A case of mosaic deletion of paternally-inherited PLAGL1 and two cases of upd(6)mat add to evidence for PLAGL1 under-expression as a cause of growth restriction
Abstract
PLAGL1 is one of a group of imprinted genes, whose altered expression causes imprinting disorders impacting growth, development, metabolism, and behavior. PLAGL1 over-expression causes transient neonatal diabetes mellitus (TNDM type 1) and, based on murine models, under-expression would be expected to cause growth restriction. However, only some reported individuals with upd(6)mat have growth restriction, giving rise to uncertainty about the role of PLAGL1 in human growth. Here we report three individuals investigated for growth restriction, two with upd(6)mat and one with a mosaic deletion of the paternally-inherited allele of PLAGL1. These cases add to evidence of its involvement in pre- and early post-natal human growth.
1 INTRODUCTION
PLAGL1 (OMIM 603044; also known as ZAC, ZAC1, or LOT1) is a zinc-finger DNA-binding protein, a transcriptional regulator with growth restriction activity (Varrault et al., 1998). Its expression was shown to be downregulated in breast and ovarian tumor cells, but could be restored through inhibition of DNA methylation (Abdollahi et al., 1999; Bilanges et al., 1999). It is expressed in multiple isoforms, and has two alternative promoters, one of which shows genomic imprinting with DNA methylation on the maternally-inherited allele and expression of the paternally-inherited allele (Arima et al., 2001; Gardner et al., 2000; Kamiya et al., 2000).
PLAGL1 is one of a group of imprinted genes whose altered expression causes imprinting disorders (Eggermann et al., 2015; Monk et al., 2019). While Plagl1-deficient mice have embryonic growth restriction, murine Plagl1 also regulates the expression of other imprinted genes, including direct transcriptional regulation of the H19-IGF2 locus and effects on expression across the imprinted gene network (Varrault et al., 2006, 2017). Furthermore, altered placental expression of PLAGL1 and the imprinted gene network influences fetal growth (Iglesias-Platas et al., 2014).
In humans, paternally-inherited deletions involving PLAGL1 are associated with growth restriction (Nowaczyk et al., 2008), but few cases have been reported and their clinical features reflect other genes involved in the deletions (e.g., Engwerda et al., 2021). Transient neonatal diabetes mellitus type 1 (TNDM1: OMIM #601410) is caused by overexpression of paternally-inherited PLAGL1, which can arise from duplication of PLAGL1, hypomethylation of the differentially methylated region (DMR) on the maternal allele, or paternal uniparental disomy of chromosome 6 (upd6pat) (Mackay et al., 2006). Aside from neonatal insulin-deficient hyperglycemia, children with TNDM1 often have severe intrauterine growth restriction (IUGR), which is unexpected for overexpression of a growth promoter, but appears to result from its expression in the human pancreas altering the development of pancreatic islets and reducing insulin secretion in late pregnancy (Arima et al., 2001).
Maternal uniparental disomy of chromosome 6 (upd(6)mat) has been identified in children with growth restriction, though it has also been detected adventitiously in people with no apparent growth disturbance in adulthood. The report of Eggermann et al. (2017) includes the first case, who came to clinical attention as an adult with renal failure, in whom early childhood was not discussed. This early case suggested that upd(6)mat had no growth phenotype.
Genomic imprinting disturbance and growth restriction are associated with maternal UPD of chromosomes 7, 11, 14, and 20; this is well established in the case of upd7mat as a cause of Silver–Russell syndrome (SRS), whose features include pre- and post-natal growth restriction. In other cases, including upd16mat and upd(6)mat, it is unclear whether IUGR results directly from the UPD, or from trisomy of the affected chromosome which persists in extrembryonic tissues but is lost in the fetus. Besides PLAGL1 there are other genomically imprinted genes on chromosome 6 (chr6), though none are currently associated with a clinical phenotype: FAM50b shows imprinted expression; IGF2R has differential methylation without imprinted expression; and LIN28B and AIM1 are imprinted in placenta (Court et al., 2014; Hanna et al., 2016; Riesewijk et al., 1996).
Here we report a child with a mosaic deletion of PLAGL1 discovered by analysis of whole genome sequence (WGS) data in the 100,000 Genomes Project (Alhendi et al., 2022), and two further cases of upd(6)mat.
2 METHODS
2.1 Editorial policies and ethical considerations
Case 1 was identified by virtue of participation in the 100,000 Genomes Project, and subsequently recruited to the research study “Imprinting disorders—finding out why” (IDFOW: approved by the Southampton and South West Hampshire Research Ethics approval 07/H0502/85). Cases 2 and 3 were also identified through participation in IDFOW.
2.2 Molecular methods
Genomic analysis and imprinting testing were performed as described (Alhendi et al., 2022).
Gene-disease association analysis was performed on genes extracted from the “TxDb.Hsapiens.UCSC.hg38.knownGene” using the GenomicRanges package in Bioconductor R (Lawrence et al., 2013). Enrichment analysis was performed using the DOSE package in Bioconductor R (Yu et al., 2015). Briefly, the enrichDGN() function was used to calculate the overpenetration enrichment score against gene-disease associations collected from several public data sources (Piñero et al., 2015). The hierarchical clustering of enriched terms was performed using the treeplot() function from the enrichplot package in Bioconductor R (https://bioconductor.org/packages/release/bioc/html/enrichplot.html). The pairwise similarities of the enriched terms were calculated using the pairwise_termsim() function, which by default utilizes Jaccard's similarity index (JC). Finally, the heatmap() function from the same Bioconductor package was used to visualize the relationship between genes and the top enriched terms.
3 CLINICAL CASE REPORTS AND MOLECULAR ANALYSIS
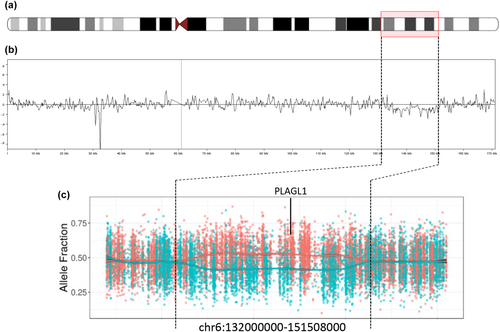
Case 1 was previously described (Alhendi et al., 2022). She was found antenatally to have short femurs and echogenic bowel, and underwent prenatal cell-free DNA analysis to exclude achondroplasia and cystic fibrosis, but no invasive prenatal testing was performed. At birth aged 37 + 1 weeks, her weight was −2.6 SDS, length −2.1 SDS, and head circumference −2.0 SDS. She was referred to clinical genetics aged 21 weeks with dysmorphism and growth restriction in the absence of feeding issues. Upon referral aged 21 weeks, her weight was −3.05 SDS, height −2.6 SDS, and OFC −2.2 SDS. Aged 13 months, her skeletal survey and development were normal. She had bilateral 5th finger clinodactyly, upturned nails on both big toes, sparse hair, mild frontal bossing, and epicanthic folds. Genetic workup included SRS testing of chr11p15.5 and upd7mat, and chromosome microarray analysis of DNA from both blood and saliva, all of which were normal. She was then recruited to the 100,000 Genomes Project. Gene panel-based diagnosis of SRS was negative, but genome sequence analysis detected a mosaic deletion involving the paternal allele of PLAGL1, and subsequent re-analysis of diagnostic chromosomal microarray data from the blood sample detected a deletion of chr6:135,183,437–149,802,477 (Hg38) affecting an estimated ~6% of cells (Figure 1). Gene-disease enrichment analysis of the genes involved in this deletion suggested that genes within the imprinted locus, PLAGL1 and HYMAI, are the main contributors to the clinical phenotypes (Figure S1).
Case 2 was conceived when her mother was 37 years of age; both parents and her elder sibling were healthy. Ultrasound scanning at 20 weeks gestation showed short femurs (below −2 SDS) and a single umbilical artery. Limb growth decelerated from 20 weeks onwards. Decreased fetal movement was noted at 35 weeks, and at 36 + 2 weeks the child was born by an emergency cesarean section for fetal distress and oligohydramnios with birth weight −3.22 SDS, and birth length and head circumference below −2.5 SDS. The placenta was described as small, with infarcts and calcification in places. Apgar scores were good (9/10/10) and breastfeeding was established while she was an inpatient for 4 weeks, but subsequent measurements identified concerns with growth and she was readmitted several times and offered additional milk supplements.
At 7.5 months, her weight was −3.7 SDS and she was diagnosed with an atrial septal defect. Investigation by chromosomal microarray showed complete loss of heterozygosity of chr6, consistent with upd(6)mat. A kidney scan was normal. She walked at 14 months but had delayed speech. Following diagnosis of growth hormone deficiency she was started on GH treatment at 2 years 2 months.
A review at 2 years 5 months her weight was −2.5 SDS, height at −2 SDS, and head circumference between −2 and −2.5 SDS. She had micrognathia, almond-shaped eyes, pectus excavatum, mild camptodactyly of the second digit of both hands, and mild radial deviation.
At 3 years, her weight and height were –2.1 SDS and –1.85 SDS, respectively. At 5 years 9 months her bone age was noted to be advanced by 1 year; GH treatment was paused at 6 years, but restarted after 6 months when growth deceleration was noted. Aged seven, she had normal IQ but required educational support for poor auditory processing, short-term working memory and executive brain function, and markers of dyslexia. On recruitment to IDFOW, targeted imprinting testing revealed gain of methylation at the PLAGL1 DMR, and microsatellite analysis confirmed upd(6)mat, being consistent with maternal isodisomy. Chromosomal microarray showed no evidence of mosaic trisomy 6, and exome sequencing revealed no pathogenic SNV likely to account for her clinical features.
Case 3 was noted at 20 week scan to have placental insufficiency, short femurs, and IUGR with reduced abdominal and head growth. She was referred to fetal medicine where notching was observed on uterine arteries. She was born at 37 + 1 weeks by emergency cesarean section with birth weight and head circumference at –2.5 SDS. After 5 days in SCBU for difficulty maintaining temperature and establishing breast feeding, she bottle-fed well and was weaned at 16 weeks. On examination at 3 years 2 months she had short stature, with weight –1.84 SDS, height –2.1 SDS and OFC +1.82 SDS, but showed good growth velocity with no evidence of asymmetry, and no developmental concerns. She was referred for diagnosis of SRS which, using MLPA analysis of chromosomes 11, 6, 7, and 14, detected gain of methylation at PLAGL1. Microsatellite analysis confirmed upd(6)mat. Postnatal chromosome analysis and microarray of mother and daughter were normal and showed no evidence of trisomy of chromosome 6; no prenatal chromosome analysis was performed.
Table 1 lists the clinical features of the Netchine–Harbison clinical scoring system (as listed in Wakeling et al., 2017) and shows that none of these three cases met diagnostic criteria for SRS.
| NH-CSS criterion | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Small for gestational age (≤–2 SDS for gestational age) | 1 | 1 | 1 |
| Relative macrocephaly at birth (head circumference ≥1.5 SDS above birth weight and/or length SDS) | 0 | 0 | 0 |
| Postnatal growth failure (height at 24 ± 1 months ≤–2 SDS or height ≤–2 SDS below mid-parental target height) | 0 | 1 | 1 |
| Feeding difficulties (BMI ≤–2 SDS at 24 months or current use of a feeding tube or cyproheptadine for appetite stimulation) | 0 (BMI at 21 weeks –1.83 SDS) | 0 (BMI at 2 years 6 months –1.84 SDS) | 0 (BMI at 3 years 2 months –0.6 SDS) |
| Protruding forehead (forehead projecting beyond the facial plane on a side view as a toddler (1–3 years)) | 0 | 0 | 0 |
| Asymmetry (leg length discrepancy [LLD] of ≥0.5 cm or arm asymmetry or LLD < 0.5 cm with at least two other asymmetrical body parts (one non-face)) | 0 | 0 | 0 |
| Total score (≥4/6 consistent with clinical diagnosis of SRS) | 1 | 2 | 2 |
4 DISCUSSION
Transient neonatal diabetes is caused by upd6pat, paternal duplication of 6q24, or hypomethylation of the PLAGL1 imprinting center. In mouse, reduced expression of PLAGL1 is associated with growth restriction (Varrault et al., 2006), but in humans an inconsistent picture is emerging from the limited reports available. In upd(6)mat cases where early growth parameters are recorded, including the cases reported here, growth failure in utero and early childhood are recurring but not universal features.
Poor growth is a non-specific feature with many underlying etiologies. Eggermann et al. (2017) propose that the features of upd(6)mat result from mosaic aneuploidy of chr6, normally confined to the placenta, that causes placental dysfunction leading to IUGR, whereas postnatal growth is normal. In reported cases where prenatal testing was performed, there was evidence of mosaic aneuploidy; in one case where mosaicism persisted to postnatal life, postnatal growth restriction was observed. No prenatal karyotype analysis was performed in these cases, but diagnostic chromosomal microarray analysis was performed on all cases presented here, and showed no evidence for (mosaic) trisomy(6) as the underlying etiology for the features of upd(6)mat. Other features of reported patients were attributed to unmasking recessive variants. Fuke et al. (2021) reported three cases of upd(6)mat in a cohort referred for diagnosis of SRS but, like the cases reported here, two of the three did not fulfill clinical criteria for SRS. Notably, Pignata et al. (2022) reported a woman with upd(6)mat and acquired isozygosity for a variant in the maternal-effect gene KHDC3L, to which was attributed the multi-locus imprinting disorder (MLID: the presence of imprinting alteration at more than one imprinted locus) in her daughter; the woman herself had no reported growth phenotype or other ill-health.
Our cases not only add to the literature, but provide observational evidence of the potential role of PLAGL1 in human growth, in an individual with mosaic deletion of the paternally-inherited allele of PLAGL1. The deletion affects both the imprinted paternally-expressed and the larger biallelically expressed isoforms, and therefore may have wider-ranging consequences than upd(6)mat which would be expected to affect only the imprinted allele. However, the patient's clinical phenotype may reflect the contribution of many other genes in her deletion; for example, TAB2, whose deletion or mutational inactivation is associated with short stature and facial features reminiscent of Case 1 (Engwerda et al., 2021).
The Decipher database lists six deletions overlapping PLAGL1; of these, we further examined three (two were not examined because they were >5 Mb in size, the number of genes involved potentially confounding their clinical interpretability; and one was established by correspondence with the contributing center to be uploaded in error). Of the three deletions considered, one of 181 kb removed both PLAGL1 isoforms (Decipher ID: 399259; chr6: 143,902,125–144,083,406, GRCh38); the patient had IUGR ex-prematurity with bilateral intraventricular hemorrhages, a VSD and a large secundum ASD. The phenotype information was provided by the patient's local clinical geneticist via personal communication. One of 17.5 kb affected only the first coding exon of PLAGL1; the only phenotype provided was increased nuchal translucency (Decipher ID: 359618; chr6: 143,947,816–143,965,332, GRCh38). The third, with a 1.45 Mb deletion, was reported to have recurrent hypoglycaemia and lipoatrophy peripheral neuropathy (Decipher Id: 359233; chr6: 143,337,585–144,784,019, GRCh38; also a chrX duplication of 430.31 kb, of unknown significance). Clinical characterization of such cases would help to clarify the significance of PLAGL1 deletion.
We also report two further cases of upd(6)mat whose prenatal histories have similarities with the child with del6: notably, midgestation growth restriction marked by short femur measurements on scan, leading to concern about achondroplasia in two of the cases. Placental insufficiency and oligohydramnios are features in common with other reported cases (Eggermann et al., 2017) and the finding of GH insensitivity is reminiscent of some cases of Prader–Willi syndrome and Temple syndrome where GH insufficiency is reported (Deal et al., 2013). While none of the individuals reported here fulfilled clinical criteria for SRS (Table 1), all three were initially clinically referred for SRS testing, illustrating how the breadth of molecular tests performed in the SRS workup can make valuable molecular diagnoses outside the current definition of SRS. A recent survey of over 16,000 imprinting disorder tests in 11 laboratories included seven cases of upd(6)mat identified among referrals for Silver–Russell syndrome testing, and suggested that upd(6)mat should be incorporated into imprinting tests for growth-restricted patients (Mackay et al., 2022). That paper made no specific recommendation about imprinting testing of PLAGL1, which is the only one of four imprinted loci on chr6 with a known role in an imprinting disorder (Monk et al., 2018), nor about testing for mosaic methylation disturbance of PLAGL1. However, taking together the numerous reports of upd(6)mat with growth restriction, and the growth restriction in this case with mosaic deletion involving PLAGL1, DNA methylation or coding changes of PLAGL1, potentially including mosaicism or hypomorphism, may warrant further consideration as a cause of growth restriction.
In conclusion, these reports add to evidence that aberrant expression at the 6q24 imprinted locus can result in growth phenotypes that are relevant to human development.
AUTHOR CONTRIBUTIONS
Ahmed S.N. Alhendi: Bioinformatic analysis and writing (including preparation of figures, writing original draft, and editing revised draft). Gabriella Gazdagh: clinical data curation, writing original draft, and reviewing and editing revised draft. Derek Lim: managing clinician of one of the reported cases, clinical data curation, reviewing and editing original draft, and reviewing and editing revised draft. Dominic McMullan: molecular data analysis and curation. Michael Wright: managing clinician of one of the reported cases, clinical data curation, reviewing and editing original draft, and reviewing and editing revised draft. I. Karen Temple: conceptualisation, funding acquisition, project direction, advising clinician of one of the reported cases, clinical data curation, and reviewing and editing original draft. Justin H. Davies: conceptualisation, funding acquisition, clinical data curation, reviewing and editing original draft, and reviewing and editing revised draft. Deborah J. G. Mackay: funding acquisition, project direction, writing original draft, and writing revised draft.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




