Neurofibromatosis- and schwannomatosis-associated tumors: Approaches to genetic testing and counseling considerations
Abstract
Neurofibromatosis (NF) and schwannomatosis (SWN) are genetic conditions characterized by the risk of developing nervous system tumors. Recently revised diagnostic criteria include the addition of genetic testing to confirm a pathogenic variant, as well as to detect the presence of mosaicism. Therefore, the use and interpretation of both germline and tumor-based testing have increasing importance in the diagnostic approach, treatment decisions, and risk stratification of these conditions. This focused review discusses approaches to genetic testing of NF- and SWN-related tumor types, which are somewhat rare and perhaps lesser known to non-specialized clinicians. These include gastrointestinal stromal tumors, breast cancer, plexiform neurofibromas with or without transformation to malignant peripheral nerve sheath tumors, gliomas, and schwannomas, and emphasizes the need for inclusion of genetic providers in patient care and appropriate pre- and post-test education, genetic counseling, and focused evaluation by a medical geneticist or other healthcare provider familiar with clinical manifestations of these disorders.
1 INTRODUCTION
Neurofibromatosis and schwannomatosis are genetic conditions characterized by the development of tumors in the nervous system. Historically, these conditions were separated into neurofibromatosis type 1 (NF1), neurofibromatosis type 2 (NF2), and schwannomatosis (SWN) with estimated incidences of 1 in 3000, 1 in 33,000, and 1 in 70,000, respectively (Evans et al., 2018; Huson et al., 1989). These conditions were differentiated by clinical manifestations, each having unique diagnostic criteria with a minimum number of criteria needed to confirm a clinical diagnosis (Baser et al., 2006; MacCollin et al., 2005; National Institutes of Health Consensus Development Conference Statement, 1988). In 2021, the diagnostic criteria for NF1 were revised and included the addition of a heterozygous pathogenic variant (PV) in NF1, the gene associated with NF1. If identified by genetic testing, an NF1 PV qualifies as one criterion; with the requirement of at least two criteria to confirm a diagnosis of NF1 (Table 1) (Legius et al., 2021). Of particular importance with the revised criteria, the identification of a PV in NF1 alone does not confirm a diagnosis of NF1; a second criterion is required. Similarly, based on phenotypic similarities and a common predisposition for schwannomas in both NF2 and SWN, revised diagnostic criteria and nomenclature were published in 2022 (Table 1) (Plotkin et al., 2022). This revision combined NF2 and SWN into one broad category of SWN with delineation by gene (NF2-related SWN, SMARCB1-related SWN, and LZTR1-related SWN) or, if the specific gene is not known, a descriptor.
| Neurofibromatosis type 1 (NF1) |
|---|
| A diagnosis of NF1 can be made when a patient has two or more of the following: |
|
|
|
|
|
|
|
|
| Note: A NF1 PV is not sufficient for a diagnosis. |
| Schwannomatosis (SWN) |
|---|
| NF2-related SWN (NF2-SWN; formerly known as neurofibromatosis type 2) |
| A diagnosis of NF2-related schwannomatosis can be made when a patient has one of the following: |
|
|
|
| Major criteria |
|
|
|
|
| Minor criteria |
| Can count more than once of each type (e.g., two schwannomas = two minor criteria) |
|
| Can count only once |
|
| LZTR1- or SMARCB1-related SWN (LZTR1-SWN or SMARCB1-SWN) | |
|---|---|
| A diagnosis of LZTR1- or SMARCB1-related schwannomatosis can be made when a patient meets one of the following criteria: | |
|
|
|
|
| 22q-related SWN (22q-SWN) | |
|---|---|
| A diagnosis of 22q-related schwannomatosis can be made when an individual does not meet criteria for NF2-related schwannomatosis, SMARCB1-related schwannomatosis, or LTZR1-related schwannomatosis, and has both of the following molecular features: | |
|
|
|
| SWN-not otherwise specified (SWN-NOS) and SWN-not elsewhere classified (SWN-NEC) |
|---|
| If genetic testing was not performed or is not available, a diagnosis of schwannomatosis-NOS (not otherwise specified) can be made if both of the following criteria are met: |
|
|
| A diagnosis of schwannomatosis-NEC (not elsewhere classified) can be made if genetic testing of unaffected tissue and at least two anatomically distinct tumors does not reveal a PV in known schwannomatosis-related genes. |
- Abbreviations: CALM, café-au-lait macules; PV, pathogenic variant; VS, vestibular schwannoma.
- Source: NF1: Legius et al. (2021); NF2/SWN: Plotkin et al. (2022).
- a If only CALM and freckling are present, the diagnosis is most likely NF1 but Legius syndrome is often possible. At least one of the two pigmentary findings (café-au-lait macules or freckling) should be bilateral.
- b Sphenoid wing dysplasia is not a separate criterion in the case of an ipsilateral orbital plexiform neurofibroma.
- c If the PV is present in clearly less than 50%, the diagnosis is mosaic NF1.
1.1 Neurofibromatosis type 1
NF1 is a multisystem disorder primarily characterized by cutaneous findings (cafe-au-lait macules, intertriginous freckling), ophthalmologic findings (Lisch nodules, choroidal abnormalities), bone abnormalities, and tumor predisposition (optic pathway glioma, plexiform and cutaneous neurofibromas, malignant peripheral nerve sheath tumors (MPNST), gastrointestinal stromal tumors [GISTs], breast cancer, and others). Less common tumors and malignancies with an elevated risk in NF1 include rhabdomyosarcoma, pheochromocytoma, and juvenile myelomonocytic leukemia. NF1 is typically diagnosed in childhood, with nearly all NF1 individuals meeting diagnostic criteria by 8 years of age (DeBella et al., 2000).
NF1 is an autosomal dominant condition with complete penetrance and variable expressivity. Approximately 50% of individuals with NF1 have a sporadic, or de novo, case which may be constitutional or mosaic NF1. Individuals with mosaic NF1 may present with manifestations of the disease generalized throughout their body (similar to constitutional NF1), distributed in a patchy pattern, or localized to a single region of the body (“segmental NF1”) (Listernick et al., 2003; Redlick & Shaw, 2004).
Although methodologies vary greatly between laboratories, germline genetic testing for NF1 typically involves the use of sequencing technology, most commonly next-generation sequencing (NGS), and deletion/duplication analysis of the entire NF1 coding region. It is most often performed on fresh blood, saliva, or DNA extracted from lymphocyte cells (Maertens et al., 2007; Messiaen et al., 2000). Germline genetic testing utilizing NGS for NF1 identifies a PV in ~95% of individuals who meet clinical diagnostic criteria. RNA-based detection techniques have also increased the sensitivity for both detection and determination of functionality and pathogenicity of deep intronic splice variants, which may account for up to 2.5% of all NF1 PVs (Koster et al., 2021; Messiaen et al., 2000). To date, there have been more than 2800 different germline NF1 PVs identified, and most are considered private, familial variants (Koczkowska et al., 2018; Messiaen et al., 2000). Variants include small deletions or duplications/insertions (28%), splicing (27%), nonsense (21%), missense (16%), total gene deletions (5%), and copy number changes (2%) (Kehrer-Sawatzki & Cooper, 2021; Kehrer-Sawatzki & Cooper, 2022; Messiaen et al., 2000). Individuals with a microdeletion typically have a more severe phenotype and an increased lifetime risk for malignant peripheral nerve sheath tumors, which is thought to be related to the loss of SUZ12 along with NF1 in the microdeletion region (Kehrer-Sawatzki et al., 2017).
In some cases, such as when the above testing is uninformative, diagnostic testing may also be completed on DNA extracted from two anatomically distinct affected areas (e.g., two tumors, a tumor and a café-au-lait macule, etc.). This allows for the identification of a common PV as both samples should have biallelic inactivation of NF1. The common PV of the two specimens can then be presumed to be the underlying molecular etiology of the constitutional or mosaic NF1, rather than a somatic NF1 mutation unique to the affected tissue.
NF1 PVs have also been identified in individuals with a solitary NF1-associated finding, such as a neurofibroma or plexiform neurofibroma (PN), which for the latter is at risk for malignant transformation (e.g., MPNST) (Maertens et al., 2006; Serra et al., 2000). In addition, somatic NF1 PVs are also seen in a wide variety of malignant tumors including melanoma, neuroblastoma, myeloid malignancies, ovarian carcinoma, and glioblastoma (Philpott et al., 2017). In these cases, the discovery of a NF1 PV is likely not indicative of a germline (constitutional) NF1 variant and therefore not sufficient to confirm a diagnosis of NF1. This reinforces the need for a focused evaluation by a medical geneticist or other healthcare provider familiar with clinical manifestations of NF1.
NF1 genetic testing may be done independently or include analysis of multiple genes associated with phenotypically similar conditions such as Legius syndrome, other RASopathy disorders (e.g., Noonan syndrome), or other cancer predisposition conditions (e.g., PTEN hamartoma tumor syndrome [PHTS], or constitutional mismatch repair deficiency [CMMRD]). Because of the overlap of these conditions but significant difference in natural history and medical implications, an inaccurate diagnosis can have devastating effects. If a diagnosis is uncertain, an evaluation by a healthcare provider familiar with these conditions (e.g., medical geneticist) including a comprehensive three-generation pedigree should be completed.
1.2 Schwannomatosis
Schwannomatosis predisposes to the development of multiple schwannomas, most commonly affecting peripheral and spinal nerves. Vestibular schwannomas (VS) are present in virtually all cases of NF2-SWN and occasionally seen in LZTR1-SWN (Smith et al., 2014), while meningiomas can be present in both NF2- and SMARCB1-SWN (Bacci et al., 2010). In addition to schwannomas and meningiomas, NF2-SWN is also associated with ependymomas and ophthalmologic abnormalities (juvenile cataracts, epiretinal membrane). Individuals with SWN have a potential for significant disease morbidity, including pain, weakness, vision loss, hearing impairment, and other neurologic complications, as well as emotional and psychological effects of having a progressive and devastating disease. All types of SWN are inherited in an autosomal dominant manner with variable expression. NF2-SWN has complete penetrance; however, other forms of SWN may have decreased penetrance, especially LZTR1-SWN (Plotkin et al., 2013; Swensen et al., 2009).
Genetic testing for SWN typically includes the use of sequencing technology, such as NGS, and deletion/duplication analysis of one or more of the associated genes, including NF2, SMARCB1, and LZTR1. Somatic mosaicism has been identified in up to 30% of NF2-SWN simplex cases (Moyhuddin et al., 2003) and as high as 60% of first-generation individuals with NF2-SWN (Evans et al., 2019). The frequency of mosaicism is not well defined in SMARCB1-SWN and LZTR1-SWN but is known to occur (Alaidarous et al., 2019; Farschtschi et al., 2016). Given mosaicism in all types of SWN, germline DNA testing may not detect the causative PV and therefore, paired germline and tumor analysis with at least two anatomically distinct tumors may be utilized to increase PV detection. Of note, individuals presenting with SWN and an intellectual disability or dysmorphic features may have a chromosomal deletion, chromosome translocation, or ring chromosome 22, which may require additional cytogenetic analysis (Kehrer-Sawatzki et al., 1997; Tommerup & Lothe, 1992; Zirn et al., 2012).
Testing of NF2 is frequently included on multi-gene somatic tumor panels, as it is a known molecular driver of tumor formation in various cancers, including mesothelioma, glioblastoma multiforme, clear cell renal cell carcinoma, and breast, colorectal, skin, hepatic and prostate cancers (Petrilli & Fernandez-Valle, 2016). In these cases, the discovery of a NF2 PV is likely not indicative of a germline (constitutional) NF2 variant and therefore not sufficient to confirm a diagnosis of NF2-SWN. An evaluation by a clinician familiar with SWN (e.g., medical geneticist) may be indicated.
1.3 Germline versus somatic testing
As molecular testing is increasingly utilized for the diagnosis and management of these conditions and their associated tumors, knowledge and interpretation of the variants identified during testing continues to evolve. One important distinction in pursuing genetic testing and interpreting the results and their implications for disease is the difference in the application of somatic versus germline testing.
In 2015, the American College of Medical Genetics and Genomics (ACMG), the Association for Molecular Pathology (AMP), and the College of American Pathologists established the first attempt to standardize the data used to classify the pathogenicity of germline genetic variants (Richards et al., 2015). These recommendations established the thresholds of informative data needed to label a variant within the five categories that have become standard terminology: “pathogenic”, “likely pathogenic”, “uncertain significance”, “likely benign”, and “benign”. These thresholds are met by using variant evidence such as population, computational, functional, and segregation data. Soon after, the NIH-based Clinical Genome Resource (ClinGen), continued efforts to standardize variant classification processes by establishing a clinical variant repository (ClinVar), gene-specific variant curation protocols using variant curation expert panels (VCEPs) (Kanavy et al., 2019; Rehm et al., 2015).
In contrast, somatic or tumor, testing by NGS is being increasingly utilized for a multitude of reasons and employs a different approach to categorize somatic sequence variations. This approach was developed in 2017 by the AMP with liaison representation from the ACMG, American Society of Clinical Oncology, and College of American Pathologists (Li et al., 2017). The classification system uses a four-tiered system to categorize somatic sequence variations based on their clinical significance: tier I (variants with strong clinical significance); tier II (variants with potential clinical significance); tier III (variants of unknown clinical significance); and tier IV (variants deemed benign or likely benign). The identification of a tier I or tier II variant in a tumor, regardless of allele frequency, is not sufficient to diagnose an individual with a genetic condition. Further evaluation and/or germline genetic testing may be necessary. This is particularly nuanced with NF1 and SWN, given the common need to complete somatic testing to aid in diagnosis, as well as the high frequency of NF1- and SWN-associated variants in sporadic tumors in the general population. As a result, paired testing using both blood and affected tissue may be needed in some cases to provide the most informative molecular analysis.
A paired testing approach using both blood and affected tissue is shifting the paradigm in hereditary cancer risk assessment. The paired testing of tumor and normal match specimen, typically a peripheral blood or saliva sample, allows the patient's own germline DNA to be utilized for comparison to the tumor DNA, improving the accuracy of somatic variant calling (Jain et al., 2016). It also has the potential of identifying patients with underlying germline or hereditary PVs, thus providing accurate risk assessment and surveillance planning to the patient and family members.
The difference between institutional approaches to using paired match somatic testing may result in different downstream models from center to center leading to inconsistent follow-up planning. Downstream models may also differ based on the age of the population served. For example, the utilization of somatic testing in pediatric oncology has unique considerations compared to its use in the adult oncologic space (Sweet-Cordero & Biegel, 2019). For pediatric cancer centers that utilize a tumor only NGS approach, identification of a somatic NF1 variant may warrant further evaluation and risk assessment by a genetics provider to assist in determination of germline testing needs. In the consideration of germline cancer predisposition testing, age, autonomy and ability to participate in counseling are some of the many factors that may affect decision making for the pediatric patient.
Collaboration with members of a comprehensive care team for NF1 and SWN patients is recommended. Given the continued advances, nuances, and implications of genetic testing, the role of a genetics provider (e.g., clinical geneticist, genetic counselor, molecular geneticist, genetics advanced practice provider) within the care team is essential. Often, GCs or medical geneticists work alongside oncologists, assisting with pre- and post- test family counseling, coordination of genetic testing, interpretation of genetic results, somatic versus germline classifications, assessment of subsequent germline risks, and final risk determination. These factors have a potential for impacting therapeutic interventions. Similarly, collaboration and coordination with pathology is recommended given the preciseness of sample collection, storage, and accurate test ordering.
Somatic genetic testing has increasing importance in the diagnostic approach, treatment decisions, and risk stratification of cancer and tumor predisposition syndromes. This is relevant to the evaluation of NF1- and SWN- related tumor types including GISTs, breast cancer, PNs with or without transformation to malignant peripheral nerve sheath tumors, gliomas, and schwannomas.
2 GASTROINTESTINAL STROMAL TUMORS
Gastrointestinal stromal tumors are the most commonly occurring gastrointestinal tract tumors of mesenchymal origin (Corless, 2014). Most GISTs arise sporadically and have multiple underlying mechanisms of tumorigenesis, often leading to variability in clinical expression. The vast majority of GISTs are driven by oncogenic variants in either KIT (75% of GIST cases) or PDGFRA (10% of GIST cases), genes that code for two tyrosine kinase receptors (Lasota & Miettinen, 2006). Routine genotyping of GISTs is often completed for management recommendations and treatment decisions based on the identification of somatic alterations (Corless, 2014). Approximately 15% of GISTs are both KIT and PDGFRA mutation negative and are deemed wild type GISTs (wt-GIST).
Approximately 3%–4% of all GISTs are considered syndromic, and an underlying inherited or hereditary etiology should be suspected in the presence of multifocal GISTs or when associated with syndromic features (Ricci, 2016). There are several hereditary cancer predisposition syndromes associated with an increased risk for GISTs including familial GIST due to germline PV in either KIT or PDGFRA, Carney Stratakis syndrome due to germline PV in genes involving the succinate dehydrogenase (SDH) pathway (SDHA, SDHB, SDHC, and SDHD), and NF1 due to PV in NF1 (Postow & Robson, 2012; Ricci, 2016). Of these syndromic etiologies, NF1 is by far the most common cause of inherited GISTs.
NF1-associated GISTs are the most common GI manifestation in individuals with NF1, occurring at a 7% prevalence rate and increasing to 25% at autopsy (Kinoshita et al., 2004; Mathias-Machado et al., 2022; Takazawa et al., 2005). NF1 is also more than 45-fold overrepresented amongst GIST patients. In comparison to sporadic GISTs, NF1-associated GISTs are often multifocal and located in the small intestine. These GISTs tend to be small, mitotically inactive tumors with a good prognosis, and are typically wt-GISTs (Fuchs et al., 2005). The average age of onset for NF1-associated GISTs is approximately 49 years of age, in contrast with sporadic GISTs which only rarely occur under the age of 50 years. GISTs are extremely rare in the pediatric population, with 0.5% occurring before the age of 20 years (Ma et al., 2015). Therefore, an inherited predisposition to GIST development is strongly suspected in pediatric cases and these individuals should be referred for genetic counseling to discuss germline testing.
NF1-associated GIST tumors result from biallelic inactivation of NF1 within the tumor. Additionally, a study by Gasparotto et al. (2017) observed that 7 of the 11 individuals in their cohort with quadruple negative GISTs for KIT, PDGFRA, BRAF, and SDH were identified to have germline NF1 variants, indicative of a possible diagnosis of NF1 (Gasparotto et al., 2017).
The identification of quadruple-negative, multifocal, and multinodular non-gastric GISTs, especially in a pediatric patient, warrants significant consideration of germline genetic testing of NF1. This analysis requires a complement of sequencing analysis with additional technologies, that is, multiplex ligation-dependent probe amplification (MLPA) and/or microsatellite markers, to evaluate for loss of heterozygosity (LOH) and biallelic NF1 inactivation.
Overall, genetics providers knowledgeable about NF1 can play a vital role in patient care when NF1 variants are identified in GIST DNA. A comprehensive evaluation with a genetics provider with consideration of germline genetic testing for NF1 can lead to diagnosis or exclusion of NF1 in the patient and significantly impact patient care, particularly with regard to use of emerging therapeutics, such as Selumetinib.
3 BREAST CANCER
Breast cancer is the most common malignancy among individuals assigned female at birth worldwide, of which approximately 5%–10% are considered hereditary, or due to a genetic predisposition (Torabi Dalivandan et al., 2021). Of breast cancers considered hereditary, approximately 25%–30% are due to a PV in a known breast cancer predisposition gene. Breast cancer predisposition genes include high-penetrance genes (e.g., BRCA1/2, CDH1, PALB2, PTEN, TP53), moderate penetrance genes (e.g., ATM, BARD1, CHEK2), and elevated risk genes (RAD51C, RAD51D) (Torabi Dalivandan et al., 2021). NF1 is considered a moderate penetrance gene for risk of breast cancer (Easton et al., 2015).
In a Finnish population-based study from 1987 to 2012, Uusitalo et al. (2016) found that women with NF1, especially those under age 40 years, demonstrated a significant risk to develop breast cancer in their lifetime compared to the general population. Further, this report demonstrated significantly poorer five-year survival and excess mortality in patients with NF1-related breast cancer versus controls, which is a finding supported in several other large studies (Evans et al., 2011; Seminog & Goldacre, 2015; Uusitalo et al., 2016). And, while there have been no definitive studies, several case reports of individuals assigned male at birth with breast cancer have been published (Lakshmaiah et al., 2014).
At this this time, the National Comprehensive Cancer Network (NCCN) recommends that individuals assigned female at birth with NF1 receive an annual mammogram, starting at age 30 years, with consideration of contrast-enhanced breast MRI between ages 30 and 50 years (NCCN, 2023). Risk-reducing mastectomy should also be considered in individuals with NF1 who are at increased breast cancer risk because of additional factors, such as family history (Stewart et al., 2018). A consultation with a genetics provider should be considered to assess and discuss risks and risk-reducing options.
Breast tumor DNA analysis is becoming more common to aid therapeutic decision making in individuals with breast cancer and has identified a wide variety of NF1 variants in breast tumor DNA. NF1 variants in breast tumor DNA are thought to contribute to tumor development as well as to the acquisition of drug resistance, including BRAF and EGFR inhibitors and tamoxifen (Philpott et al., 2017). While the spectrum of NF1 variants in sporadic tumors generally differs from both germline and tumor variants commonly seen in individuals with NF1 (Bewley et al., 2022), alarm for a possible underlying diagnosis of NF1 may be raised in a patient with a somatic NF1 variant identified in breast tumor DNA. It is important for providers to be aware that an NF1 variant in breast tumor DNA by itself is not indicative of a diagnosis of NF1. Rather, it warrants further evaluation by an NF1 provider. In rare instances, an adult individual with breast cancer may be diagnosed with NF1 through this evaluation subsequent to their breast cancer management.
Genetics providers knowledgeable about NF1 can play a vital role in the interpretation of NF1 variants identified in breast tumor DNA. A comprehensive evaluation might include a physical examination, germline genetic testing, and analysis of family history, leading to a diagnosis or exclusion of NF1 in the patient. This would significantly impact patient care, particularly regarding cancer risk assessment, surveillance, potential therapeutic options, and reproductive risks.
4 PLEXIFORM NEUROFIBROMAS AND MALIGNANT PERIPHERAL NERVE SHEATH TUMORS
Plexiform neurofibromas (PNs) are identified in up to 50% of individuals with NF1 and associated with an overall ~8%–13% lifetime risk of developing an associated malignant peripheral neve sheath tumor (MPNST). MPNST account for approximately 10% of all soft tissue sarcomas in the general population, and approximately half of all individuals with a MPNST have an underlying diagnosis of NF1 (Bates et al., 2014; Fuchs et al., 2005). While the primary treatment of MPNSTs is currently tumor resection followed by radiation and chemotherapy, genotyping may be completed to identify underlying molecular mechanisms contributing to tumorigenesis. Tumors with NF1 variants may necessitate consideration of further evaluation for NF1 through clinical evaluation by a healthcare provider familiar with NF1 and/or germline genetic testing.
Plexiform neurofibromas are peripheral nerve sheath tumors caused by bi-allelic inactivation of NF1 in the neural crest-derived Schwann cell lineage leading to absence of the neurofibromin protein and aberrant Ras activation (Evans et al., 2002; Maertens et al., 2006; Serra et al., 2000). Malignant transformation of PN to MPNST requires biallelic loss of functional NF1 and the loss of other tumor suppressor genes (e.g., TP53, CDKN2A/B, PTEN, SUZ12, EED, RB1) and/or the amplification of receptor tyrosine kinase (RTK) oncogenes (e.g., EGFR, IGFIR, ERBB2, c-KIT, MET) (Brohl et al., 2017; Evans et al., 2002; Kaplan et al., 2018; Katz et al., 2009; Lemberg et al., 2020; Pemov et al., 2019). Specifically, the loss of CDKN2A/B has been proposed as a key driver of MPNST progression and has been identified in ~50% of atypical neurofibromatosis neoplasms of uncertain biological potential (ANNUBP), formerly referred to as “atypical neurofibromas”, which are thought to be the intermediate state between PN and MPNST (Beert et al., 2011; Chaney et al., 2020; Miettinen et al., 2017).
Genetics and oncology colleagues can work together to interpret MPNST somatic test results. This may provide information about prognosis and guide treatment decisions, as well as identify patients with a NF1 PV who should be further evaluated by a healthcare provider, such as a medical geneticist, familiar with NF1. Genetics providers may further aid the oncology patient care team as emerging genetic technologies are put to use to aid earlier diagnosis and treatment of MPNST, potentially reducing MPNST related mortality.
One such advancement is cell-free DNA (cfDNA) analysis, which several recent studies have utilized to distinguish patients with cancer from healthy individuals (Cohen et al., 2018; Cristiano et al., 2019; Luo et al., 2020). Specific to NF1, Szymanski et al. (2021) used ultra-low-pass whole genome sequencing (ULP-WGS) to analyze plasma cfDNA in an attempt to distinguish benign PN from MPNST for more rapid detection of malignant transformation (Szymanski et al., 2021). This multi-institutional study at the National Cancer Institute (NCI) and Washington University in St. Louis, included 53 patients with NF1. Using this technology, they were able to differentiate MPNST from PN with 86% pretreatment accuracy (91% specificity, 75% sensitivity) and 89% accuracy on serial analysis (91% specificity, 83% sensitivity). The researchers also proposed serial cfDNA testing, as tumor fraction levels in plasma correlated with treatment response to therapy and detection before relapse. Their findings support the use of cfDNA fragment analysis with ULP-WGS has the potential to be developed as a future biomarker for early detection and treatment response of MPNST.
Genetics provider knowledge of cfDNA technologies may become vital as cfDNA screening for malignancy becomes commonplace in oncology clinics. Genetics providers can partner with the oncology team to help determine appropriate candidates for testing, provide patient education, and aid in result interpretation.
5 GLIOMAS
Gliomas are the most common primary central nervous system CNS) tumor among children and adults with NF1, found in approximately 15%–20% of individuals (Seminog & Goldacre, 2013; Uusitalo et al., 2016). Gliomas in individuals with NF1 are heterogenous, with variable tumor location, age of onset, symptomatology, clinical behavior, and grade (Costa & Gutmann, 2019; D'Angelo et al., 2019). Malignant gliomagenesis is thought to occur later in life, supported by the observation that NF1-associated low-grade gliomas (NF1-LGG) are typically diagnosed in children, whereas NF1-associated high-grade gliomas (NF1-HGG) are typically diagnosed in adolescents and adults (Gutmann et al., 2002; Helfferich et al., 2016). The increased uptake of germline genetic testing and molecular tumor DNA analysis of gliomas raises several important genetic testing and counseling considerations for pediatric and adult patients with suspected or confirmed NF1.
Pediatric low-grade gliomas (LGG) are the most common CNS tumors in children, representing approximately 40%–50% of all childhood CNS tumors (Ostrom et al., 2015; Ostrom et al., 2019). Among children with an apparently isolated LGG, particularly optic pathway gliomas (OPG), NF1 is the most significant risk factor. It is estimated that approximately 33%–60% of children with OPGs have an underlying diagnosis of NF1 and that 40%–66% of OPGs are sporadic (Czyzyk et al., 2003; Grill et al., 2000; Khafaga et al., 2003; Kornreich et al., 2001; Listernick et al., 1995; Nicolin et al., 2009; Robert-Boire et al., 2017; Singhal et al., 2002; Tow et al., 2003; Varan et al., 2013; Wright et al., 1989). While tumor biopsy is not widely utilized for pediatric OPG, germline genetic testing for NF1 may be considered as part of their comprehensive evaluation to aid management recommendations.
Approximately 15% of children with NF1 have an OPG, with an average age of diagnosis of four and half years (Blanchard et al., 2016; Listernick et al., 1994). In contrast to OPG in the general population, NF1-associated OPG (NF1-OPG) are often asymptomatic and nonprogressive. They may not require intervention. However, as many as 50% of children with NF1-OPG experience ophthalmologic and/or endocrinologic symptoms, including vision loss and precocious puberty, which may require treatment (Listernick et al., 1994). Due to the different nature of NF1-OPG, comprehensive evaluation with a healthcare provider knowledgeable about NF1, such as a medical geneticist, should be considered for pediatric patients presenting with an apparently isolated OPG. Diagnosis of NF1 in these children would significantly impact medical management with regard to their OPG treatment and surveillance.
In contrast to the increased occurrence of OPGs among children with NF1, adults with NF1 more often develop non-optic gliomas. Notably, Sellmer et al. (2017) found that in their cohort of adults with NF1, approximately 4.3% of individuals (24/562) were diagnosed with a non-optic glioma—a level 1.5–3 times higher than previously published (Sellmer et al., 2017). Most of these tumors were asymptomatic and detected by MRI and like non-optic gliomas in children with NF1, these gliomas were primarily located in the posterior fossa (Sellmer et al., 2017).
Non-optic gliomas in adults with NF1 have variable histology but are often classified as HGG and associated with progressive clinical symptoms and shorter survival (Byrne et al., 2017; Guillamo et al., 2003; Packer et al., 2020; Sellmer et al., 2017). HGG represent one of the most common causes of death among adults with NF1 (Evans et al., 2011; Rasmussen et al., 2001). Due to the progressive nature of HGG, adults with or without a clinical diagnosis of NF1 who present with a glioma may undergo molecular tumor DNA analysis to aid in the determination of glioma management and selection of therapeutic intervention (Collins et al., 2015; Jones et al., 2012; Northcott et al., 2015). And, while molecular characteristics of NF1-associated gliomas are leading to emerging therapeutics in this population, the increase in molecular analysis of gliomas raises an important genetic testing and counseling consideration. NF1 is a somatic tumor suppressor gene in the general population and haploinsufficiency or nullizygous loss of NF1 has been documented in numerous sporadic malignancies, including sporadic gliomas (D'Angelo et al., 2019). Therefore, analysis of apparently sporadic gliomas may identify NF1 variants at varying allele frequencies leading to suspicion of NF1. However, the presence of an NF1 variant in an apparently isolated glioma is not sufficient by itself to diagnose a patient with NF1 and further evaluation with a NF1 specialist may be warranted to assess whether a patient formally meets diagnostic criteria. Genetics providers can aid in this process by interpreting somatic genetic test results, communicating result information to families, discussing NF1 information and diagnosis, and coordinating and interpreting potential germline genetic testing and evaluation.
6 SCHWANNOMAS
Schwannomas are benign nerve sheath tumors derived from myelinating Schwann cells of the cranial and peripheral nerves (Belakhoua & Rodriguez, 2021; Louis et al., 2021). Schwannomas may develop at multiple body sites, however, when intracranial, they most often involve the vestibular branch of cranial nerve VIII and are therefore referred to as VS (Belakhoua & Rodriguez, 2021). In most instances, schwannomas associated with SWN are almost indistinguishable histologically from sporadic schwannomas. Histologic features seen more consistently in SWN-associated schwannomas include whorling patterns, myxoid change, and nerve edema and/or infiltration (Belakhoua & Rodriguez, 2021). Despite intralesional variability, SWN-associated schwannomas typically have mosaic loss of immunohistochemical SMARCB1/INI1 expression. Specifically, Caltabiano et al., (2017) observed informative SMARCB1/INI1- expression in 10%–70% of schwannomas from individuals with SWN-associated non-vestibular schwannomas. Thus, immunohistochemical SMARCB1/INI1 expression is a reliable marker of SWN and often utilized to determine if an isolated schwannoma is more likely to be associated with a schwannoma predisposition syndrome (Caltabiano et al., 2017; Patil et al., 2008). The exception is NF2-SWN associated VS, in which there is positivity for SMARCB1/INI1 (Caltabiano et al., 2017). Therefore, SMARCB1/INI1 expression is not useful to differentiate NF2-SWN from other forms of SWN.
Worth noting are the differences between peripheral or cranial schwannomas and hybrid nerve sheath tumors, which are a subset of benign nerve sheath tumors with features of more than one recognized nerve sheath tumor subtype. The most frequent hybrid nerve sheath tumor is the schwannoma/perineurioma, which commonly occurs in the extremities and appears as a subcutaneous or dermal mass. Histologically, the cells display schwannoma-like morphology, but the architecture of the tumor resembles a soft tissue perineurioma. Immunohistochemically, schwannoma/perineurioma hybrid tumors are positive for S100 and EMA (Belakhoua & Rodriguez, 2021). Hybrid neurofibroma/schwannoma tumors often involve large peripheral nerves. Molecularly, neurofibroma/schwannoma hybrid tumors are frequently observed to have monosomy of chromosome 22 (Stahn et al., 2016). And, among these hybrid tumors from patients with SWN, recurrent ERBB2 PVs have been observed (Ronellenfitsch et al., 2020).
The genetic drivers of schwannoma formation are complex. NF2, SMARCB1, and LZTR1 are tumor suppressor genes that likely affect a common cytoplasmic signaling cascade that regulates essential cellular processes; when constitutional or somatic PVs exist in these genes, cellular processes are altered, leading to the development of schwannomas (Evans et al., 2017). In NF2-SWN, the associated schwannomas fulfill Knudson's two-hit hypothesis through biallelic inactivation of NF2 in the tumor, with a heterozygous NF2 variant serving as a common driving variant, which also may be present in the germline (Woods et al., 2003). In contrast, schwannomas associated with SMARCB1-SWN and LZTR1-SWN display a “three-event, four-hit” process and require biallelic inactivation of two associated genes on chromosome 22q (Figure 1). With PVs affecting both copies of the driving gene (SMARCB1 or LZTR1) as well as NF2, this signature pattern can provide assurance that a schwannoma is indeed schwannomatosis related (Evans et al., 2017; Hulsebos et al., 2007; Piotrowski et al., 2014; Plotkin et al., 2022; Sestini et al., 2008; Smith et al., 2015).
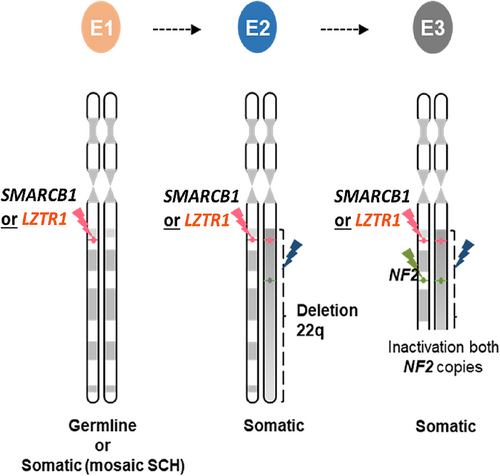
Traditionally, this is accomplished by a PV in the driving gene (SMARCB1 or LZTR1), representing the 1st event and 1st hit. This is followed by a partial deletion of chromosome 22q in trans of the first event, representing the 2nd event and 2nd and 3rd hits as the deletion encompasses SMARCB1/LZTR1 and NF2. The 3rd event and 4th hit is a spontaneous PV in NF2 in cis of the 1st hit variant in SMARCB1 or LZTR1.
Mosaicism has long been recognized in tumor predisposition syndromes (Bourn et al., 1994; Kovar et al., 1992) and leads to an added complexity for testing and diagnosis of SWN disorders. For instance, mosaicism in NF2-SWN may account for approximately 24.8%–33% of de novo cases in patients without VS and meeting the previous schwannomatosis (non-NF2-associated) diagnostic criteria (Evans et al., 2007; Kluwe et al., 2003). In 2020, Evans et al. used high read depth NGS to perform NF2-SWN genetic testing in the founding affected family members in multi-generation families. A mosaic NF2 variant was detected in 59.7% of first-generation cases. While this is much higher than previously published rates of mosaicism within NF2-SWN, it is important to note that this study specifically evaluated the parent of a child with constitutional NF2-SWN. Age of onset also appears to be an important factor, with only 21.7% of individuals with mosaic disease being under age 20 years at diagnosis, compared to 80.7% of individuals with mosaic disease presenting at age 60 years or older (Evans et al., 2020). Importantly, patients presenting with bilateral vestibular schwannoma (BVS) later in life are less likely to have NF2-SWN; specifically, approximately 25% of BVS cases over age 50 years and approximately 50% over age 70 years with no other features of NF2-SWN are more likely to have developed BVS by chance occurrence rather than due to an underlying NF2 PV (Evans et al., 2015). The frequency of mosaicism is not well defined in SMARCB1-SWN and LZTR1-SWN but is known to occur (Alaidarous et al., 2019; Farschtschi et al., 2016).
Individuals who present with unilateral vestibular schwannomas (UVS), with or without additional schwannomas, pose a diagnostic challenge given the overlapping phenotypic spectrum of SWN. An individual with a UVS and two additional findings associated with NF2-SWN most often will have a diagnosis of mosaic NF2-SWN, which is best diagnosed with paired tumor normal matched analysis of two anatomically distinct tumors to assess for biallelic inactivation of NF2 in the tumor. However, when an individual has an apparently isolated UVS without additional features typical of NF2-SWN, the likelihood of identifying a germline NF2 or LZTR1 variant is equally 10% (Smith et al., 2017).
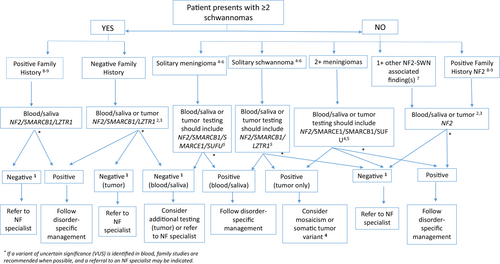
Accurate diagnosis is critical for determining appropriate prognosis, screening recommendations, and treatment options for individuals with SWN (Blakeley & Plotkin, 2016; Evans et al., 2017). A genetic testing strategy for SWN is outlined in Figure 2. Paired testing with tumor(s) and saliva/blood is most likely to result in improved diagnostic clarity for individuals with SWN Burns et al. (2022) found 13 times greater odds of diagnosis for patients who underwent paired testing with analysis of blood and two or more tumors, compared to patients who underwent germline-only testing (p < 0.01) and a six and a half greater odds of diagnosis for patients who underwent paired testing with analysis of blood and one tumor (p < 0.01) (Burns et al., 2022).
Age is an important factor when considering genetic testing in individuals with an apparently isolated schwannoma or meningioma. Specifically, those under the age of 25 years at the time of diagnosis of an apparent isolated schwannoma or meningioma should undergo further evaluation for an associated tumor predisposition syndrome due to important clinical implications and possibility of familial disease. Pathmanaban et al. (2017) found that 29% (44/153) of individuals with a solitary schwannoma before age 25 years were diagnosed with SWN and 54% (34/63) of individuals with a solitary meningioma before age 25 years had a constitutional PV in a known meningioma predisposition gene. Of those with a solitary meningioma, 40% (25/63) had NF2-SWN and 14% (9/63) had a PV in SMARCE1 or SUFU, two additional genes associated with susceptibility to meningioma (Pathmanaban et al., 2017).
The increased use of large gene panel testing and exome/genome sequencing as routine genetic testing may lead to the identification of “incidental” presumed PVs in SMARCB1 and LZTR1 in individuals without a personal or family history suggestive of SWN (Radtke et al., 2020). SMARCB1 is commonly included in intellectual disability and autism gene panels due to its association with Coffin-Siris syndrome and intellectual disability. SMARCB1 may also be included in large cancer gene panels due to an association with rhabdoid tumor predisposition syndrome (atypical teratoid/rhabdoid tumor). LZTR1 is associated with autosomal dominant and autosomal recessive Noonan syndrome and is therefore often included in RASopathy gene panels and many others. Very few (<1%) of these individuals are likely to develop SWN and surveillance is not recommended. In this instance, genetic counseling is vital and should be guided by blood and tumor molecular testing, and address disease penetrance and family planning considerations (Evans et al., 2022). Notably, penetrance associated with LZTR1-SWN is thought to be 40%–50% and possibly higher for SMARCB1-SWN (Evans et al., 2022).
The molecular characteristics of schwannomas may identify variants at varying allele frequencies leading to suspicion of SWN. However, the presence of a variant in a SWN-associated gene, particularly in a single schwannoma is not sufficient by itself to diagnose a patient with SWN. Further evaluation with a SWN specialist may be warranted to assess whether a patient formally meets diagnostic criteria. Genetics providers can facilitate this process by interpreting somatic genetic test results, communicating result information to families, discussing SWN information and diagnosis, and coordinating and interpreting potential germline genetic testing and evaluation.
7 DIAGNOSTIC EVALUATION
Given the complexities of these disorders, the diagnostic evaluation for NF1 and SWN requires an experienced provider who understands the nuances of these conditions and their differential diagnoses. Depending on the initial presentation of the patient, the evaluation might include examination of different body systems including the skin and eyes, as well as radiologic screening, biopsy, and genetic testing.
The diagnostic evaluation is often done by a medical geneticist but may also be completed by other specialists from an NF clinic or cancer center. In some situations, a patient may be initially referred to a subspecialist with a focus on care specific to the presenting feature. It is important that these providers recognize the possibility of a specific diagnosis and make referrals to the appropriate service if needed.
In the oncology setting, genetics providers who specialize in cancer have a unique skill set to assist in the identification of patients who may be at risk of NF1 and SWN based on a new tumor diagnosis or a family history of one of these conditions or their associated tumors. With the increased use, utility, and complexity of genetic testing, these providers can be especially poised in the interpretation of genetic testing with specific analysis of somatic and germline results and individual and familial risk assessment. The genetics provider often acts as a key advocate for the patient within the multidisciplinary team and provides context for how molecular results may be utilized in various aspects of care.
8 PSYCHOSOCIAL CONSIDERATIONS
Individuals with NF1 and SWN often report a variety of psychosocial experiences throughout the lifespan, which might be related to the visibility, severity, progressive nature, uncertainty of the condition, and in some cases shortened life expectancy (Merker et al., 2016; Vranceanu et al., 2013; Wiener et al., 2018). In addition, there can be elevated rates of anxiety and depression, deficits in executive functioning, and learning challenges. This can be further complicated by chronic pain with significant interference in quality of life (Evans et al., 2022; Wolters et al., 2015).
Likewise, the identification of gene variants that have unexpected or uncertain molecular results with potential implications for the patient's germline can pose new psychosocial challenges (Cushman-Vokoun et al., 2022). A practice resource published by the National Society of Genetic Counselors in 2020 emphasizes the importance of a psychosocial assessment and counseling in NF1 and SWN patients and details the various facets of providing support to this population (Radtke et al., 2020). GC involvement with affected individuals may begin at various points of time, most commonly during an initial pediatric evaluation. The genetic counselor—patient relationship during childhood and adolescence can serve as a powerful aid in effective healthcare transition from adolescence to adulthood, with distinctive opportunities to increase autonomy and improve quality of life (Radtke et al., 2020; Radtke et al., 2023). Genetic counselors can also provide necessary psychosocial support to individuals undergoing adult diagnostic evaluation, as well as in an oncology setting after a tumor diagnosis, family risk assessment, family planning conversations, or to those with an incidental finding on broad genomic sequencing.
9 CONCLUSION
NF1 and SWN are genetic conditions characterized by the development of tumors in the nervous system throughout the lifespan. Advances in genetic testing technology have led to an increased frequency of tumor based genetic testing in the diagnosis, treatment, and risk stratification of NF1 and SWN. Important testing and genetic counseling considerations should be considered when providing care for patients with NF1, SWN, or associated tumors. This review discussed how genetic testing pertains to NF1 and SWN related tumor types, including GISTs, breast cancer, PNs with or without transformation to malignant peripheral nerve sheath tumors, gliomas, and schwannomas.
AUTHOR CONTRIBUTIONS
Allison Goetsch Weisman: project administration, conceptualization, writing–original draft, writing–review and editing. Shelly Weiss McQuaid: writing–original draft, writing–review and editing. Heather B. Radtke: conceptualization, writing–original draft, writing–review and editing. Jessica Stoll: writing–original draft, writing–review and editing. Bryce Brown: writing–review and editing. Alicia Gomes: writing–original draft, writing–review and editing. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Kristiyana Kaneva, Robert Listernick, Ludwine Messiaen, and Carlos Prada for their support of this project and their constructive feedback in the finalization of the manuscript.
CONFLICT OF INTEREST STATEMENT
Jessica Stoll is an employee of Tempus Labs, Inc. Bryce Brown and Alicia Gomes are employees of University of Alabama Medical Genomics Laboratory. Allison Goetsch Weisman, Shelly Weiss McQuaid, and Heather B. Radtke declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




