The implementation of an enhanced clinical model to improve the diagnostic yield of exome sequencing for patients with a rare genetic disease: A Canadian experience
Grace Uwaila Ediae and Gabrielle Lemire contributed equally to this work.
Funding information: Genome Canada; Ontario Genomics Institute, Grant/Award Number: OGI-147; Canadian Institutes of Health Research, Grant/Award Number: FDN-154279; Ontario Research Fund; Genome Alberta; Genome British Columbia; Genome Quebec; Children's Hospital of Eastern Ontario Foundation; CHAMO (Children's Hospital Academic Medical Organization); Tier 1 Canada Research Chair in Rare Disease Precision Health
Abstract
The introduction of clinical exome sequencing (ES) has provided a unique opportunity to decrease the diagnostic odyssey for patients living with a rare genetic disease (RGD). ES has been shown to provide a diagnosis in 29%–57% of patients with a suspected RGD, with as many as 70% remaining undiagnosed. There is a need to advance the clinical model of care by more formally integrating approaches that were previously considered research into an enhanced diagnostic workflow. We developed an Exome Clinic, which set out to evaluate a workflow for improving the diagnostic yield of ES for patients with an undiagnosed RGD. Here, we report the outcomes of 47 families who underwent clinical ES in the first year of the clinic. The diagnostic yield from clinical ES was 40% (19/47). Families who remained undiagnosed after ES had the opportunity for follow-up studies that included phenotyping and candidate variant segregation in relatives, genomic matchmaking, and ES reanalysis. This enhanced diagnostic workflow increased the diagnostic yield to 55% (26/47), predominantly through the resolution of variants and genes of uncertain significance. We advocate that this approach be integrated into mainstream clinical practice and highlight the importance of a coordinated translational approach for patients with RGD.
1 INTRODUCTION
Rare diseases are individually rare, but, collectively, affect up to one in 16 Canadians; over 39% are attributed to an underlying genetic etiology (Ferreira, 2019). Patients living with a rare genetic disease (RGD) often have multiple specialist consultations, several misdiagnoses, and a variety of assessments spanning years before receiving an accurate diagnosis—an experience often referred to as a “diagnostic odyssey” (Global Commission to End the Diagnostic Odyssey for Children with a Rare Disease, 2019). Exome sequencing (ES) has been shown to be an effective diagnostic tool, providing a diagnosis in 29%–57% of patients with a suspected RGD (Clark et al., 2018). The diagnostic yield is influenced by the clinical indication, technology used, timing of testing in the diagnostic pathway, sequencing strategy (singleton vs. trio), and whether the analysis was done in a hospital-based or reference laboratory (Clark et al., 2018). For many Canadians, genome-wide sequencing has the potential to shorten the diagnostic journey. In the province of Ontario clinical ES is offered as a diagnostic test funded by the provincial government health insurance plan, and is available primarily to patients who are evaluated by a physician practicing in the field of genetics and are found to meet specific eligibility criteria.
It is estimated that up to 50% of patients undergoing appropriate diagnostic genetic tests will remain without a definite molecular diagnosis, receiving either a negative or a nondiagnostic result (Shashi et al., 2014). The increasing identification of variants of uncertain significance (VUS) from broad genetic testing such as ES can lead to unclear, or nondiagnostic, results. In addition, the fact that there are currently thousands of RGD with unknown causes significantly contributes to negative results (Bamshad et al., 2019). Several research programs have been dedicated to identifying the molecular etiologies of patients with undiagnosed RGD, including the Care4Rare/FORGE Canada Consortium (Canada) (Beaulieu et al., 2014), the Deciphering Developmental Disorders Study (United Kingdom and Republic of Ireland) (Deciphering Developmental Disorders Study, 2017), the Solve-RD Consortium (across Europe) (Zurek et al., 2021), the Center for Mendelian Genomics Consortium (Baxter et al., 2022), and the Undiagnosed Disease Network (Gahl et al., 2015) (both in the United States). For the past decade, these and other research programs have been using genomic sequencing technologies (primarily ES) to identify novel genes, and to investigate VUS and genes of uncertain significance (GUS) using research-based methods such as deep-phenotyping, international data sharing and collaboration, exome reanalysis, extensive cascade Sanger segregation in families, and functional studies. Recently, these groups have shifted focus towards studying the potential utility of new genetic technologies such as genome sequencing, RNA sequencing, epigenomics, metabolomics, and lipidomics. As such, we recently proposed that many of these earlier research methods could be well suited for use by the clinical care team, and should be implemented as part of an enhanced diagnostic workflow for undiagnosed RGD patients (Hartley et al., 2020). Unfortunately, this type of approach is not typically integrated into most genetic clinics due to limited time, resources, and/or knowledge; as a result, the testing process for many patients with RGD ends after clinical ES, even if the result is nondiagnostic.
In January 2019, the Regional Genetics Program at the Children's Hospital of Eastern Ontario (CHEO) launched a new clinical model, which aimed to integrate genomics at the outset of clinical care, and provide an enhanced diagnostic workflow, including the clinical integration of what were traditionally research opportunities, for patients living with a complex undiagnosed RGD. As a final step, the CHEO Exome Clinic offered undiagnosed patients research opportunities through the “Care4Rare SOLVE” research program, a research study under the Care4Rare Canada consortium. Care4Rare SOLVE is a large-scale project with multiple objectives, one of which is to use multiomic approaches and functional studies to facilitate diagnoses for undiagnosed patients with RGD. In the present article, we discuss the workflow of the CHEO Exome Clinic, present the 47 families who underwent clinical ES in the first year of the clinic, and describe the outcomes of the enhanced diagnostic workflow for families who remained undiagnosed after clinical ES.
2 MATERIALS AND METHODS
2.1 ES eligibility
The clinic accepted referrals for patients who, based on the information provided in the referral, were suspected to meet the clinical ES eligibility criteria from the Ontario Ministry of Health (Genetic Testing Advisory Committee, 2016). Patients were eligible for clinical ES if, upon evaluation in the clinic, they met the criteria outlined in Table 1.
| Criteria 1—Clinical Presentation (must meet two or more of the following) |
|
|
|
|
|
|
|
|
|
| Criteria 2 —Management Impact (must meet one or more of the following) |
|
|
|
|
- Abbreviation: ES, exome sequencing.
2.2 Clinical assessment
The Exome Clinic team is composed of a clinical fellow, a clinical assistant, a genetic counselor, and two alternating medical geneticists. Patients suspected to have a RGD were referred by a primary care physician or a specialist to the CHEO Regional Genetics Program for evaluation. The clinical fellow attended the departmental referral triage meeting, and identified patients who were appropriate for the Exome Clinic. Eligible patients were offered an appointment in the Exome Clinic within 1–3 months from the time of referral. The clinical assistant contacted the family for a preappointment telephone consultation; to briefly explain what to expect at the appointment, and to highlight the importance of both biological parents being present at the appointment and/or available for sample collection, if feasible. The appointment in the Exome Clinic involved review of medical history, family history, and a physical examination. With consent, facial photographs were taken of patients, in addition to photographs of other relevant features from the dysmorphology assessment. During the appointment, the clinical team would confirm if the patient was eligible for funded ES, at which point the genetic counselor met with the family to offer ES and obtain informed consent. Those patients that were not eligible for ES were offered other standard-of-care diagnostic genetic tests or relevant investigations when appropriate. A summary is outlined in Figure S1.
2.3 Clinical ES results workflow
In 2019, ES for the Exome Clinic patients was performed at GeneDx Diagnostic Laboratory, a commercial clinical laboratory (Clinical Laboratory Improvement Amendments [CLIA]—certified, College of American Pathology [CAP]-accredited), which followed the American College of Medical Genetics and Genomics (ACMG)/Association for Molecular Pathology (AMP) Interpretation Standards and Guidelines (Richards et al., 2015). Reported variants were classified as pathogenic, likely pathogenic, or a VUS. Genes not yet definitively associated with a human disease were reported as a GUS by the clinical laboratory, and all variants reported in those genes were classified as VUS. Once results were available, the clinical team would meet to evaluate whether the result was diagnostic, partially diagnostic, or nondiagnostic, and determine a follow-up plan. A follow-up appointment was offered within 2 weeks of receiving the laboratory report to discuss the results with the family. For patients who received a diagnosis from ES, the family was provided with a personalized “diagnosis navigator” which was created by the Exome Clinic team (Figure S2). This document described the identified variant and gene, the key features of the condition, the mode of inheritance, recurrence risk, the specific recommendations for management and surveillance, available community support(s), and opportunities for participation in ongoing research on the specific disorder (if available). Patients with a nondiagnostic or partially diagnostic result were offered additional clinical and/or research investigations. Furthermore, to offer additional psychosocial support, the genetic counselor followed up with all families, by phone, 2 weeks after the results disclosure appointment. The purpose of the follow-up call was to review the ES results and the follow-up plan that was discussed, and answer any additional questions that the family had in the interim.
2.4 Enhanced diagnostic workflow
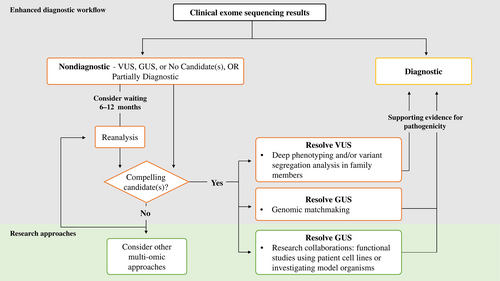
The enhanced diagnostic workflow incorporated, as appropriate, deep-phenotyping of family members, variant segregation analysis, data sharing, and exome reanalysis. Familial deep-phenotyping and variant segregation was performed for all compelling candidates identified by clinical ES—whether they be in known disease genes (e.g., VUSs) or potential novel disease genes (e.g., variants in GUSs). All candidate genes (GUSs) and high-level phenotypic information were submitted to PhenomeCentral (Osmond, Hartley, Johnstone, et al., 2022), which is a connecting node of the Matchmaker Exchange (MME), with the purpose of trying to identify additional families with rare DNA variants in the same gene, and overlapping phenotypes. MME is a federated network that connects the genotypic and phenotypic information from different gene matching platforms and thus facilitates data sharing (Philippakis et al., 2015). In addition, the testing laboratory was contacted to inquire about researchers or clinicians who follow patients with variants in the GUS, in hopes that it could enable a connection with potential collaborators. All families who received a nondiagnostic result were offered reanalysis of their data ~6–12 months after the initial nondiagnostic result was reported.
2.5 Facilitating research opportunities
Families with a nondiagnostic or partially diagnostic result were offered enrollment into the Care4Rare-SOLVE research study to share phenotypic and genomic data internationally for gene discovery purposes. Families with a compelling GUS were also offered enrollment to facilitate functional studies using patient-derived cell lines. The protocol for Care4Rare-SOLVE was approved by the institutional research ethics board at CHEO (CTO-1577). The family's raw genomic data were retrieved from the clinical laboratory that had performed the ES, and processed through the Care4Rare research bioinformatic pipeline, which has been described elsewhere (Kernohan et al., 2018). The generated variant calling file was subsequently uploaded in a Canadian rare disease research platform called Genomics4RD (Driver et al., 2022). In parallel to data retrieval, the clinical assistant extracted relevant demographic, family history, phenotypes, and variants reported from ES from the medical chart, and entered it into Genomics4RD, which is a build-out of PhenoTips software and stores phenotypic information using standardized Human Phenotype Ontology terms (Köhler et al., 2021). The re-processed ES data of each undiagnosed patient was reanalyzed by two analysts (clinical fellow, genetic counselor, and/or a local clinical laboratory scientist). The outcome of the reanalysis was presented at an “exome reanalysis rounds,” in the presence of the medical geneticist who had previously evaluated the patient and ordered the clinical ES. GUSs identified by clinical ES or exome reanalysis were further investigated using expression assays and/or functional studies (such as pathway-specific assays or protein interaction/localization studies) on patient's cell lines by the Care4Rare experimental research team. Highly compelling novel candidate genes were submitted to the Canadian Rare Diseases Models and Mechanisms (RDMM) Network to enable collaboration with a basic scientist who could study the gene of interest in a model organism (Boycott et al., 2020). If no strong candidate genes were identified after exome reanalysis by our team and a family remained undiagnosed, other multiomics technologies (genome sequencing, RNA sequencing, metabolomics, or epigenomics) were considered as part of the “Care4Rare-SOLVE” research program. Figure 1 summarizes the enhanced diagnostic workflow and approach to facilitating research opportunities.
3 RESULTS
3.1 Exome clinic cohort characteristics
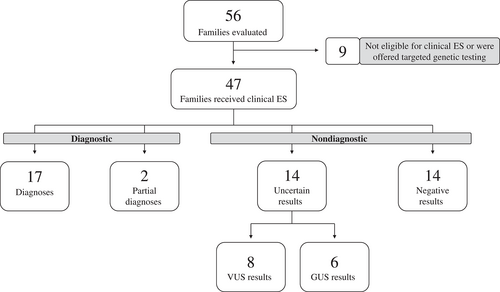
A total of 56 families were evaluated in the Exome Clinic between January 2019 and December 2019 (inclusive). A total of 47 of the 56 families were offered clinical ES. Of the nine families not offered ES, four were offered a targeted genetic test, two were not offered any genetic testing, and three were recruited directly into the Care4Rare research program for research ES or research multiomic sequencing as they were not eligible for clinically funded ES (i.e., ES was not covered by patient's provincial healthcare insurance plan), or the most appropriate next investigations were available only through research. All 47 families offered clinical ES consented to this testing. Three families had multiple affected family members, which resulted in a total of 51 patients. A majority of families (74%) underwent ES with both biological parents included (trio, quadruplet, or quintuplet sequencing strategy). A majority of patients (73%) were under the age of 18 (children/youth) and 96% presented with neurological impairment (a neurodevelopmental disorder and/or an anomaly of the nervous system), either as the sole finding or in addition to other syndromic features. Additional patient demographics are presented in Table 2 and further details related to the 47 families are in Table S1.
| Number of patients (n = 51) | Percentage | |
|---|---|---|
| Sex | ||
| Female | 26 | 51% |
| Male | 25 | 49% |
| Age | ||
| Children/Youth (<18 years old) | 37 | 73% |
| Adults (≥18 years old) | 14 | 27% |
| Number of families (n = 47) | ||
|---|---|---|
| Phenotypic category | ||
| Congenital anomalies without intellectual disability (ID) | 8 | 17% |
| Multisystemic disorder without ID | 14 | 30% |
| Single system without ID | 6 | 13% |
| Syndromic ID | 19 | 40% |
| Sequencing Strategy | ||
| Trio (proband and parents) | 33 | 70% |
| Trio (two affected siblings and parents) | 1 | 2% |
| Duo (proband and parent) | 8 | 17% |
| Singleton | 3 | 6% |
| Quadruplet (two affected siblings and both parents) | 1 | 2% |
| Quintuplet (three affected siblings and both parents) | 1 | 2% |
| Consanguinity | ||
| Yes (second cousins or closer) | 7 | 15% |
| No | 40 | 85% |
3.2 Diagnostic yield and results from clinical ES
The diagnostic yield of clinical ES for the first 47 families seen in Exome Clinic was 40% (19/47). All 19 of these families received a pathogenic or likely pathogenic result that fit the associated inheritance of the reported disease; 17 of the 19 families received a diagnosis that explained their entire clinical presentation (Figure 2 and Table S2). A diagnosis explaining only a portion of the patient's phenotype, referred to as a partial diagnosis, was identified in two families. A diagnosis navigator was provided to each of these 19 families. In total, 28 families received a nondiagnostic result: 14 of these had no variants reported (negative), and 14 received an uncertain result. The uncertain results included VUS in known disease-associated genes (eight families), or variants in GUSs (six families; Figure 2).
Furthermore, 45/47 families opted to receive ACMG secondary findings (2016 version) if identified in the proband (Green et al., 2013), with such findings reported in 6% of families (3/45). There were three maternally inherited pathogenic variants identified—two different variants in the BRCA1 gene (found in two patients and their asymptomatic mothers), and one in the DSC2 gene for arrhythmogenic right ventricular dysplasia (found in one proband and their asymptomatic mother). These patients all had an unremarkable personal and family history regarding the occurrence of cancer or cardiac abnormalities.
3.3 Enhanced diagnostic workflow
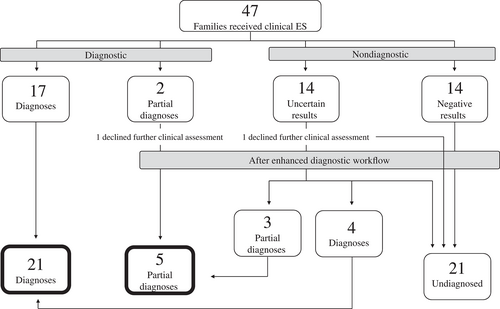
Following clinical ES, 28 of the 30 families with a nondiagnostic result opted to continue to investigate their RD etiology. Two families (one with a partial diagnosis and one with a VUS) declined further clinical or research investigations (Figure 3). The enhanced diagnostic workflow, mainly deep-phenotyping of family members along with variant segregation studies, and genomic matchmaking, led to identifying the most probable diagnoses for 7/28 families who were undiagnosed following clinical ES (Table 3). More specifically, diagnoses were identified in 7/14 families (50%) who previously had uncertain results—4 full diagnoses and three partial diagnoses (Table 3, Figure 3, and Table S1). Of these seven diagnoses, requested clinical reanalysis of the ES data identified a variant of interest in a known gene ESRRB) that was not reported by the initial clinical ES in one family, the remainder was the resolution of a VUS or a GUS reported by the clinical laboratory. The clinical team consider these seven variants to be diagnostic, based on additional genetic and/or functional evidence, and phenotypic overlap with the patient's presentation, but some of these variants in disease-associated genes do not yet meet the threshold to be classified as likely pathogenic or pathogenic as per ACMG guidelines (Richards et al., 2015). There were six GUSs reported by the clinical laboratory, of which, four led to a successful match when submitted to MME (matched with a patient with a similar or overlapping genotype and phenotype). In total, 14 families had a negative result after clinical ES and remained undiagnosed. The enhanced diagnostic workflow increased the overall diagnostic yield to 55% (26/47).
| Clinical ES reported variants | Evidence from enhanced diagnostic workflow | Supporting evidence from research | Exome Clinic team conclusions | Updated ACMG variant classification |
|---|---|---|---|---|
| Diagnoses | ||||
| VUS in candidate gene MTSS2 | Genomic matchmaking | Functional studies, model organisms study supported by MOSC | Diagnosis - published (Huang et al., 2022) | VUS (MTSS2 is a GUS) |
| VUS in candidate gene WNT9B | Segregation studies, genomic matchmaking | Diagnosis - published (Lemire et al., 2021) | VUS (WNT9B is a GUS) | |
| VUS in GH1 | Segregation studies | Partial diagnosis which explains short stature. | VUS | |
| VUS in ARR3 | Segregation studies | Partial diagnosis which explains high myopia. | Likely pathogenic | |
| VUS in candidate gene ZNF292 | Genomic matchmaking | Diagnosis - affected families have been published since clinical ES result (Mirzaa et al., 2020). Currently a definitive gene-disease relationship. | VUS | |
| VUS in candidate gene SCAI in 2 siblings | Segregation studies, genomic matchmaking | Functional studies, model organisms study supported by RDMM | Diagnosis - manuscript in preparation. | VUS (SCAI is a GUS) |
| VUS in PNPK/WDR62 and NUP62 | Reanalysis identifies a homozygous variant in ESRRB | The three VUSs are not clinically relevant. Partial diagnosis which explains hearing loss | VUS (variant in ESRRB) | |
| Potential diagnoses | ||||
| VUS in TAF1 | Segregation studies and X inactivation testing | Strong candidate variant | VUS | |
| VUS in PMP22 | Reanalysis identifies variants in KIF26A, segregation studies, genomic matchmaking | Functional studies, model organism study supported by RDMM | VUS in PMP22 is not clinically relevant. Strong candidate (KIF26A) | VUS (KIF26A is a GUS) |
- Abbreviations: ES, exome sequencing; GUS, genes of uncertain significance; MOSC, Model Organisms Screening Center of the Undiagnosed Disease Network; RDMM, Rare Diseases Models and Mechanisms Network; VUS, variants of uncertain significance.
A potential diagnosis was identified in two families and these were not counted in our diagnostic yield. The first family had a VUS in TAF1 identified by clinical ES, which was considered to be a strong candidate based on the results of familial segregation and X chromosome inactivation studies (Table 3 and Table S1). In the second family, a novel gene candidate (KIF26A) was identified after exome reanalysis, and was considered a strong candidate after an additional affected family was identified through the MME (Table 3 and Table S1). Further evidence is required before these variants would be considered diagnostic by the clinical team.
3.4 Facilitating research opportunities
There were 18 families that remained undiagnosed after the enhanced diagnostic workflow (Figure 3). All families had their raw data retrieved from the clinical laboratory and realigned and reanalyzed using the infrastructure of the Care4Rare Consortium; their data is now available for discovery via the Genomics4RD data sharing platform (Driver et al., 2022). Variants in candidate novel gene(s) were identified in five families after reanalysis, and candidate variants in a known gene were identified in two families. There was no further evidence to suggest these seven variants were causal, as there were no compelling matches identified through genomic matchmaking for the novel candidate genes. Moreover, familial segregation was nonconcordant in two of these families and the candidates were ruled out. Of the 18, 8 were offered additional research-based sequencing technologies: 6 are currently undergoing genome sequencing, and two are undergoing both RNA and genome sequencing. No further research investigations were offered for one patient, as after further evaluation, the clinical team did not believe their phenotype was likely to be explained by a Mendelian genetic etiology.
3.5 Discussion
We set out to generate evidence related to the value of an enhanced diagnostic workflow that is further improved by close relationships with research teams for additional studies. The diagnostic yield of clinical ES in the first year of the clinic was 40% (19/47). There were 17 diagnoses that explained the entirety of a patient's presentation and two that were partial diagnoses. The clinical criteria used to determine which patients were eligible for ES in our clinical model were more stringent than those used in some other published ES cohorts (Baldridge et al., 2017; Taylor et al., 2019). The majority of our patient population presented with severe functional impairment and a multisystemic disorder, which is a population known to have a high likelihood of genetic conditions (Li et al., 2021). Moreover, one of the objectives of the Exome Clinic was to ensure that whenever possible, both biological parents were available for sample collection, to aid in clinical and/or future research analysis. A sequencing strategy involving both biological parents improves the outcome of clinical ES (Clark et al., 2018; Farwell et al., 2015). These factors could explain why this cohort had a higher diagnostic yield than the average yield of clinical ES of 31% reported in the literature (Clark et al., 2018).
Our diagnostic yield increased to 55% (26/47) after the enhanced diagnostic workflow. This study demonstrated that pursuing segregation studies alongside phenotyping of family members, genomic matchmaking, and ES reanalysis have resulted in a 15% increase in the diagnostic yield in this cohort, which is likely a more significant increase than additional genomic technologies, such as genome and RNA sequencing, would have added to this cohort. Of the 26 families that had a diagnostic result, five were partial diagnoses; however, we concluded that the additional clinical features that were not explained by the genetic result were likely not due to a second underlying Mendelian genetic etiology, and thus these five patients were considered to be diagnosed (Figure 3). Two families had potential diagnoses (Table 3) that may, with time, become definitive and further increase the yield. The reanalysis was primarily conducted by the clinical team in this study. This opportunity may not be feasible for all medical genetic centers, given the costs to build a similar infrastructure are not typically financially supported by clinical institutions. However, many clinical labs offer reanalysis for patients after 1 year.
The enhanced diagnostic workflow increased the diagnostic yield, however the approaches included require additional time and resources. A considerable amount of time was dedicated to investigating VUSs in known disease genes which required phenotyping and obtaining samples from multiple family members from varying geographical locations, and arranging segregation testing in the clinical laboratory. The GUSs reported on the clinical ES reports required genomic matchmaking approaches, primarily through the MME, necessitating multiple email exchanges with other groups to determine if the match is compelling or not. The reality of investigating GUSs through MME is that many will result in false positive matches, which are ruled out after further discussion. A recent study by the Care4Rare research network reported that only 15% of novel candidate genes submitted to MME resulted in collaborations (Osmond, Hartley, Dyment, et al., 2022). As part of the enhanced diagnostic workflow, 67% (4/6) of the GUSs reported by the clinical laboratory resulted in collaborations. These GUSs are likely to have some existing supporting evidence in the literature, or the clinical laboratory may have other identified families within their internal database. Therefore, GUSs reported by a clinical laboratory are likely to be compelling candidates and warrant further investigation as part of the enhanced diagnostic workflow. Genomic matchmaking helped foster collaborations with other clinical teams to promote novel discoveries for five genes (MTSS2, WNT9B, KIF26A, ZNF292, and SCAI). We published our findings describing the association of biallelic variants in WNT9B with renal agenesis/hypoplasia/dysplasia in two families (Lemire et al., 2021), and reported MTSS2 as a cause of syndromic intellectual disability (Huang et al., 2022). Manuscripts describing the other novel disease–gene relationships are in preparation. Segregation analysis and genomic matchmaking proved to be very useful as part of the enhanced diagnostic workflow, steps that can be performed by a clinical team even with no access to a research infrastructure. This work also highlights the importance of clinical laboratories reporting GUSs with some evidence that they may be causative.
The integration of the Care4Rare research pipeline with the Exome Clinic enabled a seamless transition to research for patients who remain undiagnosed after the enhanced diagnostic workflow. Research exome reanalysis requires access to a well-established research pipeline to obtain, process, and analyze the raw sequencing data from the clinical laboratory. The reanalysis has allowed our team to identify and evaluate variants not reported by the clinical laboratory as well as ensure that the data is available for discovery in Genomics4RD. Functional studies were pursued to further explore the novel disease–gene relationships for several GUSs (MTSS2, SCAI, and KIF26A): two GUSs (KIF26A [yeast] and SCAI [fly] through submission to the RDMM Network [Boycott et al., 2020]) and one (MTSS2 [fly]) to the Model Organisms Screening Center (MOSC) of the Undiagnosed Disease Network (Baldridge et al., 2021). This enabled collaborations with basic scientists to study these three genes in model organisms and has provided additional mechanistic insight into these RDs. Families remaining without a diagnosis were offered additional multi-omic approaches through the Care4Rare SOLVE research protocol.
Our work highlights the value of an enhanced diagnostic workflow that incorporates approaches, particularly with respect to data sharing, which have traditionally been considered part of research. The diagnostic yield of clinical ES increased by 15% when VUSs and GUSs were pursued using segregation studies and genomic matchmaking, and clinical ES reanalysis was performed for undiagnosed patients in our clinical cohort. We advocate for the integration of these approaches into mainstream clinical practice, as these additional steps are accessible to clinical teams without requiring a research infrastructure, and have the potential to reduce the diagnostic odyssey for patients.
AUTHOR CONTRIBUTIONS
Conceptualization: Grace Uwaila Ediae, Gabrielle Lemire, Caitlin Chisholm, Alison Eaton, Taila Hartley, Meredith Gillespie, Sarah L. Sawyer, and Kym M. Boycott. Data curation: Grace Uwaila Ediae, Gabrielle Lemire, Caitlin Chisholm, Alison Eaton, and Samantha K. Rojas. Formal analysis: all authors. Investigation: Grace Uwaila Ediae, Gabrielle Lemire, Alison Eaton, Caitlin Chisholm, Lijia Huang, Taila Hartley, and Matthew Osmond. Methodology: Grace Uwaila Ediae, Gabrielle Lemire, Caitlin Chisholm, Alison Eaton, Taila Hartley, Sarah L. Sawyer, and Kym M. Boycott. Supervision: Gabrielle Lemire, Caitlin Chisholm, Kym M. Boycott, and Sarah L. Sawyer; Visualization: Grace Uwaila Ediae and Gabrielle Lemire. Funding Acquisition: Taila Hartley, Kym M. Boycott, and Sarah L. Sawyer, Writing-original draft: Grace Uwaila Ediae and Gabrielle Lemire. Writing-review and editing: all authors.
ACKNOWLEDGMENTS
We thank the families for their participation. Part of this work was performed under the Care4Rare Canada Consortium funded by Genome Canada and the Ontario Genomics Institute (OGI-147), the Canadian Institutes of Health Research, Ontario Research Fund, Genome Alberta, Genome British Columbia, Genome Quebec, and Children's Hospital of Eastern Ontario Foundation. Gabrielle Lemire and Alison Eaton were supported by a CHAMO (Children's Hospital Academic Medical Organization) clinical fellowship award through the Children's Hospital of Eastern Ontario. K.M.B. was supported by a CIHR Foundation Grant (FDN-154279) and a Tier 1 Canada Research Chair in Rare Disease Precision Health. We thank GeneDx for providing the raw exome sequencing data.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




