Influences of sex chromosome aneuploidy on height, weight, and body mass index in human childhood and adolescence
Elisa Guma and Armin Raznahan contributed equally to this study.
Abstract
Sex chromosome aneuploidies (SCAs) are collectively common conditions caused by carriage of a sex chromosome dosage other than XX for females and XY for males. Increases in sex chromosome dosage (SCD) have been shown to have an inverted-U association with height, but we lack combined studies of SCA effects on height and weight, and it is not known if any such effects vary with age. Here, we study norm-derived height and weight z-scores in 177 youth spanning 8 SCA karyotypes (XXX, XXY, XYY, XXXX, XXXY, XXYY, XXXXX, and XXXXY). We replicate a previously described inverted-U association between mounting SCD and height, and further show that there is also a muted version of this effect for weight: both phenotypes are elevated until SCD reaches 4 for females and 5 for males but decrease thereafter. We next use 266 longitudinal measures available from a subset of karyotypes (XXX, XXY, XYY, and XXYY) to show that mean height in these SCAs diverges further from norms with increasing age. As weight does not diverge from norms with increasing age, BMI decreases with increasing age. These findings extend our understanding of growth as an important clinical outcome in SCA, and as a key context for known effects of SCA on diverse organ systems that scale with body size.
1 INTRODUCTION
Sex chromosome aneuploidies (SCAs) are a set of neurogenetic conditions defined by carriage of a sex chromosome dosage other than the typical XX or XY complements. These conditions are collectively common (affecting ~1:400 newborns [Nielsen & Wohlert, 1991]) and can impact multiple body systems (Gravholt et al., 2017; Hong & Reiss, 2014; Rau et al., 2021; Skuse et al., 2018; Zitzmann et al., 2021). Many SCAs are associated with changes in diverse aspects of biological structure and function like metabolism, endocrine function, cardiac function, and brain anatomy (Chen et al., 2013; Salzano et al., 2016; Tartaglia et al., 2020; Warling et al., 2020). Variations in body size present an important context for understanding these changes because several of the phenotypes involved vary with human body size in health (Berry et al., 2023; Bhatti et al., 2014; Chen et al., 2019; Cole & Henry, 2005; Cook & Hamann, 1961; Hepper et al., 1960; Jørgensen et al., 2015; Reardon et al., 2018; Salzano et al., 2016; Warling et al., 2020; Zöller et al., 2016), and it has been well established that SCAs are themselves associated with changes in body size (Counts et al., 2021; Oetjens et al., 2019; Ottesen et al., 2010). Specifically, a nonlinear association between height and sex chromosome dosage has been previously described by Ottesen et al (Ottesen et al., 2010). Above average height is observed until the total number of chromosomes reaches 4 (in females) or 5 (in males), whereas below average height is observed with additional chromosomes. To date, however, available anthropometric studies in SCA have emphasized height rather than weight and also been cross-sectional rather than longitudinal in nature (Counts et al., 2021; Oetjens et al., 2019; Ottesen et al., 2010). Addressing these gaps in knowledge would help to improve our understanding of body growth in SCA as an important clinical outcome in its own right, while also providing important allometric context for other reported phenotypes such as metabolism, endocrine function, and brain anatomy (Chen et al., 2013; Salzano et al., 2016; Tartaglia et al., 2020; Warling et al., 2020). Pursuing such research in SCA would also provide a rare window onto X- and Y-chromosome influences on human growth—which may, in turn, be relevant for well described sex differences in human size and tissue composition more generally (Brown et al., 2022; Fish et al., 2017; Makris et al., 2013; Mankiw et al., 2017; Newman et al., 2020).
Here, we leverage a rare collection of anthropometric data from 177 individuals with diverse SCAs representing 8 different karyotypes (XXX, XXY, XYY, XXXX, XXXY, XXYY, XXXXX, and XXXXY), and aim to build on the foundation provided by prior work in two key directions. First, in addition to height, which has been previously examined in individuals with SCAs (Counts et al., 2021; Oetjens et al., 2019; Ottesen et al., 2010), we also examine weight and body mass index (BMI). Including these additional anthropometric measurements allows for a more comprehensive investigation of SCAs by comparison of body metrics against each other to test for dissociable effects. Second, we extend beyond a cross-sectional analysis to also include longitudinal data, which enable us to test for the first time if deviations of height, weight, and BMI vary with age in SCA. Longitudinal effects of SCA are critical to understand, since the severity of phenotypes may exacerbate or diminish over time, and throughout critical developmental periods of adolescence and young adulthood. Our goal is to more robustly characterize anthropometric measurements in various SCA karyotypes as important phenotypes in their own right, and as a key context for analysis of other phenotypes that are impacted by SCA and also vary with body size.
2 METHODS
2.1 Participants
The cross-sectional dataset for this study comprised 177 individuals aged 4–19 years with varying SCAs: 61 XXY, 32 XYY, 32 XXX, 25 XXYY, 16 XXXXY, 8 XXXY, 2 XXXX, and 1 XXXXX (cross-sectional sample characteristics detailed in Table 1). This sample was used to assess body composition across the maximum number of SCA karyotype groups available. A subset of 149 participants within the cross-sectional sample—representing the four largest SCA karyotype groups included—also had repeat measures of height, weight, and BMI (totaling 266 measurement points), which enabled us to complete the first longitudinal tests for age-related variations of these phenotypes in SCA (longitudinal sample characteristics detailed in Table 2). All individuals studied had karyotypically confirmed SCAs lacking evidence of mosaicism based on 50 metaphase spreads in peripheral blood. Head injuries or conditions that would result in gross brain abnormalities were exclusionary. Participants were recruited via advertisements on the NIH website and parental support groups as part of an ongoing deep-phenotypic study of SCA at the National Institute of Mental Health (NIMH) Intramural Research program.
| Karyotype | XXX | XXXX | XXXXX | XXY | XYY | XXYY | XXXY | XXXXY | Statistical tests | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | 32 | 2 | 1 | 61 | 32 | 25 | 8 | 16 | ||
| Age (years) | Mean | 9.4 | 11.4 | 6.6 | 11.3 | 10.7 | 10.3 | 10.5 | 11.4 | F = 0.8 p = 0.6 |
| SD | 4.28 | 8.36 | – | 4.23 | 3.58 | 4.26 | 5.17 | 4.74 | ||
| Range | 5.0–19.5 | 5.5–17.3 | – | 5.2–19.2 | 5.7–18.2 | 4.9–18.4 | 4.3–18.7 | 5.1–17.2 | ||
| Tanner stage (counts) | 1 | 16 | 1 | 1 | 23 | 14 | 15 | 4 | 7 | X2 = 13.1 p = 0.5 |
| 2–3 | 10 | 0 | 0 | 23 | 12 | 7 | 3 | 2 | ||
| 4–5 | 6 | 1 | 0 | 15 | 6 | 3 | 1 | 7 | ||
| IQ | Mean | 93 | 58 | 60 | 97 | 94 | 85 | 74 | 59 | F = 10.7 p = 7.0e−11 |
| SD | 15.1 | – | – | 14.9 | 17.4 | 13.5 | 5.64 | 8.35 | ||
| Range | 61–129 | – | – | 61–127 | 63–141 | 68–119 | 50–72 | 50–72 | ||
| SES | Mean | 41 | 39 | 51 | 56 | 55 | 50 | 58 | 59 | F = 2.2 p = 0.04* |
| SD | 17.0 | – | – | 20.3 | 21.3 | 25.2 | 20.3 | 27.1 | ||
| Range | 20–76 | – | – | 20–110 | 20–101 | 20–101 | 20–82 | 27–115 | ||
- Note: SES is socioeconomic status from Hollingshead 2-factor index of parental occupation and education level. SD is standard deviation.
- * p < 0.05
- *** p < 0.001.
| Karyotype | XXX | XXY | XYY | XXYY | |
|---|---|---|---|---|---|
| Total observations | 58 | 111 | 59 | 38 | |
| Unique participants | 32 | 60 | 32 | 25 | |
Observations per individual F = 3.4, p = 0.002** |
1 obs. | 6 | 24 | 12 | 15 |
| 2 obs. | 26 | 26 | 16 | 8 | |
| 3 obs. | 0 | 7 | 2 | 1 | |
| 4+ obs. | 0 | 1 | 1 | 1 | |
| Mean | 1.81 | 1.85 | 1.84 | 1.52 | |
| Range | 1–2 | 1–5 | 1–5 | 1–5 | |
| Age | Range | 5.0–19.5 | 5.2–19.9 | 5.7–19.9 | 4.9–19.1 |
- Note: In all reported karyotype groups, the average number of observations per individual is greater than 1.5. Karyotype is a significant predictor of observations per individual.
- ** p < 0.01.
2.2 Editorial policies and ethical considerations
Informed consent was obtained from all individuals, and the study was approved by the National Institutes of Health (NIH) Institutional Review Board.
2.3 Anthropometric measures
Height and weight were measured once per time point on all participants using standardized instruments at the NIH Clinical Center. Body mass index (BMI) was calculated from height and weight (BMI = kg/m2). Height, weight, and BMI were re-expressed as z-scores standardized for age and sex using the Centers for Disease Control and Prevention (CDC) United States Growth Chart norms (“Percentile Data Files with LMS Values”, 2009). LMS parameters, which describe the skew (L for lambda), median (M for mu), and coefficient of variation (S for sigma), representing each month in the growth chart, were used to calculate z-score given the following equation, where X is an observed value: . The LMS method allows one to fit longitudinal and cross-sectional anthropomorphic data to obtain normalized percentile curves (Fenton & Sauve, 2007).
2.4 Demographics measures
During their visits to the NIH, participants also underwent cognitive assessments and neuropsychiatric evaluations. FSIQ was estimated using the age appropriate Wechsler scale. The majority of individuals received the Wechsler Abbreviated Scale of Intelligence and other scales included the WAIS-R, WISC-R, and WIC-III (Wechsler, 1999). Socioeconomic status (SES) was quantified using the Amherts modification to the Hollingseah two-factor index (Hollingshead, 1957; Watt, 1976) as previously reported (McDermott et al., 2019). The majority of our sample was of European descent (94%) with the exception of 11 participants.
2.5 Statistical analyses
To describe participant characteristics, we used the mean and standard deviation for continuous variables, and counts for categorical variables. Comparisons of participant characteristics across SCA groups were achieved using analyses of variance (ANOVA) with follow-up pairwise group comparisons for continuous variables and chi-squared tests for categorical variables.
In the cross-sectional dataset, height, weight, and BMI z-score differences between karyotypes were analyzed using 1-way ANOVA with follow-up of pairwise t-tests (Tukey–Kramer corrected). Linear models were used to estimate the relationship between height and weight z-scores.
In the absence of a significant F-test for the interaction between age and karyotype, the interaction term was dropped to include just the main effects of age and karyotype. Finally, the model was simplified further to include only age given a statistically insignificant omnibus F-test for the karyotype term.
All statistical analyses were performed using R version 3.6.0, and packages used include lme4, dplyr, forcats, ggpubr, nortest, rstatix, and effects. All visualizations were created using ggplot2.
3 RESULTS
3.1 Cross-sectional associations between SCA and variation in height, weight, and BMI
For the cross-sectional dataset, sample sizes by karyotype were congruent with known differences in prevalence between SCA subtypes (Berglund et al., 2019; Nielsen & Wohlert, 1991; Sánchez et al., 2023)—so that the 4 largest sample sizes were available for the trisomies (XXY, XYY, and XXX) and XXYY (Table 1). Variation in SCA karyotype did not show a statistically significant association with age or Tanner stage but was significantly associated with variation in IQ and SES (IQ: F = 10.73, p = 7.0e−11, SES: F = 2.15, p = 0.041) as previously reported (Tartaglia et al., 2017; Warling et al., 2020).
We first examined the distribution of body metric z-scores in each SCA group and compared observed mean z-scores against the expected value of 0 (excluding the 2 karyotypes with less than 3 observations: XXXX and XXXXX (Table 3). Body metric z-scores were variable in each SCA group, with standard deviations for both height and weight generally >1). BMI z-scores had the highest within-group variation, with an average standard deviation of 1.42. For several karyotypes, mean z-scores for height (XXX, XXY, XYY, and XXYY) and weight (XXX, XXY, and XYY) showed statistically significant differences from 0—with generally large z-score differences (|z| > 0.6) for significant increases and moderate z-score differences (|z| > 0.3) for significant decreases. Differences in z-score for changes of mean BMI z-score in SCA were generally smaller than those for height and weight, which combined with greater interindividual variation in BMI z-scores to result in SCAs not being associated with statistically significant alterations in BMI.
| Measurement | Karyotype | Mean Z-score | Standard deviation | T-value | Adj. p-value |
|---|---|---|---|---|---|
| Height | XXX | 0.77 | 1.04 | 4.20 | 0.001** |
| XXY | 0.78 | 1.30 | 4.69 | 0.0001*** | |
| XYY | 0.90 | 1.20 | 4.26 | 0.001** | |
| XXYY | 0.80 | 1.32 | 3.04 | 0.04* | |
| XXXY | 0.71 | 1.11 | 1.80 | 0.8 | |
| XXXXY | −0.34 | 1.37 | −0.99 | 1 | |
| Weight | XXX | 0.43 | 0.86 | 2.79 | 0.05* |
| XXY | 0.52 | 1.34 | 2.99 | 0.02* | |
| XYY | 0.72 | 1.23 | 3.34 | 0.02* | |
| XXYY | 0.74 | 1.52 | 2.43 | 0.09 | |
| XXXY | 0.90 | 0.99 | 2.57 | 0.1 | |
| XXXXY | 0.13 | 1.59 | 0.34 | 0.7 | |
| BMI | XXX | 0.14 | 1.02 | 0.80 | 1 |
| XXY | 0.10 | 1.60 | 0.50 | 1 | |
| XYY | 0.37 | 1.34 | 1.57 | 0.9 | |
| XXYY | 0.45 | 1.53 | 1.47 | 0.9 | |
| XXXY | 0.70 | 1.33 | 1.49 | 0.9 | |
| XXXXY | 0.15 | 1.71 | 0.35 | 1 |
- Note: Mean height z-score is significantly different from 0 for karyotypes XXY, XXX, XXYY, and XYY; mean weight z-score is significantly different from 0 for karyotypes XXY, XXX, and XYY; and no BMI z-scores are significantly different from 0. p-values are Bonferroni (BF) corrected (* BF p < 0.05, ** BF p < 0.01, and *** BF p < 0.001). Karyotypes XXXX and XXXXX are not included due to small sample sizes (2 and 1, respectively).
We next examined whether alterations of body metrics showed significant differences as a function of SCA karyotype (Table 3). Omnibus F-tests for karyotype effects on z-scores were statistically significant for height (Figure 1a,f = 3.2, p = 0.004) but not for weight (Figure 1b,f = 1.1, p = 0.4) or BMI (Figure 1c,f = 0.43, p = 0.9). Post hoc pairwise SCA group comparisons showed that significant SCA group effects in height z-score were driven by significantly elevated height in XXY and XYY relative to other groups, and reduced height in the higher grade X-chromosome aneuploidy groups, including XXXXX, XXXXY, and XXXX (Figure 1a).
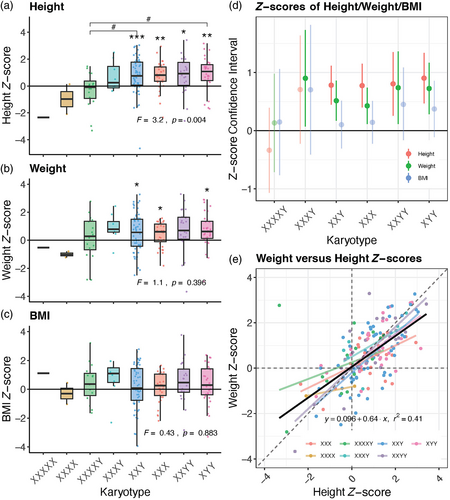
Although SCA karyotype was not associated with statistically significant z-score variation in weight or BMI, we observed a qualitative coordination of SCA group mean z-scores for height, weight and BMI z-scores (Figure 1d). Specifically, z-scores for height, weight and BMI all showed the same rank-ordering in the trisomies and XXYY, whereas this motif was lost in the higher-count karyotypes, including XXXXY and XXXY. Thus, comparison of mean z-score shifts suggested a qualitative coordination of height, weight, and BMI alteration in the more common SCA groups, which is loosened in rarer, higher grade SCAs. We further probed the coordination of body metrics in SCA through the lens of interindividual variation—by comparing height and weight z-scores across individuals (Figure 1e). A linear model failed to find karyotype as a statistically significant modulator of the relationship between height and weight z-scores, instead supporting a collective linear model with a slope of 0.644 (p < 2e−16 versus null of 0), meaning that an increase of 1 z-score for height corresponds to an increase in 0.644 z-scores for weight.
3.2 Dynamic alterations of anthropometric measurements in SCA over adolescence
Sample characteristics for participants represented in the longitudinal dataset are displayed in Table 2. Our longitudinal dataset included a total of 149 individuals (32 XXX, 60 XXY, 32 XYY, 25 XXYY) with a combined total of 266 longitudinal sets of height, weight, and BMI data points (with an average of 1.81 observations for XXX individuals, 1.85 for XXY, 1.84 for XYY, and 1.52 for XXYY).
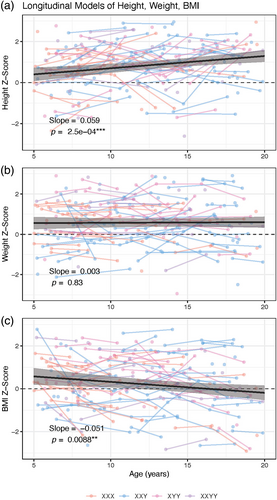
Using linear mixed-effects modeling, we found that neither the karyotype by age interaction, nor the main effect of karyotype were significant in models of height, weight or BMI z-score, so we used a simpler model testing for the effects of mean-centered age on these measurements with a random intercept per participant. This revealed a statistically significant effect of age on height z-score (p = 0.00025 vs. null of 0), wherein SCA individuals were taller than would be predicted by CDC norms at age 6 years (estimated mean height z = 0.56, p = 3.4e−5 vs. null of 0), and continued to grow at a quicker rate than predicted by CDC norms (increasing by 0.059 z-scores per year, p = 0.00025 vs. null of 0, Figure 2, Table 4) achieving a mean height z-score of 1.57 by age 19 years (p = 4e−13 vs. null of 0). We did not detect a statistically significant effect of age on weight z-score, but rather observed a developmentally stable increase in weight z-score above 0 (estimates mean weight z = 0.58, p = 6e−8 vs. null of 0, Figure 2, Table 4). In keeping with the age-related increase in height z-score and the lack of age-related variation in weight z-score, we observed a statistically significant negative effect of age on BMI z-score (p = 0.0088 vs. null of 0) in SCAs. On average, SCA individuals had a higher BMI than would be predicted by CDC norms at age 6 years (estimated mean height z-score = 0.44, p = 0.0052 vs. null of 0), and z-scores decreased by 0.051 per year (p = 0.0088 vs. null of 0, Figure 2, Table 4) to fall within CDC norm expectations by age 19 years (z = −0.22, p = 0.22 vs. null of 0).
| Measurement | Term | Coefficient | Standard error | p-value |
|---|---|---|---|---|
| Height | Intercept | 0.92 | 0.096 | <2e−16*** |
| Age | 0.059 | 0.016 | 0.00025*** | |
| Weight | Intercept | 0.58 | 0.10 | 6e−08*** |
| Age | 0.003 | 0.014 | 0.83 | |
| BMI | Intercept | 0.26 | 0.11 | 0.26 |
| Age | −0.051 | 0.019 | 0.0088** |
- Note: The intercept term, which represents the predicted z-score for the sample at mean height, is significantly elevated for height and weight (vs. null of 0). Age is a significant predictor of z-score for height and BMI.
- ** p < 0.01
- *** p < 0.001.
4 DISCUSSION
In this study, we leverage both cross-sectional and longitudinal anthropometric data from individuals with varying sex chromosome aneuploidies (SCAs) to both reproduce and extend our current understanding of how SCA affects body anthropometry.
Our cross-sectional analyses independently replicate the nonlinear relationship between height and number of sex chromosomes that was previously reported by Ottesen et al. (Ottesen et al., 2010). We find that average height is increased with addition of sex chromosomes (up to a total count of 4 for female karyotypes or 5 for male karyotypes), while average height is decreased with additional sex chromosomes. This pattern is revealed by considering cross-karyotype trends while comparison against CDC norms for individual karyotypes detects statistically significant elevations in height for trisomy karyotypes (XXX, XXY, and XYY) and for the XXYY karyotype. By extending beyond analysis of height alone, we establish that the relationship between weight and SCA karyotype is qualitatively similar to that of height. Thus, like height, weight also tends to be elevated in SCA trisomies and XXYY, but decreases with higher sex chromosome dosages — although the greater interindividual variation in weight as compared to height mutes statistical power for detection of significant SCA influences in weight as compared to height. In line with the concordant trend for SCA effects on height and weight in this cross-sectional analysis, the mathematical dependency of BMI on these two features, and the high within-group variance in BMI, none of the SCA groups examined showed BMI z-scores that were statistically significantly deviated from CDC norms. Thus, we find that there are coordinated cross-sectional effects of SCA on both height and weight, with effects being more penetrant for height than weight.
We extend beyond cross-sectional analyses by leveraging longitudinal data to ask if age modulates alterations of height, weight, and BMI. We find that for trisomy karyotypes and XXYY, age does in fact modulate alterations in height and BMI, but not weight. On average, individuals with SCA experience faster age-related height increases than CDC norms over the age range examined, which combined with a static elevation in weight relative to CDC norms leads to a age-related deviation of BMI from CDC norms such that mean BMI is elevated above CDC norms in mid-childhood, but within CDC expectations by late adolescence. Of note, increased BMI z-scores above CDC norms were also detected for the trisomies and XXYY in cross-sectional analyses (Table 3), but these increases did not reach statistical significance—likely due to the averaging of age-varying values in cross-sectional analyses, the smaller number of observations, and the lack of repeat measures in cross-sectional as compared to longitudinal analyses. Taken together, these results extend our understanding of SCD effects on body anthropometry in development and highlight the need for expanded research in this area as discussed below.
The above findings have relevance for elucidating mechanistic models of SCA effects on body size and composition. The inverted-U relationship between SCD and height (and to a lesser extent weight) constitutes a nonlinear phenotypic effect of increases in X- and Y-chromosome count. Because few studies have profiled dimensional phenotypes across the full range of SCDs, it is not clear if such nonlinearities are also at play for other aspects of SCA. To our knowledge, the only other report of nonlinear SCD effects is a qualitative inverted-U association between SCD and parieto-occipital surface area (Raznahan et al., 2016). The mechanistic basis for such nonlinearities is unclear. Because they appear regardless of gonadal context and similarly for the X- and Y-chromosome they are likely to reflect direct dosage effects of factors common to both sex chromosomes. This observation focuses attention on: (1) genes within the pseudoautosomal regions (PARs), which show full sequence homology and undergo recombination between the X- and Y-chromosome, and (2) X-Y gametolog genes, which are widely expressed and dosage sensitive genes that have retained high-sequence homology between the X- and Y-chromosome despite no longer undergoing recombination (Bellott et al., 2014; Raznahan & Disteche, 2021; Sayres et al., 2013; Trombetta et al., 2014). However, available studies of gene expression across SCAs do not find an inverted-U relationship between SCD and expression of PAR and gametolog genes (Liu et al., 2023; Raznahan et al., 2018). Thus, the causal basis for nonlinear effects of SCD must either emerge downstream of SCD effects on gene expression, or via pathways other than the direct cis-effects of SCA on gene expression (e.g., trans- effects on expression or regulatory effects of noncoding regions of the sex chromosomes) (Maier et al., 2021; Meiklejohn et al., 2014; Stocks et al., 2015).
A major proximal driver of increasing height in development is longitudinal bone growth through growth plate chondrogenesis—a process governed by the complex interplay of nutritional, hormonal, cytokine, and protein factors, which are also regulated by genetic and environmental factors (Jee & Baron, 2016). To date, SCA has been theorized to impact height through two potential pathways. First—given that sex hormones both accelerate bone growth and ultimately cause the cessation of growth via epiphyseal fusion (Counts et al., 2021)—alterations of growth in SCA could be linked to the hypogonadism seen in some SCAs. However, because we see height alterations in SCA before puberty, and in karyotypes with intact gonadal function, it seems that there must also be nongonadal mechanisms at play. Growth alterations in SCA have also been hypothesized to arise from the direct effects of altered dosage of SHOX—which resides on the pseudoautosomal region of X- and Y-chromosomes and, therefore, increases linearly with SCD (Marchini et al., 2016; Ottesen et al., 2010). Consistent with this idea, SHOX is expressed in chondrocytes at the growth plate, and haploinsufficiency of SHOX is known to cause short stature (Counts et al., 2021; Fukami et al., 2016; Hristov et al., 2014; Munns et al., 2004; Rao et al., 1997). Our results indicate that future mechanistic research on causes for growth alterations in SCA should search for factors that both operate in a nonlinear fashion (to account for the inverted-U relationship between SCD and stature) and vary over time (to account for the longitudinal exacerbation of increased height over adolescence). Mechanistic dissection of SCD effects on growth will ultimately rely heavily on work in animal models including those that can model X- and Y-chromosome dosage variation (such as the Four Core genotype mouse model) and those that can model variation in specific genes such as SHOX (e.g., zebrafish (Beiser et al., 2014; Fukami et al., 2016; Marchini et al., 2016; Yokokura et al., 2017)).
The SCD effects on height and weight reported here also carry broader relevance for clinical research in SCAs. In particular height covaries with many other body systems, including the size and output of the heart (Jørgensen et al., 2015; Salzano et al., 2016), volume and capacity of the lungs (Bhatti et al., 2014; Cook & Hamann, 1961; Hepper et al., 1960), metabolism (Cole & Henry, 2005), bone density (Chen et al., 2019), brain volume (Warling et al., 2020), and venous thromboembolism (Berry et al., 2023; Salzano et al., 2016; Zöller et al., 2016), which are all important to consider from a clinical perspective. More research on the longitudinal effects of aneuploidy on these features that also incorporate information on body growth will be important.
The findings in this study should be interpreted in the context of several limitations and caveats. First, small sample sizes limit our statistical power to detect differences for those more rare SCA karyotypes. Second, our focus on clinical groups with supernumerary sex chromosomes prevents us from comparing the effects of X-chromosome haploinsufficiency alongside the effects of X-chromosome gain. Third, the findings in our clinical cohort are subject to potential ascertainment biases. Specifically, it is estimated that 50%–85% of people with XXY and XYY are never diagnosed (Sánchez et al., 2023). Some reasons for diagnosis include delayed developmental milestones, intellectual disability, learning disability, or pubertal or gonadal failure (Hong & Reiss, 2014; Tartaglia et al., 2017). Therefore, it is possible that our dataset is subject to sampling bias, where the included individuals represent a more extreme phenotype than the full population of individuals with SCA. However, it is not clear that body size and composition are relevant to this ascertainment bias: for the ascertainment bias to influence the findings above, either (1) height/weight/BMI determines whether an individual gets tested for SCA, or (2) height/weight/BMI is correlated with a phenotype that would cause someone to get tested. Finally, our current report only considers height and weight, and it will be important for future research to examine more granular measures of body composition in SCA. In particular it would be important to obtain: more diverse and more precise measures of body composition aside from BMI (e.g., bioimpedance or DEXA scans to derive measures of percentage of total body fat, lean mass, and bone density; Adedia et al., 2020; Dekkers et al., 2019; Jaffrin, 2009; Kettaneh et al., 2005); more dense longitudinal data to allow curve fitting of trajectories that may be nonlinear and to better capture rapid pubertal changes; data covering a wider age range to investigate longitudinal changes from childhood to adulthood to late life; and accompanying phenotypic measures across different body systems that are known to vary with body size and SCA (e.g., brain anatomy, metabolism, bone density) so as to allow analyses of the coordinated SCA effects. Finally, we compare our SCA sample to norms derived from the standard CDC growth charts that are used as part of standard clinical care within the US. Nevertheless, while these norms are designed to be representative of the US population in 2000 (Kuczmarski et al., 2002), it is important to consider how different norms can influence conclusions regarding growth in comparison groups (Raznahan et al., 2013) Notwithstanding the above caveats and limitations, our study expands understanding of SCA effects on body growth. We replicate a nonlinear relationship between added sex chromosomes and height (Ottesen et al., 2010) and newly describe patterns of weight and BMI. We also establish longitudinal trajectories of these anthropometric factors for the first time, and we find that there is a dynamic effect of SCA on height—which is transmitted to BMI given the developmentally static increases in weight. These results highlight the need for regular monitoring of growth in SCA, motivate more detailed studies of body composition and its relationship with other SCA outcomes, and point toward complex nonlinearity and temporal dynamism in the biology of X- and Y-chromosome influences on human growth.
AUTHOR CONTRIBUTIONS
Claire Hanson, Armin Raznahan, and Elisa Guma conceptualized this study. Funding was acquired by Elisa Guma and Armin Raznahan. Jonathan Blumenthal and Liv Clasen were responsible for data acquisition. Jonathan Blumenthal was also responsible for project administration, and Liv Clasen was responsible for data curation. Formal analysis was conducted by Claire Hanson. Writing, review, and editing of the manuscript were completed by Claire Hanson, Elisa Guma, and Armin Raznahan.
ACKNOWLEDGMENTS
This study was supported by the intramural research program of the National Institute of Mental Health (project funding: 1ZIAMH002949-03; clinical trials identifier: NCT00001246; clinicaltrials.gov; protocol: 89-M-0006) and the National Institute of Child Health and Disease (R01HD100298). EG also receives salary support from the Fonds de Recherche du Québec en Santé.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The participants of this study did not give written consent for their data to be shared publicly. For that reason, the data that supports this research is not publicly available.




