5p13 microduplication in a malformed fetus and his unaffected father
Abstract
The 5p13 microduplication syndrome is a contiguous gene syndrome characterized by developmental delay intellectual disability, hypotonia, unusual facies with marked variability, mild limb anomalies, and in some cases brain malformations. The duplication ranges in size from 0.25 to 1.08 Mb and encompasses five genes (NIPBL, SLC1A3, CPLANE1, NUP155, and WDR70), of which NIPBL has been suggested to be the main dose sensitive gene. All patients with duplication of the complete NIPBL gene reported thus far have been de novo. Here, we report a 25-week-old male fetus with hypertelorism, wide and depressed nasal bridge, depressed nasal tip, low-set ears, clenched hands, flexion contracture of elbows, knees, and left wrist, and bilateral clubfeet, bowing and shortening of long bones and brain malformation of dorsal part of callosal body. The fetus had a 667 kb gain at 5p13.2 encompassing SLC1A3, NIPBL and exons 22–52 of CPLANE1. The microduplication was inherited from the healthy father, in whom no indication for mosaicism was detected. The family demonstrates that incomplete penetrance of 5p13 microduplication syndrome may occur which is important in genetic counseling of families with this entity.
1 INTRODUCTION
Copy number variants (CNVs) are recognized as an important cause of developmental delay/intellectual disability, autism spectrum disorders, and congenital anomalies (Miller et al., 2010). Gains or losses of genomic material exceeding 1 kb are defined as CNVs (Redon et al., 2006). Itsara et al. (2009) found that approximately 65–80% of normal individuals have CNVs of at least 100 kb, 5–10% have a CNV of at least 500 kb, and 1% has a CNV of at least 1 Mb. Obviously, it is essential to determine the clinical significance of these CNVs. A scoring system has been developed by American College of Medical Genetics and Genomics to help clinicians determine the pathogenicity of CNVs. The system classifies CNVs into pathogenic, likely pathogenic, variant of uncertain significance, likely benign, and benign. Factors that suggest pathogenicity of a copy number gain are overlap with known triplo-sensitive regions; the breakpoint is within a gene known to be haplo-insufficient; the phenotype is consistent with loss of function (LOF) of the gene; the copy number gain contains more than 34 genes; the phenotype is specific to the gene or genome region; the copy number gain co-segregates with a phenotype seen in the patient's family; and increase in observation of cases compared to controls (Riggs et al., 2020). A high score indicates that pathogenicity is more likely. If the copy number gain is found in another unaffected family member, it has a negative score and suggests it being benign. However, examples of (possibly) pathogenic CNVs inherited from a healthy parent, or being present in a control population have been reported, such as duplications of distal 1q21.1 (Mefford et al., 2008), distal 16p11.2 (Jacquemont et al., 2011; Weiss et al., 2008), proximal 16p11.2 (Giaroli et al., 2014), and 15q13.3 (Cooper et al., 2011).
For most genes, there is a high tolerance for dosage change, but gene dosage changes can have deleterious effects for some genes (Rice & McLysaght, 2017). If more than a single gene is duplicated, the phenotype is the result of the various consequences of these duplications (Newman et al., 2015). A duplication disrupting the coding sequence of a gene can act differently, as an LOF as consequence (Newman et al., 2015).
Duplications within the region 5p13 of 1 Mb or larger have been reported in several patients, with variable clinical findings (Avansino et al., 1999; Cervera et al., 2005; Lorda-Sánchez et al., 1997; Loscalzo et al., 2008; Oexle et al., 2011; Rethoré et al., 1989). The duplications were identified by classical karyotyping or fluorescent in situ hybridization techniques. Advanced techniques such as oligo array comparative genomic hybridization have allowed identification of smaller duplications in the 5p13 region (Carrascosa Romero et al., 2012; Lucarelli et al., 2017; Novara et al., 2013; Walters et al., 2010; Yan et al., 2009). NIPBL was suggested to be the major dose sensitive gene in this region (Carrascosa Romero et al., 2012; Lucarelli et al., 2017; Novara et al., 2013; Walters et al., 2010; Yan et al., 2009). This allowed recognition of the 5p13 microduplication syndrome [MIM #613174] as a contiguous gene syndrome ranging in size from 0.25 to 1.08 Mb. Since NIPBL is the major dose sensitive gene in the region and since duplication of only part of the gene might lead to a completely different phenotype as no protein might be formed or, in case of an intragenic duplication, might lead to a nonfunctional NIPBL from that allele, we have considered only cases with a duplication of the complete NIPBL gene as having the 5p13 microduplication syndrome. We mention the other cases here for completeness as having a 5p microduplication, but not having the syndrome (Table 1). All patients with 5p13 microduplication syndrome reported thus far have been de novo. Here, we describe a fetus that inherited 5p13 microduplication from his unaffected father and describe the various mechanisms that can explain this.
| 5p13 microduplication syndrome patients | 5p13 microduplication patientsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yan et al. (2009) | Carrascosa Romero et al. (2012) | Novara et al. (2013) | Lucarelli et al. (2017) | Present patient | Yan et al. (2009) | Walters et al. (2010) | ||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 1 | Patient 2–7b | |
| Sex | F | F | F | M | M | F | F | M | F | 3F/2M |
| Age | 18 years | 6 years | 30 years | 8 months | neonate | 9 months | 5 years | 25 weeks of gestation | 5 years | 8–48 years |
| Weight at birth | NA | <5% | <5% | 1% | 50–75% | “nl” | NA | 29% | 50% | NA |
| Length at birth | NA | 3% | 3% | NA | 50–75% | “nl” | 15% | 49% | NA | NA |
| Height | “nl” | “nl” | nl | <5% | 50–75% | 10–25% | 97% | − | 3% | 3–50% |
| Head circumference | “macrocephaly” | NA | NA | NA | 50–75% | 25–50% | 97% | 55% | NA | 25–90% × 4 90–97% |
| Hair | NA | NA | Low posterior hairline | Sparse | Sparse | NA | Thin | nl | NA | NA |
| Eyes | Upslanting palpebral fissures exotropia |
Astigmatism strabismus |
Hypotelorism unilateral epicanthus |
Hypertelorism proptosis |
Hypertelorism sparse eyebrows |
Downslanting palpebral fissures mildly pale retina |
− | Hypertelorism | Short palpebral fissures | NA |
| Nose | Bulbous tip broad root |
Flat, wide base | NA | NA | Broad root | Wide bridge, depressed and bifid tip, short columella | Broad nasal bridge | Wide, depressed bridge | Groove above root | Broad × 1 |
| Philtrum | Short | Short | Short | Long | Poorly defined | NA | ||||
| Mouth | Downturned corners narrow palate |
nl | Highly arched palate | Highly arched palate | Thin lips | Thin upper vermilion everted lower vermilion | nl | Thin upper vermilion | Absence of several incisors | NA |
| Chin | Prognathism | nl | Micrognathia | NA | Micrognathia | NA | Micrognathia | Micrognathia | NA | Micrognathia × 4 |
| Ears | Low set | Low-set, posteriorly rotated, cupped | NA | Low set, small simple | Low set small flat helix |
Low-set, protruding | Low-set, large | Low-set | Ear pits Hearing loss |
Low-set × 3 |
| Limbs | Long fingers; large hands/feet; deviated thumb | Long fingers, genu valgum | nl | nl | Large hands/feet, long fingers | Long fingers/toes | Valgus feet | Clenched hands, contracture of elbows/knees/wrist, clubfeet, long fingers/toes | Short fifth fingers | nl |
| Behavior | Self-injuries stereotypy, obsessive- compulsive |
“nl” | Pervasive disorder | NA | NA | “nl” | NA | NA | NA | 3/5 autistic behavior, ADHD ×2 ADD × 1 |
| Seizures | − | Staring spells | NA | − | − | Abnormal EEG | Abnormal EEG, single febrile convulsion | NA | + | NA |
| Hypotonia | − | + | NA | + | + | + | + | NA | + | − |
| Intellectual disability | + | + | + | NA | mild | + | + | NA | + | − |
| Brain MRI | NA | NA | NA | Agenesis of corpus callosum | Agenesis of corpus callosum | Small corpus callosum, periventricular white matter hyperintensities | Periventricular white matter hyperinten-sities | Dorsal dysgenesis of corpus callosum | NA | NA ×3 “nl” × 2 |
| Other | Scoliosis | Craniosynostosis | Unilateral renal agenesis | Sleep apnea | Absent labia minora | Maternal alcohol use in first trimester | Obesity × 4 | |||
- Abbreviations: ADD: attention deficit disorder; ADHD: attention deficit hyperactivity disorder; F: female; M: male; NA: not available; nl: normal.
- a Patients with partial duplication of the NIPBL gene are separately shown as 5p13 microduplication patients.
- b Mother and four offspring.
2 CLINICAL REPORT
The proband is the result of the first and only pregnancy of a healthy, first-cousin Iranian couple. First trimester chromosomal screening at 12 + 5 weeks showed a 1-in-11 risk for trisomy 13/18. Noninvasive prenatal testing at 13 + 5 weeks showed low risk for trisomy 13, 18, 21, and numerical anomalies involving X and Y chromosomes. Ultrasound exam at 19 weeks showed pleural effusion, and at 25 + 2 weeks oligohydramnios, some ascites, mild bowing and shortening of the lower limbs, clubfeet, and possibly a malformed corpus callosum. The parents decided to terminate the pregnancy.
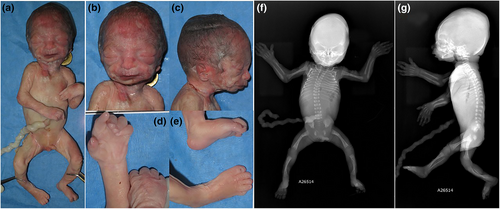
An autopsy performed showed a 25-week male fetus weighing 733 g (29th centile), with a crown-heel length of 34.0 cm (49th centile), crown-rump length of 24.5 cm (57th centile), and a head circumference of 25.0 cm (55th centile). The face showed hypertelorism, a wide and depressed nasal bridge, depressed nasal tip, underdeveloped malar regions, thin upper vermillion, low-set ears, and retrognathia (Figure 1). The hands were clenched with bilateral single palmar crease, fingers and toes were long, and there were flexion contractures of elbows, knees and left wrist, and bilateral clubfeet (Figure 1). The brain was normal except for a malformed dorsal part of the callosal body. Radiology demonstrated mild bowing and shortening of femora, tibiae and fibulae, and metaphyseal dysplasia of femora and tibiae (Figure 1). The placenta showed several thrombotic veins and large area of subchorionic hemorrhage.
3 MATERIALS AND METHODS
Genomic DNA was extracted from a muscle biopsy of the fetus using a salting-out method. Whole genome oligo-array CGH was performed using CYTOCHIP ISCA 4X44K whole genome oligo array version 1.1 (Illumina San Diego, CA), and was analyzed using BlueFuse Multi software. The array consists of 44,000 spots with average resolution of 75 kbs. The sample was hybridized twice in a dye swap experiment against male and female samples as controls. DNA from lymphocytes and epithelial cells in saliva from the father and DNA from lymphocyte from the mother were analyzed on the Agilent 8X60K whole genome oligo array ISCA design.
Aliquots of 4000 ng of the patient and reference DNAs were double-digested with RsaI and AluI (Illumina) for 2 h at 37°C. After heat inactivation of restriction endonucleases at 65°C for 20 min, each digested sample was labeled using the dCTP Labeling Kit (Illumina) for 4 h at 37°C, using Cy3-dUTP for the patient DNAs and Cy5-dUTP for the reference DNAs. The efficiency of the labeling was measured using a nanodrop spectrophotometer. The DNAs were properly combined with 25 μg of Cot-1 DNA. The DNA denatured for 5 min at 95°C. Pre-annealing was performed for 30 min at 37°C. The samples were hybridized at 65°C for 24 h in hybridization oven with rotator, and then the non-hybridized DNA was removed using two post-hybridization washing. Images of the chips were acquired using the Innopsis scanner at 5 μm resolution. Microarray image files were analyzed using Blufuse Multi software for the 44K microarray and Agilent feature extraction followed by Agilent Cytogenomics software for the 60K microarray.
To minimize false positive calls those genomic imbalances that include at least three clones were called. On the 44 K array, this results in CNVs ranging from 150 kb (in regions with high-density probe coverage) to 250 kb (in regions with low density probe coverage) and on the 60K array, this results in resolutions ranging from 25 to 225 kb. Each CNV was filtered for common variants against the database for genomic variants. Those CNVs that differed by at least 100 kb on either side or a total of 100 kb on both sides were additionally compared and screened against in house database which consists of over 10,000 prenatal and postnatal samples. The postnatal samples are comprised of healthy individuals, patients referred for developmental delay and intellectual disability, multiple congenital anomalies, autism spectrum disorders, and similar indications, and prenatal samples consist of fetal samples indicated following first trimester screening. CNVs occurring with frequencies equal to or exceeding 1/1000 were excluded. The second filtering step removes variants that may be specific to our population.
4 RESULTS
The CGH array showed a 667 kb gain of short arm of chromosome 5 at 5p13.2, between 36,520,895 and 37,187,880 (GRCH37/hg19). The father was found to have the same gain at 5p13.2 tested on DNA extracted from lymphocytes and epithelial cells of saliva, without clues for mosaicism, the mother did not show any gains or losses. The father also had a loss of 476 kb on 9q21.12q21.12 of uncertain significance (ACMG). The region overlaps only TRPM3 (Transient Receptor Potential Cation Channel subfamily M member 3) as OMIM gene (*608961). TRPM3 has a function in renal calcium homeostasis but is not known to be associated with a disorder.
The duplicated region in fetus and father contains the MIM genes 600,111: SLC1A3 (Solute Carrier Family 1 A4), 608,667: NIPBL (Nipped-B-Like), and exons 22–52 of CPLANE1. Subsequently the father was evaluated clinically but no manifestations fitting the 5p13 microduplication syndrome were present.
5 DISCUSSION
The 5p13 microduplication syndrome (MIM 613174) is a contiguous gene syndrome. The NIPBL gene located in the region has been highlighted as the main dose sensitive gene (Carrascosa Romero et al., 2012; Lucarelli et al., 2017; Novara et al., 2013; Walters et al., 2010; Yan et al., 2009). NIPBL pathogenic variants, which are associated with Cornelia de Lange syndrome 1 (CdLS; MIM #122470), were duplicated partially or completely in all reported cases (Table 2). Four cases have been reported in the DECIPHER database with microduplication less than 1.5 Mb overlapping NIPBL and adjacent genes, ranging in size from 0.4 to 1.29 Mb (264,548; 406,539; 249,589; 270,011). Unfortunately, there was only limited or no description of the phenotype (in two patients autistic features and eye anomalies were indicated). Therefore, we were unable to compare data to the other patients in Table 1. The Database of Genomic Variants does not list individuals with a normal phenotype and a duplication of NIPB (MacDonald et al., 2014). SLC1A3 (MIM #600111), CPLANE1 (also indicated as C5ORF42 or FLJ13231; MIM *614571), NUP155 (Nucleoporin 155-KD; also indicated as KIAA0791; MIM #606694), and WDR70 (WD repeat-containing protein 70; MIM #617233) have been the other genes involved in patients with 5p13 microduplication syndrome (Figure 2). SLC1A3 is associated with autosomal dominant ataxia type 6 (MIM #612656) but clinically this has not been mentioned in the two patients reported to have a duplication of this gene. CPLANE1 is associated with autosomal recessive Joubert syndrome 17 (MIM #614615), and autosomal recessive Orofaciodigital syndrome VI (MIM #277170). Homozygous or compound heterozygous variants in NUP155 can cause familial atrial fibrillation type 15 (MIM #615770) (Oberti et al., 2004). Variants in WDR70 are not known to be associated with a disorder.
| Patient | Paper | Duplication size (Mb) | Genomic region | Genes |
|---|---|---|---|---|
| 1 | Yan et al. (2009) | 0.39 | 36,845,462–37,231,819a | NIPBL, CPLANE1 (exons 3–39UT) |
| 2 | Yan et al. (2009) | 0.39 | 36,845,462–37,231,819a | NIPBL, CPLANE1 (exons 3–39UT) |
| 3 | Yan et al. (2009) | 1.07 | 36,487,985–37,556,112a | SLC1A3, NIPBL, CPLANE1, NUP155, WDR70 (exons 1–9) |
| 4 | Yan et al. (2009) | 0.25 | 36,870,689–37,120,908a | NIPBL |
| 5 | Yan et al. (2009) | 0.33 | 37,084,359–37,417,228a | NIPBL (exons 39–47), CPLANE1, NUP155, WDR70 (exons 1–2) |
| 6 | Carrascosa Romero et al. (2012) | 0.9 | 36,255,202–37,167,438a | SCL1A3 and NIPBL CPLANE1 |
| 7 | Novara et al. (2013) | 0.26 | 36,809,705–37,073,754 | NIPBL |
| 8–12 | Walters et al. (2010) | 0.341 | 36,669,569–37,010,749 | Exon 3/4–9/10 of SLC1A3, exons 1–20 of NIPBL |
| 13 | Lucarelli et al. (2017) | 0.549 | 36,609,093–37,158,361 | SLC1A3, NIPBL, CPLANE1 |
| 14 | Present | 0.667 | 36,520,895—37,187,880 | SLC1A3, NIPBL CPLANE1 |
- a Analyzed using build 36.1, the remaining were analyzed with GRCh37/hg 19.
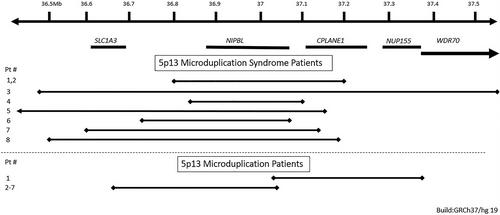
Yan et al. (2009) were the first to describe five patients with a small duplication of part of the 5p13 region who all had developmental delay and intellectual disability, a variably unusual face and limb defects. The duplicated region contained in all part or the complete NIPBL gene. Heterozygous variants leading to haploinsufficiency in NIPBL can cause CdLS, which is characterized by developmental delay and intellectual disability, limb abnormalities and an unusual face that is distinct (synophrys, short nose, convex nasal ridge, long and smooth philtrum, thin upper vermillion, and downturned corners of mouth) and differs from the characteristics in the 5p13 microduplication syndrome (Kline et al., 2018). Additional patients with a small 5p13 microduplication including all or part of NIPBL, with similar features, have been reported (Table 1) (Carrascosa Romero et al., 2012; Lucarelli et al., 2017; Novara et al., 2013; Walters et al., 2010). The main clinical characteristics of the patients are the delayed development and intellectual disability, hypotonia, and variable facial characteristics, of which low-set ears and a wide nasal bridge, wide nasal ridge and wide nasal tip seem the most constant features. Mild brain malformations are not uncommon, and several hand limited limb anomalies such as long fingers and toes and foot position anomalies (Table 1).
The overlapping duplicated gene in all these 14 cases is NIPBL (Table 1). Three of the cases/families have partial overlap of NIPBL gene. Patient 5 from Yan et al., 2009 report has a partial duplication of exons 39–47. Yan et al., 2009 suggests that most probably the partial duplication of NIPBL does not cause increased expression because of the 3′ location of the duplicated material and the phenotype could be caused by alcohol ingestion of the mother during pregnancy (Yan et al., 2009). The duplication reported in DECIPHER 264548 covers 2–47 exons but the increased expression is not known (Firth et al., 2009). The family reported by Walters et al. (2010) had 338—341 kb duplication of 5′ portion of NIPBL, exons 1–20 including the promoter region and transcription start site suggesting overexpression of NIPBL. However, attribution of the phenotype to the partial NIPBL duplication could not be confirmed since expression studies were not performed (Walters et al., 2010). Walters et al. (2010) reported a mother and her four affected offspring (two daughters and two sons) the duplication contained next to part of NIPBL also the 3′ portion of SLC1A3 (MIM:600111), and the duplicated region was inserted into the X-chromosome. The two female offspring had milder features compared to the affected males. Skewed X-inactivation in the mother was suggested as explanation for the very mild features in the mother, the absence of skewing in the female offspring was suggested to explain their relative mild features compared to their affected brothers.
We included all patients with a microduplication of less than 1.5 Mb of the 5p13 region in this paper, however we suggest only patients with a duplication of less than 1.5 Mb and containing duplication of the complete NIPBL gene to be included in the definition of 5p13 microduplication syndrome. One of the patients reported by Yan et al. (2009), the duplication reported in DECIPHER 264548 and the mother and four offspring reported by Walters et al. (2010) all have partial duplication of NIPBL gene and overexpression of NIPBL could not be confirmed in any of these patients therefore we believe they do not fulfill our criteria for 5p13 microduplication syndrome.
All patients with 5p13 microduplication syndrome reported thus far were de novo. Here, we report a fetus with a 5p13 microduplication who demonstrates abnormal clinical features of face, limbs, and brain that are compatible with this syndrome; however, the limb anomalies were somewhat more marked than in previously reported patients (Table 1). In our case, the microduplication was inherited from the healthy father. Several mechanisms may explain the reduced penetrance in the father. One is mosaicism in the father which is unlikely sinceoa-CGH on different tissues (lymphocyte and epithelial cells derived from saliva) confirmed the same duplication. A second possible explanation for the incomplete penetrance is that a second event is required for a detectable phenotype (Girirajan et al., 2011). In the fetus, no additional CNV was evident. Another possibility is that the 5p13 microduplication is not always fully penetrant and that other genetic or environmental influences are needed to express the full phenotype (Girirajan et al., 2011). There is no clue for an environmental influence for the present fetus. Searching for additional genetic influences cannot be reliably done in a single patient but will need a series of patients (Girirajan et al., 2011), in whom whole exome or genome sequencing needs to be performed searching for variants within the duplicated region and elsewhere in the genome, followed by functional studies for candidate genes. The variation in the phenotype of a 5p13 microduplication may then be explained by differences in the additional genes involved.
Irrespective the pathogenesis we conclude that a 5p13 microduplication syndrome can have incomplete penetrance. Obviously, this has implications in counseling similar families. We recommend a thorough evaluation of parents and other at risk relatives be considered for other patients in which 5p13 microduplication is detected.
AUTHOR CONTRIBUTIONS
The authors confirm contribution to the paper as follows: study conception and design: Ariana Kariminejad, Raoul C. M. Hennekam, data collection: Ariana Kariminejad, Siavash Ghaderi-Sohi, Soheila Gholami, Kimia Najafi, Roxana Kariminejad, analysis and interpretation of results: Ariana Kariminejad, Siavash Ghaderi-Sohi, Soheila Gholami, Kimia Najafi, Roxana Kariminejad, draft manuscript: Ariana Kariminejad, Raoul Hennekam: All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENT
The authors would like to thank the family for their collaboration.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




