Substantial incidence of bladder dysfunction in patients with VACTERL association: Implications for surveillance
Abstract
VACTERL association is defined as the nonrandom co-occurrence of a minimum of three of the following six key components: Vertebral anomalies, Anal atresia, Cardiac malformations, Tracheo-Esophageal fistula, Renal anomalies, and Limb abnormalities. Patients presenting with two components may also belong in the same spectrum. Additional components have been associated with VACTERL defects, including single umbilical artery, tethered spinal cord (TSC), and genital malformations. We observed a significant proportion of patients with bladder dysfunction (often called neurogenic bladder in the medical record) when reviewing a cohort of patients with VACTERL defects at our clinical center. Our finding calls attention to bladder dysfunction as an additional VACTERL phenotypic component. The prevalence of bladder dysfunction is greatest in those with genital anomalies, anorectal malformations, sacral dysplasia, renal anomalies, and TSC. We propose that patients with two or more VACTERL malformations be monitored for symptoms of bladder dysfunction if one or more of the identified risk factors are present until the achievement of urinary continence.
1 INTRODUCTION
Initially referred to as VATER association by Quan and Smith (1972) and defined as a spectrum of associated defects, including Vertebral defects, Anal atresia, Tracheo-Esophageal fistula (TEF) with esophageal atresia, Radial and Renal dysplasia, the spectrum of anomalies was broadened to include cardiac and limb malformation and the designation VACTERL association was adopted to reflect the six key components: Vertebral defects, Anal atresia, Cardiac anomalies TEF with or without esophageal atresia, Renal anomalies, and Limb defects (most often involving the radial ray) (Khoury et al., 1983). Various extensions to the definition of VACTERL have been suggested, such as vascular, auricular, and rib anomalies (Barnes & Smith, 1978; Baumann et al., 1976). Several cases of VACTERL association with less frequent congenital anomalies have also been reported, including annular pancreas (Reutter et al., 2014) and Scimitar syndrome (Fritz et al., 2015). A single umbilical artery (SUA) has been reported in approximately 20% of cases and has also been indicated as a possible addition to the spectrum of the VATER/VACTERL association (de Jong et al., 2008; Rittler et al., 1996; Solomon et al., 2011; Temtamy & Miller, 1974). Genital anomalies have been suggested as another component of the VACTERL association by several authors (Husain et al., 2018; Solomon et al., 2011; van de Putte et al., 2020). More recently, Amelot et al. (2020) proposed adding “S” to include spinal defects as an integral part of the association as they have found a rate of 69.2% of spinal abnormalities (including short cord/caudal regression, lipoma of the filum terminale, and tethered spinal cord [TSC]) in a subset of 52 patients with VACTERL having an anorectal malformation (ARM). As there is no clear consensus on the definition of VACTERL association, the definition being used in this study is: three or more core components was designated as VACTERL association. Because the requirement for three defects is arbitrary, we also evaluated patients who had just two core components, and we termed those partial VACTERL (pVACTERL).
The incidence of the VACTERL association is approximately 1 in 10,000 to 1 in 40,000 live-born infants (Bartels et al., 2012; Botto et al., 1997; Czeizel & Ludányi, 1985; Khoury et al., 1983). The etiology of VACTERL association is unknown, and the differential diagnosis includes many conditions. Microarray analysis has shown to unveil possible pathogenic anomalies in a limited (3%–5%), yet noteworthy, fraction of patients with VACTERL association (OMIM #19235) and testing for Fanconi anemia is both cost effective and efficient, and therefore should be considered (Solomon et al., 2012). When large cohorts were evaluated, rare patients were found to have probable disease-causing copy number variants at a variety of loci (reviewed by Reutter et al., 2016; Zhang et al., 2017) or sequence variants in TRAP1, PCSK5, FOXF1, DLL3, PTEN, LPP, HOXD13, FGF8 (reviewed by Chen et al., 2016) ZIC3 (Hilger et al., 2013) or HSPA6 (Kause et al., 2019). However, for most patients, the etiology of VACTERL remains idiopathic.
Renal anomalies are reported in approximately 50%–80% of the cases; vertebral anomalies have been reported in 60%–80% of patients; ARMs, such as imperforate anus/anal atresia with or without fistula, occur in 55%–90% of patients; cardiac malformations occur in 40%–80% of patients; TEF is seen in 50%–80% of patients; genitourinary anomalies in up to 25%; and limb malformations have been reported in approximately 40%–50% of patients (Solomon et al., 2011). The presence of any VACTERL association defect typically prompts a search for the other key components.
Currently, the management of patients with VACTERL association focuses on surgical correction of the presenting congenital anomalies in the immediate postnatal period (Solomon et al., 2012), though subsequent surgical care may be required, and long-term follow-up and management may be important for certain manifestations, such as some types of gastrointestinal, renal, or genitourinary anomalies.
Lower urinary tract symptoms are common in children and can stem from a variety of etiologies and comorbidities, including dysfunctional voiding secondary to constipation, urinary tract infections, vesicoureteral reflux, neuropsychiatric diagnoses, as well as anatomic and neurologic (congenital or acquired) deficits. Strictly defined, neurogenic bladder (NB) is bladder dysfunction due to a defect in the central or peripheral nervous system (Sturm & Cheng, 2016). NB in children can have deleterious effects on the upper urinary tract and lead to renal dysfunction. Therefore, recognizing NB and promptly instituting treatment is essential to avoid significant morbidity and preserve renal function (Bauer, 2008; Wishahi, 2021). In practice (personal observation), the term NB is often used loosely to include those who have impaired voiding function without proven neuropathic bladder (such as surgically repaired cloacal malformation, where the impairment could be intrinsic to the anatomy or secondary to denervation injury).
While reviewing a cohort of patients with two or more VACTERL defects at our clinical center for an unrelated project, the casual observation was made that NB was a relatively common comorbidity. Therefore, we undertook a study to systematically investigate the frequency of NB in patients with two or more VACTERL malformations to determine the prevalence and identify specific risk factors for its occurrence.
2 METHODS
2.1 Editorial policies and ethical considerations
2.1.1 Subject ascertainment
Institutional Review Board approval was obtained for a retrospective chart review at two separate institutions (University of California San Diego/Rady Children's Hospital-San Diego [UCSD/RCHSD, IRB #200444] and Valley Children's Hospital [VCH, IRB #HSC2267]).
Cases with visits occurring between January 1, 2010 and April 25, 2021 were gathered from both centers; an additional 6-month period to October 16, 2021 was included for RCHSD. Interrogation of the respective institutional electronic medical records was done to ascertain subjects.
Since VACTERL association does not have a specific ICD-10 code, we used the codes Q87.89 (other specified congenital malformation syndrome) and Q87.2 (congenital malformation syndromes predominantly involving limbs), the latter being commonly used to identify those with VACTERL association even in the absence of a limb defect. Additionally, varying combinations of codes were used to identify subjects with only two core component malformations (see Table 1).
| Anomaly | ICD-10 code(s) | ICD-9 code(s) | Description |
|---|---|---|---|
| Vertebral anomaly | Q76.* Q79.9 |
745.2, 756.10, 756.13, 756.14, 756.15, 756.9 | Congenital malformations of the spine and bony thorax; Congenital malformation of the musculoskeletal system, unspecified |
| ARM | Q42.* | 751.1, 751.2 | Congenital absence, atresia, or stenosis of large intestine |
| EA/TEF | Q39.* | 750.3, 750.4 | Congenital malformations of esophagus |
| Renal anomaly | Q60.* Q63.* Z90.5 |
753.0, 753.3 | Renal agenesis or other reduction defects of the kidney; Other congenital malformations of kidney Acquired absence of kidney |
| Limb (upper) anomaly | Q71.4* Q71.81* Q71.89* Q69.1 Q73.8 |
755.26, 755.01, 755.4 | Longitudinal reduction defect of radius Congenital shortening of upper limb Other reduction defect of upper limb Accessory thumb Other reduction defect of unspecified limb |
- Abbreviations: ARM, anorectal malformation; EA/TEF, esophageal atresia and/or tracheoesophageal fistula.
- * denote that it includes all of the sub-codes in the series.
2.2 Inclusion/exclusion criteria
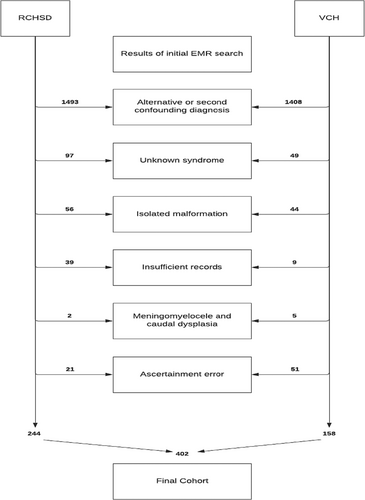
Review of 3676 resulting medical records (n = 1952 RCHSD, n = 1724 VCH) was performed to exclude the following from further consideration: subjects with an alternative or second confounding diagnosis (including teratogenic exposures, except for diabetes, which has been associated with the embryopathogenesis of VACTERL; Stevenson & Hunter, 2013); subjects with unknown syndromes (even when VACTERL features were present) such as those with growth restriction, dysmorphic features, seizures or developmental delay/intellectual disability; subjects with isolated malformations; subjects ascertained in error; and subjects with incomplete records for review. Subjects with myelomeningocele or caudal axis malformations (dysplasia/regression) having truncated spinal cords were excluded because of the known association with bowel and bladder dysfunction. A manual review of the remaining records was undertaken to catalog the presence or absence of NB; the core features of VACTERL; associated features such as genital anomalies, SUA, or TSC; and additional variables of interest, including sacral dysplasia and maternal diabetes. The final cohort consisted of 402 cases (244 RCHSD, 158 VCH) (Figure 1).
2.3 Definitions
Subjects with three or more core components (Vertebral anomalies, Anal atresia, Cardiac malformations, TEF, Renal anomalies, Limb abnormalities) were designated VACTERL, while those with only two core components were designated partial VACTERL (pVACTERL). Those subjects meeting criteria for VACTERL or pVACTERL whose records showed compelling evidence of poor maternal diabetic control in the first trimester of pregnancy were instead designated as diabetic embryopathy (IDM). Thus, the three diagnostic categories were VACTERL, pVACTERL, and IDM.
Subjects were considered to have NB if the urologist caring for the subject designated their bladder dysfunction as such and if there was supporting evidence from diagnostics (urodynamic studies) or management techniques (e.g., intermittent catheterization, appendicovesicostomy, ileovesicostomy).
2.4 Outcomes
The primary outcome was to assess the incidence of NB in patients with VACTERL association. The secondary outcome was to identify preferential associations between the key components of VACTERL and NB.
2.5 Statistical analysis
The chi-square test was used to determine possible associations between the occurrence of NB and the core features of VACTERL, as well as associated features and features of interest. A p-value less than 0.05 was considered statistically significant. For features demonstrating a statistically significant association with NB, an odds ratio (OR) of finding NB was generated for each feature and for combinations of features.
3 RESULTS
Of the 402 patients, 229 patients (57.0%) were diagnosed with VACTERL, 122 were diagnosed with pVACTERL (30.3%), and 51 (12.7%) were classified as IDM. One hundred ninety-one (47.5%) patients were female, and 210 (52.2%) were male; one patient with ambiguous genitalia died before studies were obtained to determine chromosomal sex. The prevalence of the core features, associated features, and features of interest in our cohort is displayed in Table 2.
| VACTERL, n = 229 | pVACTERL, n = 122 | IDM, n = 51 | |
|---|---|---|---|
| Vertebral anomaly | 161 (70.3%; 64.4%–76.2%) | 61 (50%; 41.1%–58.9%) | 42 (82.4%; 71.9%–92.8%) |
| ARM | 141 (61.5%; 55.3%–67.9%) | 51 (41.8%; 33.1%–50.6%) | 18 (35.3%; 22.2%–48.4%) |
| Cardiac anomaly | 142 (61.6%; 55.3%–67.9%) | 27 (22.1%; 14.8%–29.5%) | 38 (74.5%; 62.6%–86.5%) |
| EA/TEF | 91 (39.7%; 33.4%–46.1%) | 21 (17.2%; 10.5%–23.9%) | 5 (9.8%; 1.6%–18.0%) |
| Renal (urinary tract) defect | 161 (70.3%; 64.4%–76.2%) | 72 (59%; 50.3%–67.7%) | 32 (62.7%; 49.5%–76.0%) |
| Limb anomaly | 73 (31.9%; 25.8%–37.9%) | 16 (13.1%; 7.1%–19.1%) | 12a (23.5%; 11.9%–35.2%) |
| TSC | 84 (36.7%; 30.4%–42.9%) | 27 (22.1%; 14.8%–29.5%) | 16 (31.4%; 18.6%–44.1%) |
| Dysplastic sacrum | 82 (35.8%; 29.6%–42.0%) | 16 (13.1%; 7.1%–19.1%) | 13 (25.4%; 13.5%–37.5%) |
| SUA | 61 (26.6%; 20.9%–32.4%) | 19 (15.6%; 9.1%–22.0%) | 14 (27.4%; 15.2%–39.7%) |
| Genital anomaly | 76 (33.2%; 27.1%–39.3%) | 22 (18.0%; 11.2%–24.9%) | 13 (25.4%; 13.5%–37.5%) |
- Note: Percentages are based on the total number of patients, including those for whom the presence or absence of the feature could not be determined, thus represent minimum percentages.
- Abbreviations: ARM, anorectal malformation; EA/TEF, esophageal atresia and/or tracheoesophageal fistula; SUA, single umbilical artery; TSC, tethered spinal cord.
- a Including two with lower limb anomalies.
Nearly 20% of the cohort had NB (n = 75, 18.6%); the incidence of NB did not differ based on sex (15.2% males vs. 22.5% females, p = 0.15). There was no statistically significant difference in the incidence of NB based on diagnostic category (p = 0.10) (Table 3).
| Diagnostic category | Total | NB: N (percentage; 95% confidence interval) |
|---|---|---|
| VACTERL | 229 | 49 (21.4%; 16.1%–26.7%) |
| pVACTERL | 122 | 15 (12.3%; 6.5%–18.1%) |
| IDM | 51 | 11 (21.6%; 10.3%–32.9%) |
| Total | 402 | 75 (18.7%; 14.9%–22.5%) |
- Abbreviations: IDM, infant of diabetic mother, used to designate diabetic embryopathy as a distinct diagnostic category; pVACTERL, those having only two of the six core defects of VACTERL; VACTERL, Vertebral defects, Anal atresia, Cardiac anomalies Tracheo-Esophageal fistula (TEF) with or without esophageal atresia, Renal anomalies, and Limb defects.
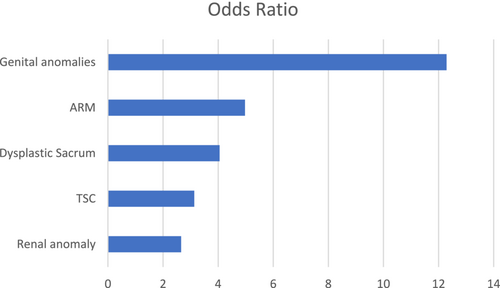
Five features (genital anomaly, anal defect, dysplastic sacrum, TSC, and renal anomaly) showed a significant association with NB (Table 4).
| Associated feature | Odds ratio | Odds ratio lower bound | Odds ratio upper bound |
|---|---|---|---|
| Genital anomaly | 12.29 | 11.42254822 | 13.15966202 |
| ARM | 4.97 | 4.359670055 | 5.589909777 |
| Dysplastic sacrum | 4.05 | 3.467707722 | 4.634856381 |
| TSC | 3.13 | 2.599320819 | 3.664142313 |
| Renal anomaly | 2.65 | 2.006472935 | 3.305364928 |
- Abbreviations: ARM, anorectal malformation; NB, neurogenic bladder; TSC, tethered spinal cord.
The OR for NB conferred by each of these features is shown in Figure 2. The presence of genital anomalies showed the highest OR, with a 12-fold risk for NB.
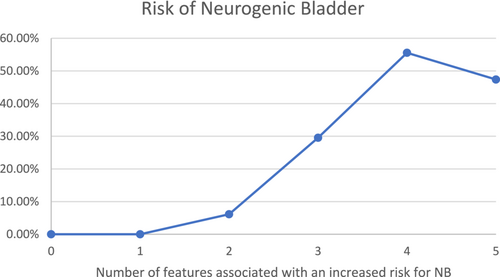
The chance of NB is significantly higher (p = 0.00045) for those patients presenting with two or more features associated with increased risk for bladder dysfunction and as high as 50% for those who have four or five of those features (Figure 3).
4 DISCUSSION
We performed a comprehensive review of a large sample of VACTERL association patients from two tertiary centers to assess the frequency of NB in this cohort and identify risk factors. We found the prevalence of the core features of VACTERL within the previously cited ranges (Amelot et al., 2020; Botto et al., 1997; Solomon et al., 2011; van de Putte et al., 2020).
Within the VACTERL cases, we found a 20% prevalence of NB. Duci et al. (2020) found a prevalence of NB of 20% in patients with complex cases of anal defects (cases of cloaca, cloacal exstrophy, urethral and bladder fistulas, and vaginal fistulas) and an overall prevalence of 12% of NB associated with any ARM. Goossens et al. (2011) also showed an incidence of 12% of urinary incontinence in patients with ARMs. The frequency we report is close to that range, with the cases of VACTERL and IDM showing a 20% incidence of NB and the pVACTERL cases showing a 12% rate of NB. It is possible that this frequency variation is related to the malformation's complexity, as suggested by Duci et al. (2020). However, our study did not aim to analyze whether the occurrence of NB is influenced by the complexity of the other VACTERL features.
Our data showed a 12-fold increased risk for NB in patients presenting with genital anomalies, a 5-fold increased risk in those with anal defects, a 4-fold increased risk with dysplastic sacrum, a 3-fold increased risk with TSC, and a 2.5-fold risk for those with renal anomalies (Figure 2). The risk of NB is statistically significantly higher for those with two or more of these risk factors by our analysis (Figure 3). These results align with the hypothesis that the more severe the embryogenic insult, the higher the possibility that multiple organs, including the bladder, are involved (Opitz, 1991). If all five of the identified risk factors are absent, the risk of NB is negligible (among the 41 patients lacking all five risk factors for whom the presence or absence of NB was known, there were no cases of NB).
It is unclear whether NB occurs secondary to a defect that disrupts innervation (such as TSC) or occurs because the bladder itself is intrinsically abnormal. We found cases of NB in the absence of TSC (30 cases of the 75), and with a variety of combinations of defects, favoring the interpretation that the bladder is primarily abnormal. The designation “neurogenic” may therefore be erroneous, as bladder dysfunction could have a muscular rather than neurogenic basis. Despite this uncertainty, we chose to use the term NB to describe the bladder dysfunction because this is the term commonly used in the medical records we reviewed.
Though genital anomalies have not been considered a core feature of VACTERL, given the significant risk of NB conferred by genital malformations, we suggest genital abnormalities should be specifically sought in all patients with two or more core VACTERL features; in females this will require diagnostic imaging of internal genitalia. Diagnosing occult renal anomalies is equally important, as these malformations may increase the risk of future morbidity such as chronic renal insufficiency, even in the absence of NB. Moreover, some studies have raised the hypothesis that patients with the VACTERL association progress to end-stage renal failure at an increased rate and present a higher incidence of complications compared with non-VACTERL patients with kidney disease and have an increased incidence of post-transplant urologic complications (Ahn et al., 2009; Diaz et al., 2019).
Akl et al. (2015) showed that NB was the cause of 25% of children with end-stage renal failure. In the study conducted by Diaz et al. (2019), 67% of a cohort of VACTERL patients that underwent renal transplants had NB. Ahn et al. (2009), in a study of children with VACTERL association and chronic kidney disease, 5 of 12 (41.6%) patients were observed to have NB. Based on the increased morbidity conferred by NB, we advocate a high index of suspicion for patients with VACTERL who have any of the five identified features associated with a higher risk of NB but especially for those with genital malformations.
In conclusion, the frequency of NB we found in a large cohort of patients with VACTERL defects is significant (18.5%), even in the pVACTERL cohort (12.3%). Our findings call attention to NB as a potential complication and possibly an additional intrinsic component of the VACTERL phenotype. Although NB occurs less frequently than other core features (in part because of how VACTERL has been defined until now), its contribution to morbidity makes surveillance for NB prudent. Therefore, we propose that patients with VACTERL, pVACTERL, and IDM, especially those with genital anomalies, should have longitudinal follow-up until the achievement of urinary continence. We recommend annual assessment of voiding pattern using a detailed voiding history to identify symptoms of bladder dysfunction, such as the questionnaire developed by the Children's National Medical Center, offered by the National Kidney Foundation (https://www.kidney.org/sites/default/files/docs/voidhist.pdf). If the questionnaire suggests voiding dysfunction or if daytime continence is not achieved by age 5 years—the age of continence in typical children according to Verkuijl et al. (2023), referral to a pediatric urologist is advised. In cases where clinical assessment is insufficient to determine the cause of voiding dysfunction, urodynamic studies should be considered.
AUTHOR CONTRIBUTIONS
Adriana Gomes: Original draft and writing, review, and editing. Lynne M. Bird: Conceptualization, data curation, data management, methodology, writing, review, and editing. Steven Swee: Formal analysis. Carolina I. Galarreta: Data acquisition, review, and editing. Riley Henderson: Data acquisition. Erin Hoyt: Data acquisition. Laura Forero Zapata: Data acquisition. All authors revised the manuscript for important intellectual content, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
The authors have no disclosures relevant to the subject matter of this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




