. It is also possible that those who elected to have GH therapy had more severe short stature.
Additionally, our study as well as others (Martinerie et al., 2012) did not demonstrate a difference in the height Z-scores of the GH-treated group and the non-treated group. We also noted that the efficacy of the GH therapy may have been diminished as all data from ages 2 to 18 were included for comparison, rather than specifically focusing on the peak times of GH therapy. Furthermore, the reduction in height Z-score over time may indicate that the GH therapy should start before the short stature is noted rather than in response to short stature.
4.3 Turner syndrome stigmata
A well-known overlap was established between the clinical characteristics of Turner syndrome and 45,X/46,XY mosaicism (Ayuso et al., 2008; Farrugia et al., 2013; Gantt et al., 1980; Knudtzon & Aarskog, 1987; Martinerie et al., 2012; Richter-Unruh et al., 2004; Telvi et al., 1999; Tosson et al., 2012). At least one feature of Turner syndrome was seen in 58% of our patients, after excluding short stature, renal, and cardiac abnormalities. Renal abnormalities were found in 19.2%, within the previously reported range of 16%–29% (Farrugia et al., 2013; Huang et al., 2019). The abnormalities consist of collecting system malformations, horseshoe kidney, and positional abnormalities, which are all observed in Turner syndrome at a rate of 30%–40% (Bilge et al., 2000; Bondy, 2007; Carvalho et al., 2010; Lippe et al., 1988). We also found a 29.5% incidence of cardiac anomalies in our population, which falls within the reported range of 21.5%–44% estimated for Turner syndrome (Carvalho et al., 2010; Gotzsche et al., 1994; Hou et al., 1993; Mazzanti & Cacciari, 1998; Prandstraller et al., 1999; Rainier-Pope et al., 1964; Völkl et al., 2005) and higher than the 16% estimate for other patients with 45,X/46,XY mosaicism (Farrugia et al., 2013). This could be also related to the limited number of patients in our cohort undergoing echocardiograms, estimated to be less than 50%. These findings demonstrate the prevalence of Turner syndrome stigmata in 45,X/46,XY mosaicism and the significance of early detection of these abnormalities.
The current management guidelines for Turner syndrome recommend renal ultrasound and echocardiogram at the time of diagnosis, as well as a follow-up echocardiograms (Gravholt et al., 2017). Given the high incidence of related abnormalities in our study population, it is crucial to incorporate these guidelines into the care of children with 45,X/46,XY mosaicism.
4.4 Psychological/ educational outcomes
Females with Turner syndrome usually have normal intellect, although some reported nonverbal learning disabilities and attention deficit disorder. While there is clinical overlap between the two conditions in these areas, the current study and two others (Fontenelle et al., 2004; Telvi et al., 1999) suggest a potential increased risk of developmental disorders or mild intellectual disability in patients with 45,X/46,XY mosaicism. This is not known to be a feature of Turner syndrome (Gravholt et al., 2017). However, the possibility of dual genetic diagnosis in these individuals cannot be excluded and was not investigated.
4.5 Prenatally diagnosed cases
In our study, 21 pregnancies were diagnosed prenatally incidentally due to non-fetal factors. None of the fetuses had abnormalities suggestive of Y chromosome mosaicism or rearrangement. Including findings noted in cases diagnosed prenatally provides an accurate assessment of the frequency of anomalies in 45,X/46,XY mosaicism without the bias associated with postnatally ascertained patients. Previous studies have shown that 90%–95% of prenatally diagnosed patients have a normal male phenotype; however, follow-up was limited (Chang et al., 1990; Telvi et al., 1999).
Postnatal review of the 21 patients diagnosed prenatally revealed some features seen in a postnatally diagnosed patient such as short stature, genital anomalies, and Turner syndrome stigmata; however, this was limited by inconsistent follow-up. Additionally, due to limited sampling, we were unable to investigate the frequency of the anomalies reported in a previous study (Chang et al., 1990). Notably, three patients initially diagnosed with a 45,X karyotype during prenatal analysis were found to have mosaic patterns with a structurally abnormal Y chromosome postnatally. This highlights the importance of a cytogenetic follow-up after birth in these cases. Even though these patients were all prenatally diagnosed, ascertainment bias could not be excluded as these patients continued to be followed at our center. Future studies with a larger sample size and consistent long-term follow-up are needed to provide further clarity on various manifestations including age at onset of puberty, mode of growth, and the risk and age of onset associated with developing gonadal tumors.
4.6 Study limitations
Our study is limited by its retrospective nature and the long study period, which resulted in inconsistent assessments and non-systematic examinations by various clinical teams, potentially under-representing the extent of stigmata. Second, there is an inherent bias of postnatally ascertained cases, similar to the previous studies. Although we attempted to minimize this bias by including prenatally diagnosed patients, the sample size was small. Third, having a population ascertained from large tertiary care centers, we could predict that we attracted individuals at the extremes of the phenotype, and many prenatally diagnosed males did not require the specialized care that a tertiary care center provides. Fourth, with an estimate of 90% of prenatally diagnosed cases being normal males, it is likely that there is a large population of men who remain undiagnosed therefore making any risk estimates inflated. Fifth, since our patients were ascertained mainly from a pediatric care center, our access to their medical records after transitioning to adult care was limited. Sixth, the included cohort in this study is relatively heterogeneous, despite the careful application of inclusion and exclusion criteria. This heterogeneity arises from the unique mosaic karyotypes found in each patient. This heterogeneity can make it challenging to draw precise conclusions about specific aspects of the condition, such as the frequency of certain clinical features, the effectiveness of GH therapy, or the risk of tumor development. Clinicians must consider this heterogeneity when interpreting and applying the findings from our study to individual patients. Last, despite being the largest study of its kind to date, the sample size of 100 patients is relatively small when considering the diversity of mosaic karyotypes and potential clinical presentations within the 45,X/46,XY mosaicism spectrum. This limited sample size may impact the precision of our conclusions and the ability to detect rare outcomes or associations.
In the future, it would be valuable to conduct an extensive prospective long-term follow-up of prenatally diagnosed cases to obtain accurate estimates of anomalies in this population, particularly regarding tumor risk, sexual dysfunction and infertility, short stature, and psychological/educational outcomes, as there is a strong ascertainment bias in postnatally diagnosed cases. An evidence-based study is needed to evaluate the effectiveness of the current management guidelines in regard to tumor risk. Lastly, this study has identified patients with psychological and educational issues, some of which overlap with Turner syndrome. A prospective study including psycho-educational assessment is necessary to determine the true frequency of these issues in this population and provide better guidance for early intervention programs.
4.7 Conclusions and future directions
To the best of our knowledge, this study represents the largest retrospective review and long term follow-up of patients with 45,X/46,XY mosaicism. Our findings highlight the clinical challenges associated with 45,X/46,XY mosaicism, particularly regarding the timing and choice of gonadectomy. We suggest that gonadectomy of streak or dysgenetic gonads may be appropriate in males with gonadal failure who are possibly infertile, or females with normal genitalia once diagnosis is established, given the risk of tumor development and limited screening methods. Our study provides important data, addressing knowledge gaps regarding outcomes, manifestations, associated risks and also provides recommendations regarding the follow-up of this group of individuals. Importantly, our study highlights an increased incidence of renal and cardiac abnormalities in these individuals, suggesting the inclusion of renal ultrasound and echocardiogram in the follow-up guidelines for children diagnosed with 45,X/46,XY mosaicism.
AUTHOR CONTRIBUTIONS
All listed authors meet authorship criteria, certifying active participation and individual contributions. They collectively took public responsibility for content, were involved in patient care, manuscript submission, and approved the final draft.
ACKNOWLEDGMENTS
This project was generously funded by the Rare Disease Foundation and the BC Children's Hospital Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




