Detecting pathogenic deep intronic variants in Gitelman syndrome
Funding information: Grants-in-Aid for Scientific Research (KAKENHI), Grant/Award Number: 20K16892; The Japan Foundation for Pediatric Research, Grant/Award Number: 19-002
Abstract
Gitelman syndrome (GS) is a rare, autosomal recessive, salt-losing tubulopathy caused by loss of function in the SLC12A3 gene (NM_000339.2), which encodes the natrium chloride cotransporter. The detection of homozygous or compound heterozygous SLC12A3 variants is expected in GS, but 18%–40% of patients with clinical GS carry only one mutant allele. Previous reports identified some pathogenic deep intronic variants in SLC12A3. Here, we report the screening of SLC12A3 deep intronic variants in 13 patients with suspected GS carrying one mutated SLC12A3 allele. Variant screening used the HaloPlex Target Enrichment System Kit capturing whole introns and the promotor region of SLC12A3, followed by SureCall variant analysis. Rare intronic variants (<1% frequency) were identified, and pathogenicity evaluated by the minigene system. Deep intronic variant screening detected seven rare SLC12A3 variants from six patients. Only one variant showed pathogenicity in the minigene system (c.602-16G>A, intron 4) through activation of a cryptic acceptor site. No variants were detected in the promotor region. Deep intronic screening identified only one pathogenic variant in patients with suspected GS carrying monoallelic SLC12A3 variants. Our results suggest that deep intronic variants partially explain the cause of monoallelic variants in patients with GS.
1 INTRODUCTION
Gitelman syndrome (GS) is an inherited tubulopathy caused by defects in the SLC12A3 gene (NM_000339.2), the apical sodium chloride transporter (natrium chloride cotransporter/NCCT) also known as the thiazide sensitive cotransporter in the distal convoluted tubule (Blanchard et al., 2017; Mori et al., 2020; Nozu et al., 2009). Gitelman et al. (1966) described a familial disorder characterized by hypokalemia, hypomagnesemia, hypocalciuria, metabolic alkalosis, and hypereninemic hyperaldosteronism. The distal convoluted tubule is responsible for 5%–10% of renal sodium reabsorption in healthy individuals (Subramanya, 2014), but SLC12A3 defects in patients with GS result in increased sodium delivery to the collecting duct, a degree of distal sodium rescue, and increased potassium and hydrogen excretion (Urwin et al., 2019).
GS is caused by biallelic pathogenic variants of the SLC12A3 gene that encodes NCCT (Simon et al., 1996). GS is inherited as an autosomal recessive trait, so homozygous or compound heterozygous variants are expected (Simon et al., 1996). However, after SLC12A3 screening, between 18% and 40% of patients with clinical GS appeared to carry only one mutant allele (Ji et al., 2008; Lemmink et al., 1998). An unidentified variant in the second allele could be explained by human error, the direct sequencing of an incomplete detection, including rearrangements, or the possibility of variants in gene-regulating elements such as promotor or enhancer segments (Nozu et al., 2016). This is supported by a previous study by Vargas-Poussou et al. (2011), which found that only 70% of unrelated patients with suspected GS had biallelic variants.
Significant findings from multiplex ligation-dependent probe amplification (MLPA) do not account for all patients with GS carrying monoallelic SLC12A3 variants. Indeed, only 6% of heterozygous patients harbored large SLC12A3 rearrangements in the form of copy number variations (CNVs; Vargas-Poussou et al., 2011). CNVs can readily be screened by pair analysis using next-generation sequencing (NGS) data (Nagano et al., 2018). However, still only monoallelic variants were detected in high numbers of patients with GS. A lack of systematic screening for such cases raises the possibility of a failure in the variant detection process (Gamba, 2005; Riveira-Munoz et al., 2008).
Genetic testing has been revolutionized by NGS. However, because the information obtained from standard NGS is limited to exons and exon–intron boundaries, the genetic cause of a disease is not always identified in a substantial proportion of patients (Vaz-Drago et al., 2017). The prevalence of GS is relatively common, at approximately 1 in 40,000 (Hsu et al., 2009), but symptoms can be unspecific and difficult to diagnose without genetic diagnosis (Cruz et al., 2001). Additionally, we recently reported total carrier frequencies of SLC12A3 pathogenic variants in the Japanese population of approximately 9%, and a calculated GS prevalence of approximately 2 in 1000 based on reference databases of genetic variations in the Japanese population (Kondo et al., 2021). Therefore, it is conceivable that many cases of GS are undiagnosed.
Deep intronic variants can activate noncanonical splice sites and alter splicing regulatory elements (Lo et al., 2011; Nozu et al., 2016; Nozu et al., 2019). Such variants also interrupt regulatory motifs and the transcription of noncoding RNA genes. Deleterious DNA variants positioned >100 bp from exon–intron junctions usually contribute to pseudo-exon inclusion. The inclusion of pseudo-exons is now considered a more common cause of disease than was previously thought (Vaz-Drago et al., 2017). Nozu et al. were first to report a single base substitution in the deep intron of SLC12A3 that resulted in the inclusion of a novel cryptic exon in the mRNA (c.1670-191C>T; Nozu et al., 2019). Lo et al. (2011) identified a different deep intronic variant in intron 21 of SLC12A3 (c.2548+253 C>T; Lo et al., 2011), and we detected another pathogenic deep intronic variant in SLC12A3 (c.1669+297T>G) in a 5-year-old girl with latent GS diagnosed by chance with mild proteinuria and electrolyte imbalance accompanied by metabolic alkalosis (Nozu et al.).
Identification of this new deep intronic variant was facilitated by NGS analysis capturing the whole intron sequence of SLC12A3 followed by the step-wise identification of the intronic variant, in silico analysis, and real-time PCR validation of aberrant transcripts (Nozu et al., 2016). Such deep intronic variants partially explain the low detection rate of biallelic variants in some patients with GS because disease may be triggered by single base substitution within the deep intron.
In the present study, we used NGS capturing the whole intron sequence and in vitro analyses to determine whether deep intronic variants in SLC12A3 were present in 13 patients with suspected GS carrying monoallelic SLC12A3 variants. We also screened the SLC12A3 promotor region in which variants can be potentially pathogenic, although none has been detected previously.
2 METHODS
2.1 Patients
Thirteen patients from our cohort with suspected GS, as determined by clinical symptoms and biochemical profiles (Blanchard et al., 2017), who had been shown by previous targeted NGS and variant calling using SureCall v.4.0 (Agilent Technologies, Santa Clara, CA) to carry only one mutated SLC12A3 allele (Smith et al., 2007) were included in this study. We classified the pathogenicity of the previous variants according to the American College of Medical Genetics and Genomics (ACMG) criteria using VarSome (Kopanos et al., 2019).
Patients were included following informed consent from each individual. All procedures involving human participants in this study were performed in accordance with the ethical standards of the Institutional Review Board of Kobe University Graduate School of Medicine (IRB approval number 301). The clinical characteristics of the patients are shown in Table 1. No significant large SLC12A3 rearrangements were found by the MLPA or CNV analysis.
| No. | Patient's ID | Age | Gender | Nucleotide change | Amino acid change | ACMG criteria | Symptom | Potassium (mEq/L) (RR 3.5–4.5) | Mg (mg/dl) (RR 1.5–3) | Urine ca/Cr (mg/mg) (RR 0.04–0.37) | Bicarbonate (mEq/L) (RR 22–28) | Renin (ng/ml/h) | Aldosterone (ng/dl) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | A004 | 22 | Male | c.1394C>T | p.(Thr564Ile) | Likely pathogenic | Polyuria | 2.5 | 2.1 | 0.0014 | 29.3 | 9.3 | 214 pg/ml | – |
| 2. | B021 | 41 | Male | c.2782C>T | p.(Arg928Cys) | VUS | Lower limb weakness and numbness | 2.9 | 1.6 | 0.0042 | 30 | 11.7 | 24.1 | Lemmin et al. (1998) |
| 3. | B065 | 11 | Male | c.2891G>A | p.(Arg964Gln) | Pathogenic | No specific symptom | 2.1 | 1.3 | 0.026a | 26.8 | ND | 1275.2 pg/ml | Simon et al. (1996) |
| 4. | B105 | 46 | Female | c.2573T>A | p.(Leu858His) | Pathogenic | Upper and lower limb weakness and numbness | 1.4 | 1.9 | 0.014 | 25.2 | 37.6 | 11.7 | Monkawa (2000) |
| 5. | B107 | 53 | Female | c.1216A>C | p.(Asn406His) | Likely pathogenic | Athralgia | 2.5 | 1.8 | 0.048 | 41.6 | 5.2 | 16.3 | Yoo (2003) |
| 6. | B191 | 48 | Male | c.2573T>A | p.(Leu858His) | Pathogenic | No specific symptom | 2.8 | 2.3 | 0.08 | 30.1 | 1.3 | 361 pg/mL | Monkawa (2000) |
| 7. | B257 | 65 | Male | c.539C>A | p.(Thr180Lys) | Likely pathogenic | No specific symptom | 2.7 | 1.9 | 0.097 | 30.8 | 2.2 | 207 pg/mL | Monkawa (2000) |
| 8. | B265 | 12 | Female | c.2573T>A | p.(Leu858His) | Pathogenic | Fatique tetany | 3.1 | 2.1 | 0.16a | 27.8 | 9.5 | 8.7 | Monkawa (2000) |
| 9. | B289 | 18 | Male | c.2573T>A | p.(Leu858His) | Pathogenic | No specific symptom | 2.1 | 2.8 | 0.0027a | 50.5 | 482.9 | 78.8 | Monkawa (2000) |
| 10. | B291 | 28 | Male | c.2029G>A | p.(Val677Met) | Likely pathogenic | Tetany | 2 | 1 | 0.036 | 30.2 | ND | ND | Syrén (2002) |
| 11. | B292 | 19 | Male | c.2612G>A | p.(Arg871His) | Pathogenic | No specific symptom | 2.3 | 1.9 | 0.007 | 39.1 | 10.9 | 116 pg/ml | Lin (2005) |
| 12. | B298 | 28 | Female | c.1868T>C | p.(Leu623Pro) | Likely pathogenic | Upper limb numbness | 2.8 | 1.4 | 0.01 | 30 | ND | 478.15 pg/ml | Takeuchi (1996) |
| 13. | B300 | 44 | female | c.1698C>A | p.(Asn566Lys) | VUS | No specific symptom | 2.5 | 1.9 | 0.199 | 37.1 | 11 | 223 pg/ml | Fujimura et al. (2019) |
- Note: Biochemical value at the time referred to our hospital.
- Abbreviations: ACMG, American College of Medical Genetics and Genomics; Ca, calcium; Cr, creatinine; Mg, magnesium; ND: no data; RR: reference range; VUS, variant of uncertain significant.
- a Normal range calcium/creatinine ratio in children (7–17 years) = 0.01–0.25 mg/mg.
2.2 Potential pathogenic deep intronic variant screening in SLC12A3
DNA was isolated from peripheral blood samples using a QuickGene DNA whole blood kit (Kurabo, Kurashiki, Japan). Samples for NGS analysis were prepared using the HaloPlex target enrichment system kit (Agilent Technologies) according to the manufacturer's instructions. SLC12A3 and other tubulopathy-related genes including deep intronic sites were sequenced on an MiSeq platform (Illumina, San Diego, CA), and variant calling was performed using SureCall v.4.0 (Agilent Technologies). The obtained SLC12A3 sequence was mapped to the human RefSeq SLC12A3 sequence (NM_000339.2). Following the step-wise identification of a previously reported intronic variant (Nozu et al., 2019), rare variants with a minor allele frequency of <1% were analyzed using a variant annotation window by Alamut Visual software, v.2.11 (Interactive Biosoftware, Rouen, France). The site of the promoter region was defined based on typical putative promoter elements (MacKenzie et al., 2001).
To confirm the pathogenicity of candidate variants, we conducted an in vitro splicing assay using the minigene system.
2.3 In vitro splicing assay
We used the H492 vector that we had developed previously to create hybrid minigene constructs (Nozu et al., 2016). The H492 vector is based on the pcDNA3 mammalian expression vector (Invitrogen, Carlsbad, CA) and mimics in vivo splicing. We cloned the intronic site of the suspected SLC12A3 variant with up- and downstream flanking exons between exon A and exon B on the H492 vector. We used the In-Fusion cloning method according to the manufacturer's instructions (Takara, Japan) and transfected the constructs into HEK293T and HeLa cells using Lipofectamine® 3000 (Thermo Fisher Scientific, Waltham, MA). After 24 h, total RNA was extracted from the cells using an RNeasy® Plus Mini Kit (QIAGEN, Hilden, Germany). Then, 1 μg of total RNA was reverse transcribed into cDNA using the RNA to cDNA EcoDry Premix (Double Primed; Takara, Japan). PCR was performed with a forward primer complementary to a segment upstream of exon A (YH307: 5′-ATTACTCGCTCAGAAGCTGTGTTGC-3′) and a reverse primer complementary to a segment downstream of exon B (Y308: 5′-CTGCCAGTTGCTAAGTGAGAGACTT-3′). The PCR products were analyzed by 1.5% agarose gel electrophoresis, followed by Sanger sequencing. When the different bands on the agarose gel were not evident, we performed capillary electrophoresis on an Agilent 2100 Bioanalyzer using an Agilent DNA1000 Kit (Agilent Technologies, Santa Clara, CA).
Positive controls for deep intronic evaluation were established by in vitro splicing of previously reported deep intronic variants in SLC12A3: positive control no. 1 c.1670-191C>T; no. 2 c.2548+253C>T; and no. 3 c.1567+297T>G (Lo et al., 2011; Nozu et al., 2019; Table 2).
| Case no. | Patient ID | Clinical characteristic | Variant in SLC12A3 | Type of aberrant splicing caused by deep intron variant |
|---|---|---|---|---|
| 1 | 28 |
|
c.818_819insG c.1670-191C>T | New donor site activation ➔ pseudo-exon inclusion |
| 2 | 29 | c.448C>T c.2548+253C>T | New acceptor site activation ➔ pseudo-exon inclusion | |
| 3 | B144 |
|
c.2927C>T c.1567+297T>G | New acceptor site activation ➔ pseudo-exon inclusion |
3 RESULTS
3.1 Patient characteristics
We enrolled a total of 13 patients (eight males and five females) with suspected GS who were 11–65 years old (Table 1). All the patients carry a previously reported heterozygous missense variant in SLC12A3, except for patient no. 1 who has a novel heterozygous missense variant. No other suspected pathogenic variant related with GS-like tubulopathy, including variants in CLCNKB, KCNJ10, FXYD2, and HNF1B, was found. Most patients presented with weakness in their extremities as the main clinical complaint suggesting a diagnosis of GS, and the patients showed variability in proposed biochemical criteria for a diagnosis of GS. Some patients had no specific symptoms and were diagnosed by chance as having hypokalemia. The possibility of non-genetic conditions related with GS (diuretic abuse, chronic laxative abuse, or chronic vomiting) was not evident.
3.2 Pathogenic deep intronic variant screening in SLC12A3
We used the HaloPlex target enrichment system to analyze deep intronic variants and detected seven candidate variants with minor allele frequencies of <1% in six patients (Table 3). One of these variants (c.602-16G>A in patient no. 1) was predicted to cause aberrant splicing by in silico analysis using SpliceSiteFinder-like and MaxEntScan tools in Alamut software (Figure S1). All identified candidate variants were novel, except for c.602-16G>A in patient no. 1 (Glaudemans et al., 2012; Table 1). No variants were detected in the promotor region of SLC12A3.
| Case no. | Patient's ID | Variant | Intron location | Total allele frequency | In silico prediction of splicing regulatory elements/score | Reference |
|---|---|---|---|---|---|---|
| 1. | A004 | c.602-16G>A | IVS4 | 0.00006047 | Creation of novel acceptor site 80.5b/9.5c | Glaudemans et al.a |
| 2. | B021 | c.2951+103C>T | IVS25 | ND | ND | ND |
| 3. | B065 | c.1568-353C>T | IVS12 | 0.00004333 | ND | ND |
| 4A. | B105 | c.1444-87C>T | IVS11 | ND | ND | ND |
| 4B. | B105 | c.2952-326A>G | IVS25 | ND | ND | ND |
| 5. | B107 | c.2952-4257A>G | IVS25 | ND | Creation of novel acceptor site/68.2b/9.2c | ND |
| 6. | B191 | c.2951+4262G>C | IVS25 | ND | ND | ND |
- Abbreviations: IVS, intron; ND, no data.
- a Glaudemans et al. (2012).
- b SpliceSiteFinder-like in silico prediction.
- c MaxEntScan in silico prediction.
3.3 In vitro splicing assay
To further investigate the seven candidate deep intronic variants, hybrid minigenes were constructed by inserting genomic DNA around the target SLC12A3 variant. We cloned intron 4–intron 5 for the suspected case no. 1; intron 24–intron 25 for suspected case nos. 2, 4B, 5, and 6; intron 11–intron 12 for suspected case no. 3; and intron 10–intron 12 for suspected case no. 4A. We also cloned intron 13–intron 14 for positive control no. 1; intron 20–intron 22 for positive control no. 2; and intron 11–12 for positive control no. 3 (Figure S2).
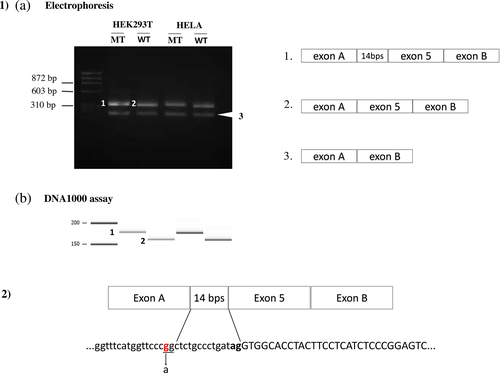
The minigene system detected aberrant splicing (i.e., inclusion of the cryptic exon) in the three positive control cases (Figures S3 and S4). Among the seven candidate deep intronic variants, only variant c.602-16G>A in patient no. 1 showed aberrant splicing that led to the inclusion of an intron fragment through the creation of a new acceptor site (Figure 1, Figures S5 and S6). We used the Agilent DNA 1000 assay to enhance visualization of the inclusion of a 14-bp intron fragment. No specific aberrant splicing was shown in the other six variants.
4 DISCUSSION
In this study, we comprehensively investigated pathogenic variants located in the deep introns and promotor region of SLC12A3 by NGS. We screened 13 patients with suspected GS carrying only monoallelic variants, and evaluated the seven candidate deep intronic variants by minigene assay. We detected only one deep intronic variant that caused aberrant splicing, and no variants were detected in the promotor region.
Recently, GS was classified as a salt-losing tubulopathy along with other tubulopathies such as Bartter syndrome, and therefore GS is not always confirmed clinically. We included patients who were clinically compatible with GS and carried a monoallelic variant in SLC12A3. For the 12 patients in which the other variant was not identified, we considered the following possibilities. The second variant was simply not detected, or these patients had the intermediate phenotype of GS with a monoallelic variant as previously reported, or they harbored other GS-like tubulopathies. Other tubulopathies caused by CLCNKB, KCNJ10, FXYD2, and HNF1B can roughly be ruled out based on the results of the standard NGS analysis. Variants of uncertain significance (VUS) may play a role in patients with GS who were substantially monoallelic (Nuñez-Gonzalez et al., 2021). The probability of such variants becoming pathogenic was estimated to be 10%–90%, with “hot” VUS having the highest probability (Richards et al., 2015).
Evidence from mRNA analysis has indicated that pathogenic variants can occur deep within the introns of over 75 disease-associated genes (Vaz-Drago et al., 2017). Deep intronic variants may be undetected until they are suspected and investigated separately or as part of a whole-genome study (Iancu & Ashton, 2020). Because sequencing that is limited to exon and exon–intron boundaries cannot detect genetic causes of diseases in a large proportion of patients (Vaz-Drago et al., 2017), we performed deep intronic targeted panel sequencing by NGS to avoid missing deep intronic variants in SLC12A3.
Introns are typically considered the least conserved regions of a gene, where most variations will accumulate without having a major impact unless they alter essential regions such as canonical donor and acceptor sites or the branch site. However, some variants may produce a novel splice site and cause the original splice site to be misidentified (Vaz-Drago et al., 2017). Moreover, when deep intronic variants are located <150 bp away from natural exon–intron junctions, weakening of the canonical splice site has been observed (Vaz-Drago et al., 2017). This is consistent with our finding that c.602-16G>A, located in intron 4 of SLC12A3, led to the inclusion of a 14-bp pseudo-exon by creating a new acceptor site (score = 80.5 using the SpliceSiteFinder-like algorithm; score = 5.2 using MaxEntScan). This variant was previously reported in a patient with clinically suspected GS (Glaudemans et al., 2012), but transcript analysis was not performed, and therefore it was not possible to determine the pathogenicity of this variant.
In the present study, pathogenicity could not be confirmed for the remaining six suspected variants by in vitro transcript analysis. This is consistent with the in silico prediction, which showed no probability of aberrant splicing or a low score of cryptic acceptor site activation in c.2952-4257 A>G. In the remaining suspected pathogenic deep intronic variants, the minigene transcript analysis identified nonspecific transcripts with inclusions of vector intron fragments and deletions of exon fragments. These transcripts can occur because the base sequence tends to create a novel acceptor/donor site in the absence of a strong splice site in the inserted sequence, and is a limitation of minigene splicing analysis.
No other notable findings could explain the heterozygosity in the remaining patient with GS, including rearrangements such as duplications or deletions, or the presence of variants in gene-regulating fragments such as promoter or enhancer segments. Ellison et al reported that intermediate phenotypes may be present in heterozygous SLC12A3 carriers, although their occurrence was not statistically significant (Ellison & Lin, 2019). This is in accordance with the findings of Wan et al. (2021) who showed that heterozygous carriers of SLC12A3 p.R642G had significantly lower serum potassium levels than noncarriers, but that levels were less severe than those seen in patients with GS. The average potassium level of 2.4 mEq/L detected in the 13 patients with suspected GS and monoallelic SLC12A3 variants was similar to the 2.5 mEq/L seen in the patients with GS in our earlier cohort (Fujimura et al., 2018).
In summary, we identified a pathogenic deep intronic variant in only one of 13 patients with suspected GS whose monoallelic SLC12A3 variant was detected by standard sequencing and CNV analysis. Our results suggest that deep intronic variants partially explain the cause of monoallelic variants in patients with GS. However, because the number of the patients in our study was relatively small, no definitive conclusion can be drawn. A larger cohort and more samples will allow more confidence in estimating the proportion of patients with GS caused by deep intronic variants. Furthermore, it is conceivable that other mechanisms of disease onset are present in patients with GS who carry monoallelic SLC12A3 variants, such as modifier genes or environmental factors.
ACKNOWLEDGMENTS
This study was supported by The Japan Foundation for Pediatric Research (grant no. 19-002 to Tomoko Horinouchi) and Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (subject IDs: 20K16892 to Tomoko Horinouchi, 19K08726 to Kandai Nozu, and 19K17710 to Tomohiko Yamamura). We thank Sarah Williams, PhD and Margaret Biswas, PhD from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




