Craniosynostosis in molecularly diagnosed Kabuki syndrome: Prevalence and clinical implications
Abstract
Kabuki syndrome (KS) is characterized by growth impairment, psychomotor delay, congenital heart disease, and distinctive facial features. KMT2D and KDM6A have been identified as the causative genes of KS. Craniosynostosis (CS) has been reported in individuals with KS; however, its prevalence and clinical implications remain unclear. In this retrospective study, we investigated the occurrence of CS in individuals with genetically diagnosed KS and examined its clinical significance. Among 42 individuals with genetically diagnosed KS, 21 (50%) exhibited CS, with 10 individuals requiring cranioplasty. No significant differences were observed based on sex, causative gene, and molecular consequence among individuals with KS who exhibited CS. Both individuals who underwent evaluation with three-dimensional computed tomography (3DCT) and those who required surgery tended to exhibit cranial dysmorphology. Notably, in several individuals, CS was diagnosed before KS, suggesting that CS could be one of the clinical features by which clinicians can diagnose KS. This study highlights that CS is one of the noteworthy complications in KS, emphasizing the importance of monitoring cranial deformities in the health management of individuals with KS. The findings suggest that in individuals where CS is a concern, conducting 3DCT evaluations for CS and digital impressions are crucial.
1 INTRODUCTION
Kabuki syndrome (KS [MIM: 147920 and 300867]), first described in 1981, is a clinically recognizable syndrome, which is characterized by growth failure, psychomotor delay, congenital heart disease, and other complications, as well as the dysmorphic features (long palpebral fissures with eversion of the lateral third of the lower eyelid; arched and broad eyebrows with the lateral third displaying notching or sparseness; large, prominent, or cupped ears; and short columella with depressed nasal tip) that are responsible for its name (Kuroki et al., 1981; Niikawa et al., 1981; Adam et al., 2011). In 2010, heterozygous pathogenic variants of KMT2D (NM_003482.3) were reported as the causative genes of KS, and KDM6A (NM_21140.4) was identified as the second gene, the pathogenicity of which can lead to the features of KS. Although not all pathogenic variants leading to the phenotype of KS have been detected, molecular genetic confirmation is considered the gold standard for diagnosis (the analytical sensitivity of sequence analysis is approximately 99%; Adam et al., 2019).
KS is associated with various phenotypes and a wide range of complications (Adam et al., 2019; Adam et al., 2022; Niikawa et al., 1988). Although KS is recognized by its characteristic clinical findings, clinical management based on a confirmed molecular diagnosis of KS is highly critical.
In the context of KS, the prevalence of craniosynostosis (CS) as a co-occurrence was previously reported to be around 6% prior to the identification of causal genetic variants (Armstrong et al., 2005). Cases with pathogenic variants in KMT2D associated with CS have only been reported in individuals with concomitant 10q22.3q23.1 deletions (Topa et al., 2017). Therefore, CS has not been regarded as a major complication of KS, and the assessment of CS has not been considered crucial in clinical management (Adam et al., 2022).
In this study, we retrospectively assessed the occurrence of CS in individuals with KS confirmed by molecular diagnosis.
2 MATERIALS AND METHODS
2.1 Study population
Individuals with KS (including clinical diagnosis) who visited the Department of Medical Genetics at the Osaka Women's and Children's Hospital (OWCH) between January 2017 and June 2023 and whose genetic testing revealed pathological variant of KMT2D or KDM6A were included. Details of the genetic test results, evaluation of CS using three-dimensional computed tomography (3DCT), clinical findings related to CS (microcephaly at birth, microcephaly, relative microcephaly, poor occipitofrontal circumference [OFC] growth, cranial dysmorphology, funduscopic findings, and irritability), diagnosis of CS, and cranioplasty for CS were investigated and reviewed retrospectively from their medical records.
2.2 Editorial policies and ethical considerations
This study focused on retrospective analysis of complications and therefore was exempted from the requirement of informed consent at the facility. Public awareness about the study was ensured through the hospital bulletin board and website. The genetic analysis conducted as part of the research was approved by the institutional ethics committee (approval no. 665). The parents of the patients provided written informed consent for genetic analysis.
2.3 Genetic analysis
For each individual, cytogenetic testing was performed using G-banding analysis to confirm a normal karyotype. Molecular genetic testing for KS has been conducted since 2013 using conventional methods and/or targeted resequencing, and whole-exome sequencing (Miyake, Koshimizu, et al., 2013). At OWCH, since 2016, we have been designing and analyzing a custom panel containing the causative genes of KS using an Ion Torrent PGM™ system (Thermo Fisher Scientific, MA), and the candidate disease-causing variants have been confirmed by Sanger sequencing using Applied Biosystems 3130 DNA Analyzer (Thermo Fisher Scientific, MA). In 2020, the Ministry of Health, Labour, and Welfare of Japan approved genetic analysis for KS to be included in social health insurance coverage. Subsequently, genetic analysis using next-generation sequencing has been performed at a clinical testing company (Kazusa DNA Research Institute, Kisarazu, Japan) (Fujiki et al., 2018).
2.4 Statistical analysis
Quantitative data are presented as median (range), and categorical variables as numbers and proportions. The chi-square tests were used to examine the differences in the occurrence of CS among individuals with KS based on sex, causative genes (KMT2D or KDM6A), and molecular sequences. In addition, we examined the presence of microcephaly at birth, microcephaly, relative microcephaly, OFC growth defects, cranial dysmorphology, fundus findings, and irritability in individuals with and without 3DCT evaluation of CS, and in individuals with and without CS requiring cranioplasty. Statistical significance was defined as p < 0.05. These analyses were performed using IBM SPSS Statistics version 18.0 (IBM, Armonk, NY).
3 RESULTS
Fifty individuals with KS (including those with clinical diagnosis only) visited the outpatient clinic of the Department of Medical Genetics at OWCH during the study period. All of them presented with typical clinical features of KS, including a distinctive facial appearance and psychomotor delay. Molecular genetic testing was performed on 43 individuals. Variants were evaluated according to the American College of Medical Genetics and Genomics/Association of Molecular Pathology (ACMG/AMP) guidelines (Richards et al., 2015), and only those classified as pathogenic or likely pathogenic were considered as confirmed cases by molecular genetic diagnosis. A variant of unknown significance in KDM6A identified in one individual was subsequently excluded based on further studies.
Out of the 42 individuals molecularly diagnosed with KS, 30 individuals (71.4%) underwent cranial 3DCT evaluation. Among them, 21 individuals (50% of those molecularly diagnosed with KS) exhibited CS, and 10 individuals (23.8% of those molecularly diagnosed with KS) required cranial reconstructive surgery (Figure 1).

We performed 3DCT on individuals with KS who were clinically suspected of having concomitant CS due to cranial dysmorphologies, such as dolichocephaly or plagiocephaly, microcephaly, poor head circumference growth, and significant psychomotor delay, which could not be explained solely based on the diagnosis of KS (details of individuals requiring cranioplasty are shown in Table 2). In some cases (Individuals 1, 4, and 7), CT scans were conducted as part of ophthalmic screening, investigation of head trauma, or evaluation of orbital deformities, leading to the identification of CS. At OWCH, 3DCT scans were interpreted by pediatric radiologists, while CS was diagnosed by neurosurgeons. Individuals diagnosed with CS were monitored by neurosurgeons, who assessed the degree of cerebral compression and determined the necessity of surgical intervention. The extent of cerebral compression was mainly assessed from digital impression findings on 3DCT, and in cases where assessment was challenging, intracranial pressure (ICP) sensors were utilized. While the presence of papilledema in the optic fundus examination was significant, it can often be challenging to examine in infants and children. In individuals with pronounced cranial deformities, cranioplasty was performed during a suitable period for corrective measures.
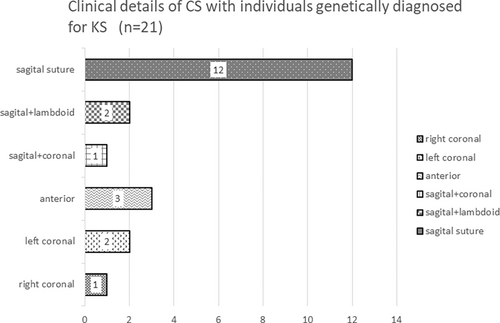
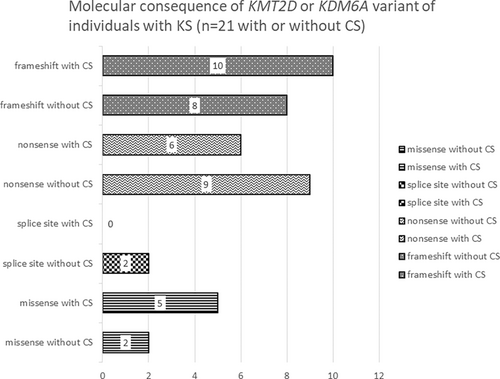
Table 1 presents detailed information of the 42 individuals, including age, sex, implicated genes, molecular findings, specifics of CS, age at initial consultation, age at KS diagnosis, age at CS diagnosis, age at CS surgery, and findings associated with suspected CS or elevated ICP. The 42 patients included 20 males and 22 females, with ages ranging from 2 years and 4 months to 32 years and 6 months (median age, 10 years 4 months). We identified a heterozygous pathological variant of KMT2D in 41 individuals and a heterozygous pathological variant of KDM6A in one individual (Table 1). The molecular consequence of the 42 variants included 18 frameshift variants, 16 nonsense variants, 2 splice site variants, and 7 missense variants. Table S1 shows the results of the variants evaluated according to the ACMG/AMP guidelines (Richards et al., 2015), some of which have been previously reported (Individuals 8, 20, 37, 40, and 42; Miyake, Koshimizu, et al., 2013; Miyake, Mizuno, et al., 2013). Of the 42 individuals, 30 (15 males and 15 females) underwent 3DCT for cranial evaluation, with 21 (50%; 12 males and 9 females) being diagnosed with CS, and among these, 10 (23.8%) required cranioplasty. Conversely, seven individuals (one male and six females) were confirmed to have no CS based on the 3DCT evaluation. Details of individuals requiring cranioplasty, reasons for CS diagnosis, findings leading to cranioplasty, and outcomes are presented in Table 2. Among these, the 3DCT images of Individuals 1, 3, 4, 5, 7, 8, and 10 are included in Figure 2. The details of CS and the number of cases per cranial suture are shown in Figure 3. Sagittal suture CS was the most common, followed by anterior suture, coronal suture, and lambdoid suture CS. Figure 4 presents the molecular consequences of the variants in individuals with KS, diagnosed using molecular genetics, with and without CS.
| Individual | Age | Sex | Gene | Details of CS | Age | Findings associated with suspected CS or elevated intracranial pressure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic analysis of KS | Initial visit | KS diagnosis | CS diagnosis (evaluation) | CS surgery | Microcephaly at birth | Microcephaly | Relative microcephaly | Poor growth of OFC | Cranial dysmorphology | Funduscopic findings | Irritability | ||||||
| 1 | 4 years 0 month | F | KMT2D | c.13320delA | p.Ile4440fs | LC, DI | 0 year 0 month | 0 year 4 months | 1 year 9 months | 1 year 10 months | − | − | − | + | + (P) | + | + |
| 2 | 7 years 7 months | M | KMT2D | c.15953_15956del | p.Leu5318Serfs*14 | A | 5 years 3 months | 5 years 5 months | 3 years 0 montha | 3 years 2 mmonths | − | − | − | + | + | NA | NA |
| 3 | 8 years 6 months | M | KMT2D | c.4135_4136del | p.Met1379Valfs*52 | S, DI | 0 year 0 month | 0 year 6 months | 4 years 1 month | 5 years 7 months | − | − | − | + | + (D) | NA | − |
| 4 | 8 years 7 months | F | KMT2D | c.9739delC | p.Leu3247fs | S, DI | 0 year 0 month | 2 years 5 months | 2 years 0 montha | 2 years 7 months | − | − | + | + | − | − | + |
| 5 | 9 years 8 months | F | KMT2D | c.12700_12701del | p.Gln4235Glyfs*98 | S, DI | 0 year 6 months | 0 year 11 months | 3 years 4 months | 3 years 5 months | − | − | − | − | + (D) | − | − |
| 6 | 11 years 0 months | M | KMT2D | c.15548 T > C | p.Leu5183Pro | A | 1 year 6 months | 1 year 10 months | 2 years 6 months | 3 years 3 months | − | − | + | − | + | − | − |
| 7 | 13 years 0 months | F | KMT2D | c.15310 T > C | p.Cys5104Arg | S, DI | 0 year 0 month | 3 years 0 month | 5 years 5 months | 5 years 6 months | + | + | + | + | + (D) | NAb | NA |
| 8 | 13 years 0 months | M | KMT2D | c.11722C > T | p.Gln3908* | LC, DI | 2 years 0 month | 2 years 4 months | 2 years 9 months | 2 years 11 months | − | − | − | + | + (P) | NA | − |
| 9 | 11 years 3 months | M | KMT2D | c.15953_15956del | p.Leu5318Serfs*14 | S, DI | 3 years 4 months | 4 years 3 months | 6 years 1 month | 6 years 11 months | + | − | − | − | + (D) | NA | − |
| 10 | 7 years 3 months | F | KMT2D | c.6334delG | p.Ala2112fs | RC | 0 year 1 months | 1 year 0 month | 0 year 2 monthsa | 0 year 2 months | − | − | − | + | + (P) | NA | − |
| 11 | 5 years 9 months | M | KMT2D | c.8974G > T | p.Glu2992* | A, DI | 0 year 10 months | 1 year 11 months | 0 year 8 monthsa | − | − | − | + | + | NA | + | |
| 12 | 8 years 0 months | F | KMT2D | c.7162dupC | p.Gln2388fs | S | 0 year 0 month | 0 year 4 months | 2 years 0 months | − | + | + | + | + | NA | + | |
| 13 | 9 years 6 months | F | KMT2D | c.1029C > A | p.Tyr343* | S, DI | 0 year 9 months | 1 year 1 month | 4 years 1 month | − | − | − | − | + (D) | − | − | |
| 14 | 10 years 4 months | F | KMT2D | c.6114G > A | p.Trp2038* | S, DI | 2 years 1 months | 2 years 1 month | 3 years 4 months | − | − | − | − | + (D) | NA | − | |
| 15 | 10 years 8 months | M | KMT2D | c.13606C > T | p.Arg4536* | S, C, DI | 5 years 5 months | 5 years 10 months | 5 years 6 monthsa | − | − | − | + | + (D) | − | − | |
| 16 | 10 years 10 months | M | KMT2D | c.8059C > T | p.Arg2687* | S, L, DI | 0 year 11 months | 1 year 11 months | 6 years 9 months | − | − | − | − | + (D) | NA | − | |
| 17 | 13 years 5 months | M | KMT2D | c.15536G > A | p.Arg5179His | S | 3 years 4 months | 12 years 11 months | 12 years 11 months | − | − | − | + | + (D) | NA | − | |
| 18 | 14 years 1 months | F | KMT2D | c.13948delG | p.Glu4650Serfs*16 | S | 5 years 6 months | 5 years 9 months | 7 years 8 months | − | − | − | + | + (D) | NA | − | |
| 19 | 7 years 7 months | M | KMT2D | c.15673C > T | p.Arg5225Cys | S, DI | 4 years 0 months | 4 years 2 months | 5 years 5 months | − | − | − | + | + (D) | NA | − | |
| 20 | 22 years 5 months | M | KMT2D | c.4127 T > G | p.Met1376Arg | S | 0 year 4 months | 12 years 5 months | 3 years 10 monthsa | NA | NA | NA | NA | NA | NA | − | |
| 21 | 8 years 5 months | M | KMT2D | c.5384del | p.Gly1795Alafs*7 | S, L | 0 year 9 months | 1 y 4 months | 8 years 2 months | − | − | − | + | + (D) | NA | − | |
| 22 | 8 years 4 months | M | KMT2D | c.1010C > A mosaic | p.Ser337* | Only DI | 4 year 0 month | 5 years 8 months | 6 years 0 months | − | + | + | + | + (P) | NA | + | |
| 23 | 10 years 4 months | M | KMT2D | c.15289C > T | p.Arg5097* | Only DI | 0 year 2 months | 0 year 7 months | 3 years 11 months | − | + | − | + | + (D) | − | + | |
| 24 | 2 years 4 months | F | KMT2D | c.8005_8018delinsTTCT | p.Met2669Phefs*19 | − | 0 year 0 months | 0 year 11 months | 2 years 11 months | − | + | + | + | − | − | − | |
| 25 | 5 years 9 months | F | KMT2D | c.9857delT | p.Leu3286Argfs*44 | − | 0 year 4 months | 0 year 8 months | 4 years 3 months | − | + | − | + | + (D) | − | + | |
| 26 | 2 years 4 months | M | KMT2D | c.14644-1_14644delinsAT | − | 0 year 2 months | 0 year 5 months | 0 year 11 months | − | + | + | + | + | NA | + | ||
| 27 | 7 years 5 months | F | KMT2D | c.16390A > C | p.Thr5464Pro | − | 0 year 9 months | 1 year 3 months | 1 year 5 months | − | + | + | + | − | − | + | |
| 28 | 10 years 4 months | F | KMT2D | c.5707C > T | p.Arg1903* | − | 0 year 0 month | 0 year 7 month | 3 years 4 months | − | + | + | + | − | − | + | |
| 29 | 7 years 8 months | F | KMT2D | c.8727_8730delAAGT | p.Ser2910fs | − | 0 year 0 month | 0 year 6 month | 7 years 8 months | − | + | + | + | − | NA | − | |
| 30 | 11 years 8 months | F | KMT2D | c.2795delC | p.Pro932Glnfs*26 | − | 0 year 0 month | 1 year 3 months | 11 years 7 months | − | + | − | + | − | − | − | |
| 31 | 8 years 10 months | M | KMT2D | c.5898del | p.Gly1967Valfs*80 | NA | 2 years 7 months | ~2 years 7 months | − | + | + | + | − | NA | + | ||
| 32 | 5 years 9 months | F | KMT2D | c.12466C > T | p.Gln4156* | NA | 0 year 9 months | 1 year 7 months | − | − | + | + | NA | NA | + | ||
| 33 | 8 years 1 months | M | KMT2D | c.9226delG | p.Ala3076Leufs*43 | NA | 0 year 6 months | ~0 year 6 months | − | + | − | + | NA | NA | + | ||
| 34 | 11 years 0 months | F | KMT2D | c.14549delC | p.Pro4850fs | NA | 5 years 0 month | 4 years 11 months | − | − | − | + | − | − | − | ||
| 35 | 12 years 2 months | F | KMT2D | c.15088C > T | p.Arg5030Cys | NA | 0 year 3 months | 1 year 5 months | − | − | − | − | − | − | − | ||
| 36 | 13 years 10 months | F | KMT2D | c.15784 + 4A > C | NA | 5 years 6 months | 7 years 8 months | + | − | + | − | − | − | − | |||
| 37 | 14 years 10 months | M | KMT2D | c.11944C > T | p.Arg3982* | NA | 2 years 11 months | 3 years 4 months | − | + | − | + | − | − | − | ||
| 38 | 16 years 6 months | M | KMT2D | c.3862A > T | p.Lys1288* | NA | 2 years 2 months | 7 years 0 months | − | − | − | − | − | − | − | ||
| 39 | 20 years 1 months | F | KMT2D | c.11386C > T | p.Gin3796* | NA | 18 years 2 months | 18 years 4 months | − | − | − | − | NA | NA | − | ||
| 40 | 23 years 6 months | F | KMT2D | c.11917C > T | p.Gln3973* | NA | 1 year 3 months | 11 years 6 months | − | − | − | + | − | − | − | ||
| 41 | 25 years 2 months | F | KMT2D | c.1215delA | p.Gln405Hisfs*15 | NA | 1 year 1 month | 14 years 6 months | + | − | − | − | − | − | − | ||
| 42 | 32 years 6 months | M | KDM6A | c.1555C > T | p.Arg519* | NA | 4 years 6 months | 21 years 5m | − | + | − | NA | − | NA | + | ||
- Note: This table provides concise details of the 42 individuals, including age, sex, genes involved, molecular findings, molecular results, presence of CS, age at initial visit, age at KS diagnosis, age at CS diagnosis, and age at CS surgery.
- Abbreviations: A, anterior craniosynostosis; CS, craniosynostosis; DI, digital impression; D, dolochocephaly; KS, Kabuki syndrome; L, lambdoid craniosynostosis; LC, left coronal craniosynostosis; NA, not evaluated; OFC, occipitofrontal circumference; P, plagiocephaly; RC, right coronal craniosysnostosis; S, sagittal craniosynostosis.
- a Individuals with craniosynostosis diagnosis preceding KS diagnosis.
- b Difficult to evaluate due to ocular complications.
| Individual | Reasons for diagnosis of CS | Findings leading to cranioplasty | Outcomes with cranioplasty |
|---|---|---|---|
| 1 | During the ophthalmic screening for complications of Kabuki syndrome, the fundus examination revealed papilledema, raising suspicion of elevated ICP (intracranial pressure). | The decision for surgical intervention by the neurosurgeon was based on the presence of cranial deformity extending to the frontal region as observed through digital impression, the presence of papilledema indicating elevated ICP, and signs of irritability even though the individual could not verbalize headache complaints. | The cranial digital impressions have improved, and neurological development is progressing well. |
| 2 | Early closure of the large fontanelle was observed during infancy, and as part of the follow-up, plagiocephaly developed. Additionally, poor occipitofrontal circumference (OFC) growth was noted, prompting attention. | Individual 2, after being diagnosed with CS through 3DCT, underwent ICP measurement using an ICP sensor, and surgical intervention was indicated. | The DQ value was not assessed preoperatively, but postoperatively at the age of 4 years, the individual exhibited a DQ of 47, which remained relatively stable at 46 at the age of 5 years, showing consistent growth. Most importantly, irritability and sensory hypersensitivity were alleviated. |
| 3 | Cranial dysmorphology (dolichocephaly), poor OFC growth, and profound developmental delay (nonverbal and unable to walk independently at 4 years old). | At the age of 3 years, Individual 3 was diagnosed with sagittal suture craniosynostosis. One year later, an exacerbation of digital impression was observed. At the age of 4 years, he has not acquired walking or speech abilities, and there is also a stagnation in OFC growth. | Individual 3 could walk without assistance, speak one word, and displayed considerable improvement in psychomotor retardation, which was previously severe a few months after the surgery. |
| 4 | During cranial investigation following a head injury, a CT scan revealed sagittal and metopic CS with prominent digital impressions. | Noting the significant digital impression, Individual 4 had inadequate ability to express headache complaints. Signs such as facial grimacing and irritability were present, raising expectations for symptom improvement through surgery. | After surgery, the digital impression improved, and the irritability seen in Individual 4 subsided. Within a year post-surgery, she developed acute encephalopathy due to a viral infection, which has made it challenging to assess the effects of the surgery on psychomotor delay due to its impact. |
| 5 | Cranial dysmorphology (dolichocephaly), poor OFC growth, relative microcephaly, and narrow forehead were observed, prompting the performance of a 3DCT to investigate suspected scaphocephaly. | The poor growth of OFC and the progression of digital impression to the frontal region raised suspicion of elevated ICP. | Prior to surgery, Individual 5 exhibited mild psychomotor delay with DQ of 69. However, post-surgery, there has been an improvement to DQ 76, moving toward the borderline range. |
| 7 | As part of the evaluation for plastic surgery regarding orbital deformation, a head CT scan was performed, raising suspicion of the presence of CS. | Individual 7 had not experienced significant OFC growth over a span of 2 years, from the age of 3 years, remaining at 45.8 cm (−2.8 SD). The presence of marked microcephaly and dolichocephaly, along with severe developmental delays, prompted the surgical intervention. ICP measurement through an ICP sensor was not conducted due to the family's wishes. | The cessation of OFC growth in Individual 7 has improved. Prior to surgery, her DQ was 34, and her psychomotor delay remained severe post-surgery with a DQ of 27. |
| 8 | Cranial dysmorphology (anterior plagiocephaly), poor OFC growth and narrowing of the lateral aspects of the head were observed, prompting the performance of a 3DCT to investigate suspected scaphocephaly. | Individual 8 exhibited poor OFC growth, observed plagiocephaly, and had pronounced left–right asymmetry in digital impression. Consequently, surgical intervention was performed for CS involving the left coronal suture. | Individual 8 exhibited moderate psychomotor delay with a preoperative DQ of 46. Post-surgery, their DQ remained stable at 46. However, there has been an increase in activity levels, and he has started speaking more fluently after the surgery. |
| 9 | Observation of cranial dysmorphology (dolichocephaly), suggesting the possibility of CS. | Individual 9 had a DQ of 90 without intellectual disability and did not report headaches. However, during the placement of an ICP sensor and subsequent measurement, elevated ICP was confirmed, leading to the decision for surgical intervention. | While Individual 9 did not exhibit intellectual disabilities prior to surgery, post-surgery, his intellectual development has been progressing well. He has been living without complaints such as headaches. |
| 10 | Observing cranial dysmorphology (plagiocephaly), suggesting the possibility of CS. | Individual 10 exhibited congenital plagiocephaly and had limited OFC growth over the course of 1 month following the diagnosis of CS. | Post-surgery, there is evident steady growth in OFC. Individual 10 contracted acute encephalopathy of unknown cause at the age of 1 year, followed by the onset of epilepsy. While the DQ value remains unassessed, severe psychomotor delay is observed. |
- Note: This table details the reasons and triggers that led to the diagnosis of CS, the reasons for surgery, and their outcomes for the KS individuals who required cranioplasty (not including Individual 6, for whom surgery was performed at another facility and detailed information was not available).
- Abbreviation: CS, craniosynostosis; CT, computed tomography; 3D, three dimensional; DQ, developmental quotient; ICP, intracranial pressure; OFC, occipitofrontal circumference.
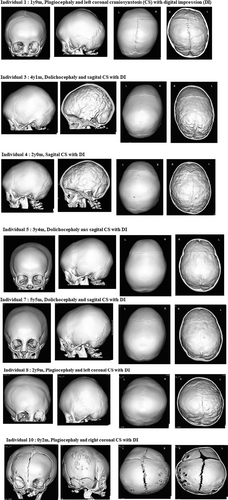
The median age at which the 42 individuals were diagnosed with KS through molecular genetic testing was 2 years and 1 month (range, 4 months to 21 years and 5 months). The median age during CS diagnosis in the 21 individuals with KS and CS was 3 years and 10 months (range, 2 months to 12 years and 11 months). The median age at which 10 individuals underwent cranioplasty was 2 years and 3 months (range, 2 months to 6 years and 11 months). No significant differences were observed in terms of sex, causative gene, and the type of molecular consequence in relation to CS complications among individuals with molecularly confirmed KS (p-values were 0.217, 0.5, and 0.25, respectively, with p > 0.05). Regarding clinical findings associated with CS, among individuals evaluated through 3DCT, a significant prevalence of cranial dysmorphology was observed. Similarly, among individuals requiring cranioplasty, a significant tendency toward cranial dysmorphology was noted. Notably, individuals requiring cranioplasty exhibited a trend of not having microcephaly.
4 DISCUSSION
In this study, among 42 individuals with genetically diagnosed KS, CS was observed in 21 cases (50%), with 10 of them requiring cranioplasty. This suggests that CS is a significant complication in individuals with KS. Furthermore, the reasons for conducting cranial evaluation through 3DCT varied, including findings from optic fundus examinations, cranial morphology and poor OFC growth, and profound psychomotor delay (Tables 1 and 2, Table S2). However, particularly, when cranial dysmorphologies, such as dolichocephaly or plagiocephaly, are present, it is suggested that performing 3DCT to diagnose CS requiring cranioplasty is desirable even in the absence of microcephaly. The location of CS varied; however, more than half of the cases involved the sagittal sutures (Figure 3). All 21 individuals possessed pathogenic variants of KMT2D, with frameshift variants being the most prevalent (p = 0.002; Table 1, Table S1). Conversely, there was no significant difference in the distribution of molecular consequences between individuals with CS and those without CS (Figure 4). Due to the retrospective nature of the study, it was challenging to extract detailed information about the reasons for suspecting CS and the rationale for performing 3DCT or undergoing surgery at the time for each individual. To investigate clinical indicators associated with the diagnosis of CS, we reviewed the data of OFC at birth and during CS evaluation for all 42 individuals (Table S2). However, observations such as the presence of microcephaly at birth, poor OFC growth, and the presence of microcephaly at CS evaluation did not lead to specific factors. These observations were consistent in both individuals diagnosed with CS but not requiring cranioplasty and those who underwent 3DCT without a CS diagnosis. Hence, these factors were considered non-decisive in determining the necessity of performing 3DCT. In other words, the absence of microcephaly or poor OFC growth does not mean that we should necessarily suspect CS in individuals with KS. The significant observation of cranial deformities in individuals requiring cranioplasty is noteworthy, highlighting the importance of assessing cranial morphology in the health management of individuals with KS. Furthermore, it was observed that individuals requiring cranioplasty typically did not exhibit microcephaly. In this regard, we emphasize the need to recognize that individuals with conditions such as dolichocephaly, even in the context of CS, may not necessarily have microcephaly.
We evaluated the median ages of KS diagnosis, CS diagnosis, and cranioplasty implementation. However, the median age at diagnosis might be influenced by the inclusion of individuals born before 2010 or 2012 despite the identification of causative genes for KS in these years (such as individuals who have been clinically diagnosed with KS and have been receiving medical care for several years). If limited to those born after the two causative genes were identified, the median age of diagnosis of KS would be much lower. Considering that the median age for cranioplasty implementation in this study was 3 years and 3 months (ranging from 2 months to 6 years and 11 months), it is crucial to pay special attention to cranial morphology and other factors during thorough examinations, particularly until around the age of 7 years for individuals diagnosed with KS. Furthermore, in individuals with suspected CS, particularly those cases requiring cranioplasty, the utilization of 3DCT for accurate diagnosis of CS is considered essential in the health management of individuals with KS. Interestingly, in one individual (Individual 2), the KS was diagnosed after the implementation of cranioplasty, while in five individuals (Individuals 4, 10, 11, 15, and 20), CS diagnosis preceded KS diagnosis. These observations indicate that CS, along with a characteristic facial appearance, can be an important clinical feature contribution to the diagnosis of KS. In this study, 12 individuals did not undergo CS evaluation with 3DCT. For these individuals, it is speculated that they encompass not only those who clinically were not suspected of CS but also those for whom CS evaluation with 3DCT was considered due to significant poor growth of OFC, but cooperation for the examination might have been challenging. Individuals with KS may experience challenges when undergoing imaging examinations, such as 3DCT, or optic fundus examinations without sedation or under sedation, due to the presence of intellectual disabilities, autism spectrum disorder, and hypersensitivity. Therefore, in the health management of individuals with KS, further investigation is crucial for determining the rationale, timing, and methods of imaging examinations, such as 3DCT, for diagnosing CS, and for assessing the frequency and approaches of assessments to consider surgical indications.
CS occurs in approximately 1 of 2500 infants, and genetic factors such as chromosomal abnormalities and pathogenic variants in fibroblast growth factor receptor families are believed to be involved in 15%–30% of cases (Boulet et al., 2008; Cohen Jr, 2000; Zollino et al., 2017). Among syndromic CS, an association between CS and RASopathy caused by the pathogenic variants of KRAS, BRAF, and PTPN11 has been reported (Ueda et al., 2017). However, for chromatinopathy, the frequency of CS is typically low (Zollino et al., 2017). To the identification of the genetic causes of KS, the prevalence of CS in KS has been reported to be approximately 6% (Armstrong et al., 2005).
The mechanisms by which CS occurs in individuals with KS are not fully understood. In a study on KS cases with CS, cranial fusion in KS was typically mild and involved the metopic suture (Martínez-Lage et al., 2010), and CS in KS was hypothesized to be related abnormalities in the development of neural crest cells, which are the precursors of the craniofacial bones. A case of KS with symptoms resembling those of C-trigonocephaly syndrome has been reported, and the possibility of overlapping genetic and developmental mechanisms in both conditions has been suggested, as both involved abnormalities in the midline structures of the face and skull (Geneviève et al., 2004). An individual with KS has been identified with concomitant KMT2D variants and a 10q22.3q23.1 deletion; the patient exhibited CS involving multiple skull sutures, which was probably owing to the fact that CS in KS may be associated with pathogenic variants of KMT2D, given the involvement of KMT2D in the development of craniofacial bones (Topa et al., 2017). Furthermore, Dong et al. (2019) investigated the expression pattern of KMT2D in murine craniofacial tissues and discovered that KMT2D was highly expressed in the developing cranial sutures. They suggested that KMT2D might play a role in regulating osteoblast differentiation and proliferation in the cranial sutures, and that mutations in KMT2D could disrupt these processes and contribute to CS.
As a limitation, this was a retrospective study using medical records, and although we found a higher incidence of CS complications in individuals with molecularly diagnosed KS, the specific mechanism by which pathogenic variants of KMT2D cause CS remains unclear. Additionally, we were unable to evaluate the association of KDM6A with CS due to the inclusion of only one individual in the study. It is anticipated that the underlying mechanism by which pathogenic variants of KMT2D causes CS will be elucidated and the association between KDM6A and CS will be examined in the future.
5 CONCLUSION
In summary, our study showed that CS is a relatively common complication in individuals with molecularly diagnosed KS. Furthermore, the research highlights the significance of being attentive to cranial deformities in the management of KS individuals and suggests the importance of selectively utilizing 3DCT for evaluation when CS is suspected.
AUTHOR CONTRIBUTIONS
Eriko Nishi diagnosed, provided clinical data, planned the study, and wrote the manuscript. Yuiko Hasegawa provided clinical data. Noriko Miyake, Kana Hosoki, and Naomichi Matsumoto provided molecular genetic data. Rie Kawamura performed statistical analysis. Nobuhiko Okamoto supervised the study. All authors reviewed the drafts and authorized the final version of the manuscript.
ACKNOWLEDGMENTS
We are deeply grateful to the patients and their families. We are also very grateful to the doctors of the Department of Radiology and the Department of Neurosurgery at Osaka Women's and Children's Hospital. We would like to thank Honyaku Center Inc. for language editing.
FUNDING INFORMATION
This work was supported by the Health and Labour Sciences Research Grant of Research on Intractable Diseases (23FC0201) from the Ministry of Health, Labour and Welfare, Tokyo, Japan, JSPS KAKENHI grant number JP22H03047; AMED under grant numbers JP23ek0109674, JP23ek0109549, JP23ek0109617, JP23ek0109648; the Takeda Science Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




