A homozygous Y443C variant in the RNPC3 is associated with severe syndromic congenital hypopituitarism and diffuse brain atrophy
Tulay Guran and Gözde Yeşil have contributed equally to this study.
Abstract
Biallelic RNPC3 variants have been reported in a few patients with growth hormone deficiency, either in isolation or in association with central hypothyroidism, congenital cataract, neuropathy, developmental delay/intellectual disability, hypogonadism, and pituitary hypoplasia. To describe a new patient with syndromic congenital hypopituitarism and diffuse brain atrophy due to RNPC3 mutations and to compare her clinical and molecular characteristics and pituitary functions with previously published patients. A 20-year-old female presented with severe growth, neuromotor, and developmental delay. Her weight, height, and head circumference were 5135 gr (−25.81 SDS), 68 cm (−16.17 SDS), and 34 cm (−17.03 SDS), respectively. She was prepubertal, and had dysmorphic facies, contractures, and spasticity in the extremities, and severe truncal hypotonia. There were no radiological signs of a skeletal dysplasia. The bone age was extremely delayed at 2 years. Investigation of pituitary function revealed growth hormone, prolactin, and thyroid-stimulating hormone deficiencies. Whole-exome sequencing revealed a novel homozygous missense (c.1328A > G; Y443C) variant in RNPC3. Cranial MRI revealed a hypoplastic anterior pituitary with diffuse cerebral and cerebellar atrophy. The Y443C variant in RNPC3 associated with syndromic congenital hypopituitarism and abnormal brain development. This report extends the RNPC3-related hypopituitarism phenotype with a severe neurodegenerative presentation.
1 INTRODUCTION
The development of the pituitary gland is tightly regulated by various signaling molecules and transcription factors. Abnormal development of the pituitary gland may result in congenital hypopituitarism (CH) (Ara et al., 2021; Gergics, 2019). Occasionally, CH may present as a component of a syndrome with extra-pituitary abnormalities. While a number of genetic mutations have been associated with phenotypes including congenital hypopituitarism, the underlying etiology of CH remains elusive in the majority of patients (Blum et al., 2018).
Pathogenic variants in components of the minor spliceosome have been associated with several diseases that include growth retardation. Pathogenic variants in RNU4ATAC are linked to microcephalic osteodysplastic primordial dwarfism type 1, Roifman syndrome and Lowry–Wood syndrome (Edery et al., 2011; Farach et al., 2018; He et al., 2011). RNPC3 codes for the U11/U12-65K protein, a component of the minor spliceosome. The minor spliceosome plays a role in the splicing of minor (U12-type) introns, which are present in ~700–800 genes in humans and represent about 0.35% of all introns (Turunen et al., 2013). Variants in RNPC3 have previously been associated with isolated growth hormone deficiency (Argente et al., 2014; Gucev et al., 2015; Yamada et al., 2021). Additionally, three siblings with novel biallelic RNPC3 variants were recently reported to manifest panhypopituitarism (Verberne et al., 2020). More recently, biallelic mutations in RNPC3 were reported in association with a phenotype including growth hormone deficiency, primary ovarian insufficiency and neuropathy (Akin et al., 2022). The phenotype associated with biallelic RNPC3 variants may therefore be variable.
Here, we report a patient with a homozygous missense variant in RNPC3, presenting with panhypopituitarism with extreme growth failure and microcephaly due to diffuse cerebral and cerebellar atrophy. The clinical, biochemical, radiologic and molecular characteristics of the patient were investigated.
1.1 Case report
A 20-year-old female presented with severe growth failure and developmental delay. She was born at term with a birth weight of 3300 g (0 SDS) to second cousin parents. At initial examination her weight, height and head circumference were 5135 g (−25.81 SDS), 68 cm (−16.17 SDS), and 34 cm (−17.03 SDS), respectively. She had the appearance of a prepubertal female. She had dysmorphic facies with extreme microcephaly, a small forehead, bitemporal narrowing, arched eyebrows, shallow orbits, long and straight eyelashes, big ears, maxillary hypoplasia, narrow palate, irregularly placed teeth, small and anteverted nose, and microretrognathia. The elbow, wrist and knee joints were restricted and had a pes equinovarus deformity with a very small, rounded toe. The skin was soft, loose, and transparent with visible veins, and was dry and fragile due to anhydrosis. Cutis marmaratus was also observed. Neurological examination revealed spasticity in all extremities, truncal hypotonia and lack of head control (Figure 1a). There were no pathological reflexes. Despite extreme short stature there were no signs of a skeletal dysplasia on imaging (Figure 1d–g). The bone age was extremely delayed at 2 years (Figure 1h).
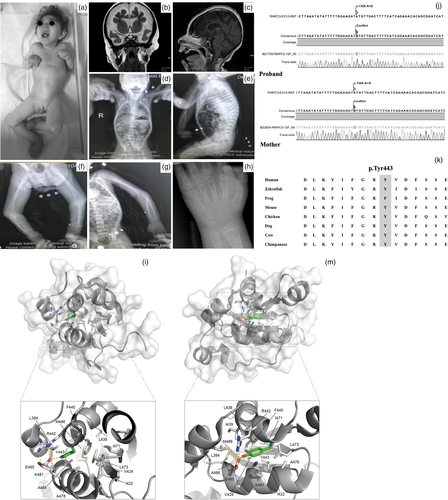
Endocrine evaluation of the patient revealed central hypothyroidism (sT4 0.25 ng/dl [0.7–1.66] and TSH 4.1 mU/L [0.51–4.8]). Early morning ACTH (13.67 pg/ml [7.2–63.3]) and cortisol (11.62 mcg/dl [Adzhubei et al., 2013; Akin et al., 2022; Argente et al., 2014; He et al., 2011; Turunen et al., 2013; Verberne et al., 2020; Yamada et al., 2021]) concentrations were normal. The serum IGF1, IGFBP3 and PRL concentrations were found to be low (IGF1: 15.2 ng/ml [127–424], IGFBP3: 0.63 mg/L [2.9–7.2] and PRL: 0.43 ng/ml [4.7–23.2]). L-dopa and clonidine growth hormone stimulation tests revealed a peak GH value of 0.03 ng/ml which reflects severe growth hormone deficiency. The basal LH value was <0.1 mIU/ml and the gonadotropin releasing hormone (GnRH) stimulation test showed hypogonadism (Table 1). There was no history of polyuria and polydipsia and the urinary specific gravity was 1015. Pituitary MRI revealed a hypoplastic anterior pituitary gland with a height of 3.1 mm. The cranial MRI showed diffuse, symmetrical cerebral and cerebellar atrophy which is most significant in frontal and temporal lobes. Decreased cortical sulci, decreased white matter volume, mega cisterna magna in posterior fossa and enlarged extra-axial cerebrospinal fluid (CSF) spaces secondary to diffuse atrophy were noted. The optic discs were atrophic and dysplastic bilaterally (Figure 1b,c). She died unexpectedly at 21 years of age due to respiratory failure.
| Measurement | Minutes | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | |
sT4 (0.70–1.66 ng/dl) |
0.25 | ||||
TSH (0.51–4.80 mU/L) |
4.1 | ||||
ACTH (7.2–63.3 pg/ml) |
13.67 | ||||
Cortisol (6–12 mcg/dl) |
11.62 | ||||
IGF-1 (127–424 ng/ml) |
15.2 | ||||
IGFBP-3 (2.9–7.2 mg/L) |
0.63 | ||||
PRL (4.7–23.2 ng/ml) |
0.43 | ||||
| GH stimulation test | |||||
| GH response to L-dopa stimulation (ng/ml) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| GH response to clonidin stimulation (ng/ml) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| GnRH test | |||||
| LH response (mIU/ml) | <0.1 | 3.97 | 4.94 | ||
| FSH response (mIU/ml) | 3.93 | 15.81 | 21.49 | ||
| E2 response (pg/ml) | 6 | 8.67 | |||
- Abbreviations: ACTH, adrenocorticotropic hormone; E2, estradiol; FSH, follicle stimulating hormone; IGF-1, insulin-like growth factor 1; IGFBP-3, insulin-like growth factor binding protein 3; GH, growth hormone; LH, luteinizing hormone; PRL, prolactin; sT4: free thyroxine; TSH, thyroid stimulating hormone.
2 MATERIALS AND METHODS
2.1 Genetic studies
The Ethical Committee of Marmara University approved the study (#01.04.2022.625). Written informed consent for publication of the clinical details and clinical images of the patient was obtained from the mother.
DNA was extracted from peripheral blood of the patient and from the only living parent, her mother. Whole exome sequencing was performed. Amplified PCR samples were used for gene libraries by using the NEXTERA XT (ILLUMINA, USA) kit protocol and ran on the NextSeq500 platform. Variants were analyzed using Illumina Variant Studio software, Alamut Visual and HGMD Professional databases and pathogenicity prediction of novel variants were evaluated using Polyphen2, SIFT and MutationTaster databases (Adzhubei et al., 2013; Vaser et al., 2016). The mean depth of coverage was 50-fold coverage and an average of 93% of target bases sequenced at ≥20× coverage.
Given the rarity of the phenotype and due to parental consanguinity, we first queried for homozygous variations which were absent in public databases like dbSNP, 1000 Genomes Project and ExAC. We then filtered for candidates that were computationally predicted to be damaging according to SIFT, Polyphen2 and mutationtaster databases. The RNPC3 gene was the only candidate gene associated with the phenotype. Besides the homozygous variant in RNPC3, there were six more homozygous variations identified in WES (SLC10A2; p.Ala190Thr, TCHH; p.Gln506_Leu507insGluArgArgGluGlnGln, KCNN3; p.Gln76_Gln80dup, KIF1A; p.Glu917del, CLDN16; p.Ala56LeufsTer16, AR; p.Gln58_Gln60del), all of which were predicted benign according to ACMG criteria or reported benign in ClinVar. We have also checked pathogenic variants in the previously described genes for growth/ pituitary hormone deficiency and central hypothyroidism (HESX1, LHX3, LHX4, POU1F1, PROP1, BTK, GH1, GHRHR, GHSR, OTX2, SOX2, SOX3, PAX6, BMP4, FGFR1, ARNT2, GLI2, FGF8, PROKR2, GPR161, IGSF1, NFKB2, PITX2, CHD7, TSHB, TRHR, THR, and GNAS; Ara et al., 2021). No disease associated mutations or variations were found in these genes. Furthermore, there were no disease associated variants in 200 genes known to be associated with peripheral neuropathy and neurodegenerative diseases.
We have compared the clinical characteristics and pituitary function test results of our patient with all other previously reported patients with RNPC3 mutations (Table 1).
3 RESULTS
We detected a novel homozygous missense change in exon 12 of RNPC3 (NM_017619.3:c.1328A>G; NP_060089.1:Y443C) by whole exome sequencing. Sanger sequencing confirmed homozygosity in the patient and heterozygosity in the mother of the given variant (Figure 1j). The variant was also absent in our in-house control exome data set of more than 100 patient with genetic and neurometabolic disorders. The variation is a variant of unknown significance according to ACMG criteria. The variant has not been reported in the gnomAD database and is predicted to be pathogenic according to nine prediction databases including BayesDel_addAF, DANN, EIGEN, FATHMM-MKL, LIST-S2, M-CAP, MutationTaster, PrimateAI, and SIFT and predicted benign by two databases DEOGEN2 and MVP. The variant also occurs in a highly conserved residue, and is predicted to be pathogenic due to the high degree of conservation of the residue (Figure 1k).
Y443 is a large aromatic residue with its side chain buried inside the protein domain. On one side the Y443 sidechain is engaged in hydrophobic interactions with the aliphatic part of R442 and K481, two solvent exposed residues, and it also forms a hydrogen bond with the side chain of E485, another solvent exposed residue (Figure 1l,m). On the other side, the Y443 contributes largely to form the hydrophobic core of the protein together with L394, I422, V424, L436, I439, F440, I471, L473, A478, A482, A486, and M496. Mutation of Y443 by a much smaller cysteine residue would have a dramatic effect on the protein hydrophobic core which has a crucial function to maintain the normal structure and function of U11/U12 65K protein, and it would also affect the conformation of R442, K481, and E485 at the surface of the protein.
4 DISCUSSION
The patient described in this report further establishes the association between syndromic congenital hypopituitarism and RNPC3 mutations, which are very rarely reported in the literature. Coexistence of severe microcephaly and anatomic brain abnormalities in our patient suggest the role of RNPC3 in pituitary and brain development.
The phenotypic spectrum associated with RNPC3 mutations has been better understood with the description of new affected patients. So far 20 patients from eight families have been reported (Akin et al., 2022; Argente et al., 2014; Gucev et al., 2015; Verberne et al., 2020; Yamada et al., 2021). Isolated growth hormone deficiency was described in the initial patients (Argente et al., 2014; Gucev et al., 2015). Akin et al. (2022) reported growth hormone, as well as variable TSH and PRL deficiencies, in their patients, as did Verberne et al. (2020). Similarly severe deficiencies of TSH, PRL and growth hormone in our patient supports the role of RNPC3 mutations in the etiology of some syndromic forms of congenital hypopituitarism, possibly with variable interindividual phenotypic expression. Compared with previously published patients, our patient had the most severe growth failure phenotype. Nevertheless ACTH and vasopressin secretion of the patient were normal at 20 years of age, and this is consistent with previously published patients, suggesting spared function of corticotrophs and vasopressin-secreting cell lineages in these patients. Gonadotropin function of previous patients was variable; some had ostensibly low gonadotrophins (Verberne et al., 2020) whereas others had elevated FSH values (Akin et al., 2022; Argente et al., 2014). Akin et al. (2022) reported five female patients from the same family with ovarian dysgenesis due to homozygous RNPC3 mutations; however, histological examination of sexually mature ovaries of Rnpc3 knockout mice revealed no abnormalities. FSH secretion increased to 21.5 mIU/ml by GnRH stimulation in our patient at 20 years of age; nevertheless her bone age was 2 years which makes the interpretation of these data challenging.
A number of other clinical characteristics were reported in patients with RNPC3 mutations including red hair, obesity and myopathy (Gucev et al., 2015), congenital cataract and intellectual disability (Verberne et al., 2020). This study, to our knowledge, is the first to describe diffuse cerebral and cerebellar atrophy associated with a hypoplastic anterior pituitary. In both mouse and human, Rnpc3/RNPC3 was expressed in the telencephalon, diencephalon, trigeminal ganglia, hypothalamus and Rathke's pouch (Akin et al., 2022). Various datasets also demonstrate the high expression of RNPC3 in cerebral cortex, cerebellum, basal ganglia, amygdala and hippocampus (https://www.proteinatlas.org/ENSG00000185946-RNPC3/tissue). Pathogenic variants in components of the minor spliceosome have been associated with several human diseases associated with microcephaly including Lowry Wood syndrome, microcephalic osteodysplastic primordial dwarfism type 1 (MOPD1) or Taybi-Linder syndrome (TALS) (Edery et al., 2011; Farach et al., 2018; Kilic et al., 2015). This suggests that aberrant/abnormal splicing of genes containing U12-type introns may impair global cell proliferation in brain. However, previous patients with RNPC3 mutations were also reported to have less severe microcephaly (Argente et al., 2014), although Yamada et al. (2021), described a patient with severe microcephaly and growth retardation due to compound heterozygous RNPC3 mutations. Cranial imaging findings of our patient support the role of RNPC3 in global brain development. Even more importantly, minor spliceosome inactivation is reported to cause microcephaly, owing to cell cycle defects and death of self-amplifying radial glial cells (Baumgartner et al., 2018) or reported to be linked with some neurodegenerative diseases like amyotrophic lateral sclerosis (Buratti, 2016; Onodera et al., 2014), and early onset cerebellar ataxia (Elsaid et al., 2017). Polyneuropathy has already been reported in the majority of the patients with RNPC3 mutations (Akin et al., 2022). Although we could not confirm the polyneuropathy by electromyography or nerve conduction studies; skin findings, anhydrosis and deformities, and spasticity in extremities in the absence of skeletal abnormalities suggest severe polyneuropathy in our patient. Together with global cerebral and cerebellar atrophy in this patient, we think that RNPC3 mutations which impair the function of minor spliceosome complex can be related to degeneration and atrophy of neural structures and that potential manipulation of the minor spliceosome pathway can be an important treatment target for neurodegenerative disorders. Phenotypic variation in microcephaly and severity of neurodegeneration in previously reported patients can be explained by the type or the effect of the RNPC3 variation, though this needs further functional evidence, which we cannot provide in this report. In summary, our findings suggest that RNPC3 is a cause of syndromic congenital hypopituitarism. The brain phenotype of minor spliceosome-related disease due to RNPC3 mutation might be broader than previously described.
AUTHOR CONTRIBUTIONS
Diğdem Bezen, Mehul Dattani, Tulay Guran and Gözde Yeşil designed the study. Diğdem Bezen, Orkide Kutlu, Tulay Guran and Gözde Yeşil clinically characterized the patient. Diğdem Bezen, Orkide Kutlu and Tulay Guran conducted and analyzed biochemical measurements. Gözde Yeşil performed and analyzed the sequencing data. Stephane Mouilleron and Karine Rizzoti analyzed the characteristics of mutant protein in silico. Diğdem Bezen, Mehul Dattani, Tulay Guran and Gözde Yeşil prepared the draft manuscript. All authors contributed to the discussion of results, and edited and approved the final manuscript.
ACKNOWLEDGMENT
We are deeply grateful to the patient and family without whom this study could not have been performed.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Written approval from Marmara University Ethics Committee was obtained prior to the short report (#01.04.2022.625).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




