Uniparental disomy of multiple chromosomes in two cases with a complex phenotype
[Correction added after first online publication on 30 July 2024. The new author “Christina L. Grant” has been added in the author byline]
Abstract
Uniparental disomy (UPD) is the inheritance of both chromosomal homologs from one parent. Depending on the chromosome involved and the parental origin, UPD may result in phenotypic abnormalities due to aberrant methylation patterns or unmasking recessive conditions in isodisomic regions. UPD primarily originates from somatic rescue of a single meiotically-derived aneuploidy, most commonly a trisomy. Double UPD is exceedingly rare and triple UPD has not been previously described. Here, we report two unrelated clinical cases with UPD of multiple chromosomes; an 8-month-old male with maternal isodisomy of chromosome 7 and paternal isodisomy of chromosome 9, and a 4-week-old female with mixed paternal UPD for chromosomes 4, 10, and 14. These cases also demonstrate that although extremely rare, the detection of AOH on two or more chromosomes may warrant additional clinical and laboratory investigation such as methylation and STR marker analysis, especially when involving chromosomes known to be associated with imprinting disorders.
1 INTRODUCTION
Whole chromosome uniparental disomy (UPD) is defined as the inheritance of both chromosome homologs from the same parent. Several mechanisms of UPD formation have been described such as trisomy rescue, monosomy rescue, and gamete complementation (fusion of a nullisomic gamete with a disomic gamete).
The inheritance of both parental homologs (heterodisomy) versus two identical homologs (isodisomy) depends on the whether the aneuploidy occurred due to nondisjunction in meiosis I or II, respectively. Furthermore, due to meiotic recombination, UPD may also present as a mixture of both isodisomic and heterodisomic regions with either a noncentromeric (meiosis I error) or centromeric (meiosis II error) region of absence of heterozygosity (AOH) (Del Gaudio et al., 2020). The most common type of UPD is maternal heterodisomy, while complete isodisomy is most often of paternal origin (Del Gaudio et al., 2020; Nakka et al., 2019; Scuffins et al., 2021).
UPD can affect any of the chromosomes, however clinical presentation depends on the chromosome involved and parent of origin (Liehr, 2022). UPD for most chromosomes does not seem to result in an abnormal phenotype unless a region of isodisomy unmasks a recessive disease-causing variant (Kotzot & Utermann, 2005). Currently, six chromosomes (6, 7, 11, 14, 15, and 20) are definitively associated with abnormal phenotypes due to altered expression of genes at imprinted loci through parent-of-origin effects (Eggermann, Perez de Nanclares, et al., 2015). Additionally, a phenotype related to either imprinted or non-imprinted chromosomes might be related or modified by a low level of aneuploidy cell line that can persist from the rescue event (Conlin et al., 2010; Eggermann, Soellner, et al., 2015).
The estimated prevalence of UPD in the general population is reported as 1 in 2000 (Nakka et al., 2019). The incidence of UPD resulting in an imprinting disorder is estimated to be 1 in 3500–5000 live births (Liehr, 2010; Robinson, 2000). Multiple chromosome UPD is extremely rare, with recent estimates suggesting that double UPD may occur in approximately 1 in 50,000 births in the general population (Nakka et al., 2019). Up-to-date, only one clinical case with double UPD has been reported and involved paternal isodisomy of chromosomes 7 and 15 in a patient with features of Beckwith-Wiedemann syndrome (Berland et al., 2021).
Here, we report two additional clinical cases with multiple chromosome UPD events, presenting with complex phenotypes: one with double UPD involving chromosomes 7 and 9, and the second with triple UPD involving chromosomes 4, 10, and 14. To the best of our knowledge, UPD of three chromosomes has not been described in either clinical or general populations.
2 RESULTS
2.1 Patient 1
2.1.1 Clinical presentation
Patient 1 was an 8-month-old male born at full term to non-consanguineous parents. He was the product of a spontaneous pregnancy to a 33-year-old mother and a 34-year-old father at the time of birth. Mother denied an exposure to any known teratogens including alcohol, smoke, or drugs during pregnancy. Both parents were of Mexican ancestry and their family history was unremarkable. Intrauterine growth restriction was noted prenatally, and he has consistently remained below the first percentile for age and sex. At birth, he weighed 2.1 kg and was 43.2 cm long. His initial examination findings were remarkable for mild hypotonia, facial dysmorphism (triangular face, small chin, prominent ears, frontal bossing), and bilateral fifth finger clinodactyly.
Echocardiogram was performed at 12 weeks of age and showed a patent foramen ovale, patent ductus arteriosus (PDA), normal segmental intracardiac anatomy, normal chamber dimensions, and normal ventricular function. The parents reported that the child was not sitting independently or babbling at 7 months, with no history of regression of skills or seizures at that time point. Subsequently, he was evaluated for failure to thrive. Computed tomography (CT) of the head was performed at 7 months of age and revealed borderline mild ventricular enlargement and no other brain malformations.
2.1.2 Molecular testing
Chromosomal microarray (CMA) was performed postnatally and demonstrated AOH for the entirety of chromosomes 7 and 9, as well as apparently low-level mosaic trisomy 21 (~20%) (arr[hg19] (7)x2 hmz, (9)x2 hmz, (21)x2-3) (Figure 1a). Polymorphic microsatellite marker analysis at loci spanning chromosomes 7 and 9 indicated homozygosity for all tested markers. Comparison to the parental genotypes showed non-Mendelian segregation of 10 informative markers (out of 12) on chromosome 7 with no paternal contribution, and 4 informative markers (out of 6) on chromosome 9 with no maternal contribution. Taken together, these results are consistent with maternal isodisomy of chromosome 7 (iUPD7) and paternal isodisomy of chromosome 9 (iUPD9) (Figure 1b). Placenta was unavailable to test for low-level aneuploidy associated with UPD affected chromosomes. No additional workup, such as whole exome sequencing to evaluate for recessive variants within any regions of homozygosity, has been performed at this time.
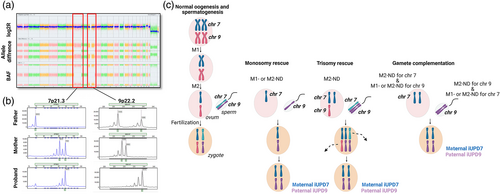
Source: Created with BioRender.com
.2.2 Patient 2
2.2.1 Clinical presentation
Patient 2 was a 4-week-old female born at 34 weeks gestation to non-consanguineous parents. Mother was of West African ancestry and father was of South Korean ancestry. Family history was unremarkable. Infant presented with omphalocele, pulmonary artery stenosis of the central branch, acute respiratory failure with hypoxia, suck-swallow incoordination (feeding difficulty), hyponatremia, hypochloremia, and hypokalemia.
2.2.2 Molecular testing
G-banded chromosome analysis was performed prenatally and showed a normal 46,XX karyotype. CMA performed postnatally showed multiple large regions of homozygosity on chromosome 4 (108.9 Mb total), chromosome 10 (53.8 Mb total), and chromosome 14 (36.5 Mb total) (arr[hg19] 4p15.1p13(31,084,801-42,831,822)x2 hmz,4q21.21q32.2(81,943,067-164,389,550)x2 hmz,4q34.1q35.2(176,176,193-190,915,650)x2 hmz,10q21.3q25.1(65,762,990-108,279,199)x2 hmz,10q26.13q26.3(124,139,766-135,434,303)x2 hmz, 14q11.2q12(20,219,082-28,492,203)x2 hmz,14q24.3q32.33(79,042,564-107,282,024)x2 hmz) (Figure 2a, b). There was no evidence of mosaic aneuploidy associated with these UPD events by SNP array on peripheral blood. Polymorphic microsatellite marker analysis demonstrated absence of maternal alleles (only paternal alleles present) for chromosomes 4, 10, and 14. Furthermore, the isodisomic regions on these chromosomes coincided with homozygous alleles, while heterozygous regions demonstrated two different alleles of paternal origin, confirming paternal mixed UPD for chromosomes 4, 10, and 14 (Figure 2c). UPD testing of chromosome 15 was also performed and showed biparental inheritance, ruling out the possibility of mosaic genome-wide UPD. CMA on formalin-fixed and paraffin-embedded (FFPE) placenta was performed to test the hypothesis of independent trisomy rescue events, however no evidence of low-level trisomy was observed for any of the UPD chromosomes. No fresh placental specimen was available for FISH analysis. No additional workup, such as whole exome sequencing to evaluate for recessive variants within any regions of homozygosity, has been performed at this time.
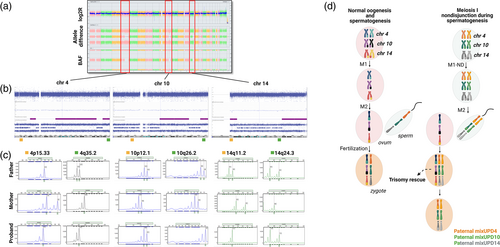
Source: Created with BioRender.com
.3 DISCUSSION
Here, we report two new cases with a rare finding of multiple chromosome UPD and presenting with complex phenotypes. In Patient 1, who presented with intrauterine growth restriction, failure to thrive, mild hypotonia, and facial dysmorphism, maternal iUPD7 and paternal iUPD9 were detected. Maternal UPD7 is associated with Russell-Silver Syndrome (RSS) and accounts for ~7%–10% of all RSS cases. Clinical presentation of RSS includes small for gestational age, postnatal growth failure, relative macrocephaly at birth, frontal bossing, which is consistent with much of this patient's phenotype (Saal et al., 2002). The PDA and clinodactyly may be related to mosaic trisomy 21.
No defined disorders of imprinting are currently associated with UPD9. Most reported cases with paternal UPD9 had abnormal phenotypes likely due to additional abnormalities such as duplication on chromosome 9 (Carvalho et al., 2015), supernumerary ring chromosome 9 (Chen et al., 2017), trisomy 9 mosaicism (Ma et al., 2015), or a pathogenic variant in a recessive gene (Yang et al., 2014). There are at least two more clinical cases reported in whom iUPD9 was noted as likely incidental and not directly contributory to phenotype (Li et al., 2019; Scuffins et al., 2021). One case of paternal iUPD9 was identified in the general population, presumably in a phenotypically normal individual (Nakka et al., 2019).
In Patient 2, who presented with omphalocele and feeding difficulties, we identified mixed UPD of paternal origin for chromosomes 4, 10, and 14. Paternal UPD14 (Kagami-Ogata syndrome) is characterized by facial abnormalities, skeletal abnormalities, abdominal wall defects, feeding difficulty with poor sucking and swallowing, and intellectual disability, which only partially explains this patient's phenotype (Kagami et al., 2012). No defined disorders of imprinting are currently associated with either paternal UPD4 or UPD10. In the literature there are 10+ cases of whole-chromosome UPD4 (one confirmed to be paternal) (Aminoff et al., 2012; Cottrell et al., 2012; Ding et al., 2012; Liu et al., 2015; Liu et al., 2020; Middleton et al., 2006; Palumbo et al., 2015; Scuffins et al., 2021; Spena et al., 2004; Yauy et al., 2020) and one case of mixed UPD10 (parental origin not reported) (Scuffins et al., 2021). In most cases, clinical phenotypes were noted to be likely associated with phenotypic expression of a recessive disorder or with pathogenic variants in other than UPD-affected chromosomes. In the general population, there are at least 21 cases of UPD4 (5 of paternal origin), three cases of paternal UPD10, and one case of maternal UPD10 identified (Nakka et al., 2019).
As described above, cases of UPD for chromosomes 4, 9, and 10 have been previously described; however, the clinical presentations were attributed to additional genetic alterations. Given that UPD for chromosome 4, 9, and 10 has also been reported in apparently asymptomatic individuals, it is possible that these findings might be incidental and not directly contributory to a clinical phenotype of the patients. However, we cannot exclude a risk of expressing recessive conditions mapping to the regions of homozygosity. Therefore, the expected phenotypic outcomes in our patients are unclear.
UPD arises from somatic correction of aneuploidy by several mechanisms such as trisomy rescue, monosomy rescue, and gamete complementation (Conlin et al., 2010). Identifying a specific mechanism underlying UPD is often challenging as several types of segregation errors can result in the same outcome. For example, complete isodisomy may result from three segregation errors: monosomy rescue, trisomy rescue, or gamete complementation (Niida et al., 2018). In Patient 1 with maternal iUPD7 and paternal iUPD9, gamete complementation would require four independent non-disjunction errors with no recombination in meiosis I for the respective chromosomes, which would be a very unlikely event. We hypothesize that the most likely mechanism forming double iUPD in this patient was fertilization of a nullisomic oocyte for chromosome 9 by a nullisomic sperm for chromosome 7 followed by monosomy rescue. In this scenario, paternal and maternal nondisjunction may have occurred during either meiosis I or II both with no crossing-over events (Figure 1c).
Mixed UPD most often is maternal in origin due to higher rates of maternal versus paternal meiotic nondisjunction, and therefore the observation of paternal mixed UPD for all three chromosomes (4, 10, and 14) in Patient 2 was unexpected. Given that regions of homozygosity on the affected chromosomes did not include pericentromeric regions, we hypothesized that paternal nondisjunction may have occurred during meiosis I followed by three independent trisomy rescue events after a formation of a trisomic zygote (Del Gaudio et al., 2020) (Figure 2d). CMA on placenta was performed to test this hypothesis, however no evidence of low-level trisomy was observed for any of the UPD affected chromosomes.
Typically, when AOH is detected on more than one chromosome, shared ancestry or consanguinity is suspected. Our second case highlights that the detection of multiple regions of homozygosity on three chromosomes should also raise suspicion of UPD in addition to consanguinity or identity by descent, particularly in the presence of terminal regions of homozygosity (Hoppman et al., 2018). Still, identifying cases suspicious of UPD might be challenging as the size of regions of homozygosity associated with heterodisomy is variable and might overlap with the sizes of regions of homozygosity associated with parental consanguinity. Of note, approximately one-third of all cases of molecularly confirmed UPD do not show extended regions of homozygosity and therefore are not detectable by CMA (Hoppman et al., 2018). Therefore, we speculate that the prevalence of multiple chromosome UPD is likely underestimated in clinical populations as many of these UPD case may either go undetected or are misinterpreted as representing identity by descent. We also anticipate that with the improved resolution of diagnostic tests, more patients will receive a molecular diagnosis of multiple chromosome UPD.
In conclusion, we describe two cases with multiple chromosome UPD involving both imprinted and non-imprinted chromosomes and presenting with complex phenotypes. We also demonstrate that the detection of regions of homozygosity on two or more chromosomes should raise suspicion of UPD in addition to consanguinity and may warrant additional clinical and laboratory investigation, especially when involving chromosomes known to be associated with imprinting disorders.
4 METHODS
4.1 Evaluation of copy number alterations and regions of homozygosity
Chromosomal microarray on DNA extracted from peripheral blood was performed using both copy number and single-nucleotide polymorphism (SNP) probes on a whole-genome array (Thermo Fisher Scientific [Affymetrix] CytoScan HD platform). CMA analysis on DNA extracted from formalin-fixed, paraffin embedded placental tissue was performed using either on molecular inversion probes on a whole- genome array (Thermo Fisher Scientific [Affymetrix] OncoScan platform) using in silico controls. All data was analyzed in Chromosome Analysis Suite (V.3.3.0.139) and reported using the February 2009 NCBI human genome build 37.1 (hg19).
4.2 Evaluation of DNA-based polymorphic markers
PCR-based analysis on the DNA from the proband and both parents was used to test for the presence of UPD of selected chromosomes. The following microsatellite markers were used: chromosome 4—D4S403, D4S405, D4S392, D4S2930; chromosome 7—D7S517, D7S641, D7S493, D7S516, D7S484, D7S510, D7S630, D7S657, D7S515, D7S530, D7S2465, and D7S242; chromosome 9—D9S288, D9S157, D9S1817, D9S283, D9S1677, and D9S1838; chromosome 10—D10S591, D10S197, D10S1652, D10S1730, and D10S217; chromosome 14—D14S261, D14S283, D14S275, D14S70, D14S288, D14S276, D14S63, D14S258, D14S74, and D14S985; chromosome 15—D15S128, D15S1002, D15S165, D15S1007, D15S1012, D15S978, D15S153, D15S131, D15S205, D15S127, D15S13, and D15S120. Electrophoresis of the amplicons was performed on ABI 3730xl DNA Analyzer and analyzed using GeneMarker Software (V.3.0.0).
4.3 Editorial policies and ethical considerations
This study was approved by The Mayo Clinic Institutional Review Board (15-007359).
AUTHOR CONTRIBUTIONS
Katarzyna Polonis, Ross A. Rowsey, Nicole L. Hoppman: conception of the case report, Katarzyna Polonis, Jaime L. Lopes, Stuart Schwartz, Linda Hasadsri, Ross A. Rowsey, Nicole L. Hoppman: data analysis and interpretation; Katarzyna Polonis: drafting the manuscript; all authors: critical revision of the manuscript and final approval of the version to be published.
ACKNOWLEDGMENTS
We thank Joseph A. Thompson (Mayo Clinic, Rochester, MN) for preparing images for this publication.
FUNDING INFORMATION
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




